ABSTRACT
Palivizumab is the only licensed respiratory syncytial virus (RSV) immunoprophylaxis (IP) available to prevent severe RSV disease in high-risk pediatric populations, including infants born at 29–34 weeks’ gestational age (wGA). In 2014, the American Academy of Pediatrics (AAP) stopped recommending RSV IP use for otherwise healthy 29–34 wGA infants and stated that 29–34 wGA infants and term infants have similar RSV hospitalization (RSVH) rates. This study aimed to compare RSV IP use and RSVH rates in 29–34 wGA infants and term infants during the 3 RSV seasons before and after the 2014 AAP policy change. RSV IP use in otherwise healthy infants 29–30, 31–32, and 33–34 wGA was estimated from pharmacy or outpatient medical claims for palivizumab. RSVH rates in the first 6 months of life were calculated per 100 infant-seasons. RSVH rate ratios were used to compare preterm infants and term infants before and after the policy change. Across infant cohorts (29–34 wGA) and chronologic age groups (<3 months and 3-<6 months), absolute decreases in RSV IP use between the combined 2011–2014 seasons and 2014–2017 seasons ranged from 7% to 38% and from 68% to 97%, respectively. Compared with 2011–2014, the RSVH risk increased 2.09-fold (P< .001) and 1.76-fold (P< .001) in 2014–2017 for infants born at 29–34 wGA and aged <6 months with commercial and Medicaid insurance, respectively. Overall, RSV IP use declined in the RSV seasons following the 2014 RSV IP policy change, and RSVH increased among 29–34 wGA infants aged <6 months.
Introduction
Respiratory syncytial virus (RSV) is a common infectious agent that can result in severe lower respiratory complications in at-risk populations, including preterm infants.Citation1 Among infants aged <1 year, RSV bronchiolitis is the leading cause of hospitalization, accounting for 20% of hospitalizations for acute respiratory infections during the peak season that extends from November through March.Citation2–4 Predictors of severe RSV infection and RSV hospitalization (RSVH) include preterm birth, chronologic age of <6 months during the RSV season, congenital heart disease (CHD), bronchopulmonary dysplasia (BPD, also known as chronic lung disease of prematurity [CLDP]), exposure to other children in the household or childcare, and cigarette smoke exposure.Citation5–7
There is no vaccine to prevent RSV disease, and treatment of this illness is supportive. Supplemental oxygen, intensive care, ventilatory support, and administration of intravenous fluids may be required until the child’s condition improves.Citation8 RSVH risk among vulnerable infants can be reduced with injections of the prophylactic antibody palivizumab.Citation9,Citation10 Palivizumab is approved for the prevention of RSVH in infants born preterm (≤35 weeks’ gestational age [wGA]) who are aged ≤6 months at RSV season start and infants and young children who are aged ≤24 months at RSV season start and who have BPD or hemodynamically significant CHD.Citation10 Among infants born at 29 to <35 wGA, without CLDP or CHD, and aged <6 months at RSV season start, palivizumab has an estimated real-world effectiveness of 74% in reducing RSV hospitalizations.Citation11
First released in 1998,Citation12 the American Academy of Pediatrics (AAP) Committee on Infectious Diseases (COID) has issued multiple recommendations for (or against) RSV immunoprophylaxis (IP) with palivizumab across different cohorts of infants (e.g., <29 wGA, 29–34 wGA, 35–36 wGA, and ≥37 wGA) based on their changing opinions of what they consider to be high-risk infants.Citation13–16 With respect to a single cohort, infants born at 29 to 34 wGA, the AAP took a more restrictive position in 2014 and stopped recommending RSV IP with palivizumab for those infants who do not have BPD/CLDP or CHD, saying that these preterm infants should no longer be considered high risk because “infants born at or after 29 weeks, 0 days’ gestation have an RSVH rate similar to the rate of full-term infants.”Citation17
In the RSV seasons following this policy change, observational studies, including some by current authors, have characterized patterns in the occurrence of RSVH and reported an increase in the risk and severity of RSVH among infants born at 29 to 34 wGA during the first 3 to 6 months of life.Citation18–22 The finding of greater RSVH risk persisted in a direct comparison of the 2 seasons before and after the change.Citation23 In an examination of the 3 seasons before and after the policy change, Krilov et al. found an increase in RSVH severity among preterm infants after 2014, with the greatest increase among those aged <3 months.Citation19 Following some of these publications, in 2018, the National Perinatal Association (NPA) updated its guidance for the use of palivizumab, recommending it for all infants born at 29 to 31 wGA and some 32 to 35 wGA infants with additional risk factors.
This study follows the previous methodology and evaluates the durability of previous results by comparing RSVH rates in the three seasons before and after the 2014 AAP policy change among commercially insured and Medicaid-insured preterm infants expected to be affected by the change.Citation23 The primary objective was to compare the use of RSV IP and RSVH rates for preterm (29 to 34 wGA) infants with those of term (>36 wGA) infants in the 3 RSV seasons before (2011–2014) and after (2014–2017) the policy change. RSVH costs were assessed as a secondary objective.
Methods
Study design and data source
This analysis was an observational retrospective cohort study of preterm and term infants in the United States with data obtained from de-identified administrative claims housed in the IBM MarketScan® Commercial and Multi-State Medicaid databases. Each of these databases captures the enrollment data, inpatient medical data, outpatient medical data, and outpatient prescription drug data for its respective covered population. The commercial database includes data for employees and their dependents covered under a variety of fee-for-service and managed care health plans. The Medicaid database contains data for covered individuals in approximately 15 geographically dispersed states. Data from the commercial and Medicaid populations were analyzed separately.
All study data were accessed with protocols that are compliant with US patient confidentiality requirements, including the Health Insurance Portability and Accountability Act of 1996 regulations (HIPAA). All data were fully de-identified and compliant with HIPAA; as such, this study was exempted from institutional review board approval.
Patient selection and cohort assignment
The initial cohort included infants born between July 1, 2010, and June 30, 2017, who could be linked to a birth hospitalization record and were discharged alive from birth hospitalization. Gestational age at birth and the presence of medical conditions associated with RSV risk were obtained using International Classification of Diseases, 9th and 10th Revision, Clinical Modification (ICD-9-CM and ICD-10-CM) diagnosis codes, diagnosis-related group (DRG) codes, and Current Procedural Terminology (CPT) 4th edition procedure codes. National Drug Codes (NDC) were used to assess the use of selected medications. Consistent with our previous studies on RSVH, a hierarchical approach was used to assign each infant to one of the following mutually exclusive risk cohorts: (1) infants with complex, rare medical conditions (e.g., infants with evidence of cystic fibrosis, immunodeficiency, congenital anomalies of the respiratory system, neuromuscular, immunologic, or genetic conditions, or organ transplants); (2) infants with CLDP; (3) infants with CHD; (4) infants born at <29 wGA; (5) infants born at 29 to 30 wGA; (6) infants born at 31 to 32 wGA; (7) infants born at 33 to 34 wGA; (8) infants born at 35 to 36 wGA; (9) term infants (>36 wGA) with major health problems (based on DRG code 793); (10) term infants without major health problems (i.e., normal newborn; DRG code 795); (11) other preterm infants with unknown wGA; and (12) other infants with unknown wGA.Citation20,Citation23
The commercial and Medicaid populations were restricted to include only otherwise healthy infants born at 29 to 34 wGA (i.e., preterm infants without evidence of the condition-based exclusions described above) and healthy term infants (i.e., term infants without major health problems). Infants in all other risk cohorts were excluded (). Demographic characteristics, including sex, health plan type, region (commercial only), and race/ethnicity (Medicaid only), were collected from the birth hospitalization record. To evaluate study outcomes, included infants were followed from birth hospitalization discharge until the earliest of the following events: end of the first 6 months of life, death, or end of continuous health plan enrollment. Infants who contributed follow-up time at <6 months old during an RSV season were further stratified into two groups based on their chronologic age during each RSV season: <3 months or 3-<6 months. Infants may have contributed time to more than one chronologic age group during the same RSV season as the infant aged. The number of infant-seasons was calculated by dividing the total number of days that an infant contributed data to a particular chronologic age group during the RSV season by 151 days (the duration of the RSV season).
Table 1. Commercially insured and medicaid-insured infants by risk cohort
Outcomes
The primary outcomes were the proportion of infants with evidence of ≥1 outpatient claim for palivizumab (i.e., RSV IP) and occurrence of RSVH during the RSV season (defined as November through March each year from 2011 to 2017). Outcomes observed during these RSV seasons were combined for the 3-season periods before and after the AAP policy change (November 2011 to March 2014 and November 2014 to March 2017). Because inpatient palivizumab doses were not captured in the source databases, RSV IP was defined by its relevant NDC code on outpatient pharmacy claims and administration codes (CPT or Healthcare Common Procedure Coding System) on outpatient medical claims. In-season RSVH were identified by ICD-9-CM (079.6, 466.11, or 480.1) and ICD-10-CM (B974, J121, J205, or J210) diagnosis codes on inpatient claims. Hospitalization rates were calculated per 100 infant-seasons. Because infants may be transferred from their birth hospital to another hospital offering more intensive care, RSVH occurring <2 days after discharge from birth hospitalization were excluded from the analysis to limit misclassification of such transfers and readmissions from birth hospitalizations. Crude rate ratios (RRs; described below) comparing hospitalization rates of preterm infants to those of term infants were calculated to account for seasonal variations in RSV circulation and severity.
RSVH characteristics (length of stay, time from admission to birth discharge, intensive care unit [ICU] admission) and costs were captured for all RSVH during the study period to provide a fuller view of the clinical and financial implications. Full-year RSVH-coded costs were captured because, while an infant’s initial RSVH may occur during the season, the infant may be readmitted or transferred, with the subsequent RSVH occurring after the end of the RSV season. Costs were based on the paid amounts of fully adjudicated inpatient claims, including insurer and health plan payments, as well as patient cost-sharing in the form of copayment, deductible, and coinsurance. All costs were inflated to 2017 US dollars using the medical care component of the Consumer Price Index.Citation24
We expect all-cause bronchiolitis hospitalization trends for preterm and term infants to mirror RSVH trends because RSV testing may not be performed, or tests may produce false negatives. As a sensitivity analysis to address potential issues related to variations in the coding of RSV over time, trends in hospitalizations for all-cause bronchiolitis were also assessed during the two sets of combined RSV seasons. All-cause bronchiolitis hospitalizations encompassed RSVH and unspecified bronchiolitis hospitalizations. The latter were identified by ICD-9-CM (466.19) and ICD-10-CM (J211, J218, or J219) codes on inpatient claims and no diagnoses of influenza, bacterial pneumonia, or other viral pathogens within 3 days of the admission. Similar to the approach used for RSVH, all-cause bronchiolitis admissions that occurred within 2 days of the birth hospitalization were excluded from the analysis.
Statistical analysis
Means and standard deviations were reported for continuous variables. Frequencies and percentages were reported for categorical variables. Data on demographic characteristics were summarized descriptively. Changes in the absolute proportions of infants with RSV IP and percentage changes in these proportions were assessed between the 2011–2014 seasons and the 2014–2017 seasons. Chi-squared tests assessed the statistical significance of differences in RSV IP proportions between seasons.
Crude (unadjusted) RRs provided an initial assessment of risk by comparing the rate of RSVH/all-cause bronchiolitis hospitalizations among preterm infants with that of term infants by season among infants aged <6 months. This comparison within each season accounts for variability in RSV circulation levels by normalizing the experience of preterm and term infants under the same exposure conditions. Crude RRs were also assessed for the combined seasons.
Difference-in-difference models were used to compare adjusted RRs of the 2011–2014 RSV seasons with the 2014–2017 RSV seasons for RSVH and all-cause bronchiolitis hospitalizations between preterm and term infants.Citation25 These models adjusted for gestational age at birth, chronologic age during the RSV season, and sex. Generalized linear models with a Poisson error distribution, log link, and a log offset term to represent exposure were used in this analysis. The following equation was used to calculate the difference-in-difference effect estimate:
Hospitalization rate for preterm infants in 2014–2017/Hospitalization rate for preterm infants in 2011–2014
Hospitalization rate for term infants in 2014–2017/Hospitalization rate for term infants in 2011–2014
Hospitalization characteristics and mean and median hospital costs were calculated separately for preterm and term infants, stratified by chronologic age at the time of the hospitalization, and summarized for the combined RSV seasons (with costs attributed to the period in which the hospitalization began). No statistical testing was conducted on hospitalization characteristics or hospital costs between periods because cohorts were not necessarily mutually exclusive, i.e., some infants could be counted in both cohorts. RSV IP and hospitalization results are presented for the overall preterm group (29–34 wGA) and the gestational age cohorts (29–30 wGA, 31–32 wGA, and 33–34 wGA). The alpha level for all statistical tests was 0.05. All data analyses were conducted using SAS version 9.4 (SAS Inc., Cary, NC).
Results
Study populations
Within the MarketScan databases, we identified a total of 1,583,610 commercially insured and 1,998,923 Medicaid-insured infants, who were born between July 1, 2010, and June 30, 2017, and discharged alive from the hospital (). After excluding infants with complex medical conditions, CLDP, or CHD, the commercially insured population included 46,868 infants born at 29 to 34 wGA and 928,288 healthy term infants. Among the Medicaid-insured population were 71,550 eligible preterm infants and 1,227,520 eligible term infants. Demographic characteristics of eligible infants who were aged <6 months are reported in .
Table 2. Characteristics of infants aged <6 months born during the study period at 29–34 wGA or term without major health problems
While <6 months of age, commercially insured preterm and term infants contributed 9,041 and 215,307 infant-seasons to follow-up, respectively, during the 2011–2014 seasons and 6,403 and 136,108 infant-seasons, during the 2014–2017 seasons (). Similarly, Medicaid-insured preterm and term infants aged <6 months contributed 13,073 and 271,815 infant-seasons, respectively, during the 2011–2014 seasons and 13,282 and 240,886 infant-seasons, during the 2014–2017 seasons. The overall MarketScan databases were smaller in the 2014–2017 period, resulting in lower infant frequencies.
Table 3. Commercially insured and medicaid-insured infants by risk cohort born between July 1, 2010, and June 30, 2017, who contributed time to the 2011 to 2017 RSV seasons while aged <6 months
RSV immunoprophylaxis
The proportion of commercially insured and Medicaid-insured infants with at least one claim for outpatient RSV IP declined across all gestational age/chronologic age category combinations following the 2014 AAP policy change (all P values comparing the 2011–2014 period with the 2014–2017 period for preterm infants were <0.001). Absolute declines in outpatient RSV IP receipt from 2011–2014 to 2014–2017 ranged from 7% (Medicaid, 33–34 wGA, 3-<6 months) to 38% (Medicaid, 29–30 wGA, 3-<6 months). Percentage declines ranged from 68% (commercial, 29–30 wGA, 3-<6 months) to 97% (commercial, 33–34 wGA, <3 months) (). Data for individual wGA cohorts can be found in the relevant tables and figures. Only 0.02% of term infants received in-season outpatient RSV IP at any point from 2011 to 2017, and the changes in RSV IP use among term infants were small in the periods before and after the AAP policy change (data not shown).
Table 4. RSV IP use among commercially insured and medicaid-insured infants by risk cohort
RSV and all-cause bronchiolitis hospitalizations
Among commercially insured preterm and term infants aged <6 months, we identified 8,223 hospitalizations in the 2011–2014 RSV seasons and 4,658 hospitalizations in the 2014–2017 RSV seasons. Of these, 2,556 (31%) and 1,468 (32%) were RSVH, contributed by 2,466 and 1,418 infants, respectively. Among Medicaid-insured preterm and term infants, we identified 16,513 hospitalizations in the 2011–2014 RSV seasons and 12,638 hospitalizations in the 2014–2017 RSV seasons. Of these, 5,558 (34%) and 4,213 (33%) were RSVH, contributed by 5,344 and 4,061 infants, respectively.
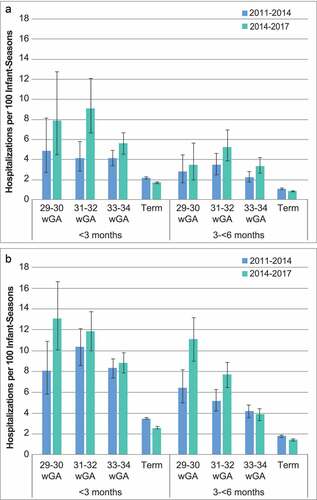
Rates of RSVH were higher among preterm infants than among term infants and ranged from 2.0 to 10.0 hospitalizations per 100 infant-seasons for infants aged <3 months and from 1.3 to 7.0 hospitalizations per 100 infant-seasons for infants aged 3 to <6 months (,)). For term infants, the rate of RSVH ranged from 1.3 to 2.5 hospitalizations per 100 infant-seasons among infants aged <3 months and from 0.6 to 1.2 hospitalizations per 100 infant-seasons among infants aged 3 to <6 months. With one exception (Medicaid infants born at 33 to 34 wGA), the RSVH rate increased in the combined 2014–2017 RSV seasons across all preterm wGA and chronologic age subgroups, whereas term rates decreased. In addition, in all preterm and term wGA and chronologic age subgroups, RSVH rates were higher among Medicaid-insured infants than among commercially insured infants.
Figure 1. RSV hospitalization rates with 95% confidence intervals for 2011–2014 and 2014–2017 RSV seasons by wGA among infants with A) commercial or B) Medicaid insurance
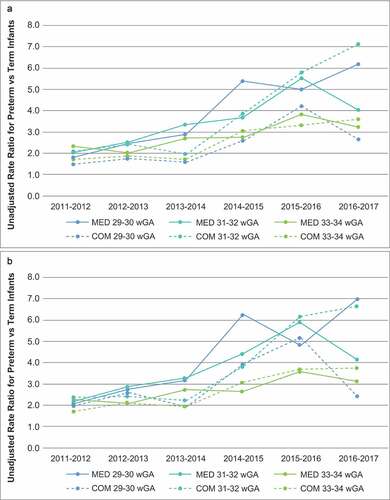
The crude RRs of RSVH rates for preterm infants vs. RSVH rates for term infants were greater than 1.0 for all wGA and chronologic age subgroups, and there was an overall trend of higher RRs in RSV seasons following the 2014 change in RSV IP policy ()).
Figure 2. Unadjusted rate ratios for (A) RSV hospitalization and (B) all-cause bronchiolitis hospitalization by season for preterm infants aged <6 months by gestational age relative to term infants aged <6 months
Difference-in-difference models estimating adjusted RRs indicated that among commercially insured infants, the risk of RSVH associated with birth at 29 to 34 wGA in the RSV seasons after the policy change was twice the risk associated with birth at 29 to 34 wGA in the RSV seasons prior to the change (RR = 2.09 [95% CI, 1.58–2.75]; P< .001). Among Medicaid-insured infants, the difference-in-difference results indicated a 76% greater risk associated with birth at 29 to 34 wGA in the RSV seasons after the policy change (RR = 1.76 [95% CI, 1.52–2.02]; P< .001) compared with the 3 seasons before the policy change (). The adjusted risk of RSVH for preterm infants relative to term infants was statistically higher in the post-policy period for all examined preterm subgroups except commercially insured infants born at 29 to 30 wGA (P= .063).
Table 5. Change in RSV hospitalization risk and all-cause bronchiolitis risk from 2011–2014 to 2014–2017 among preterm infants aged <6 months compared with the change in risk among term infants aged <6 months
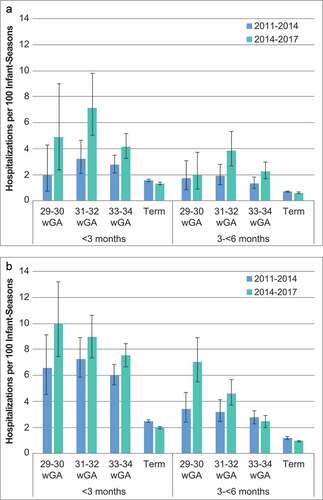
All-cause bronchiolitis hospitalization results are presented in and and . Crude RRs for all-cause bronchiolitis hospitalizations comparing preterm infants to term infants were greater than 1.0 for all wGA and chronologic age subgroups, and there was an overall trend of higher RRs in the RSV seasons following the 2014 policy change ()). Trends in the rates of all-cause bronchiolitis hospitalizations were similar to those observed in the rates of RSVH (). Difference-in-difference model results indicated that the adjusted risk of all-cause bronchiolitis hospitalization for 29 to 34 wGA infants, overall and by each GA subgroup, compared with term infants increased in the 2014–2017 RSV seasons compared with the 2011–2014 RSV seasons ().
Hospitalization characteristics and costs
As noted earlier, comparisons of hospitalization characteristics and hospital costs between periods are nominal only, without statistical tests. With one exception (29–34 wGA, 3-<6 months), the mean number of days from birth discharge to first RSVH and the mean length of RSVH stay were largely unchanged during the 2 periods for preterm and term infants in the commercial and Medicaid-insured populations (). The mean time from birth discharge to first RSVH fell for infants in the 29–34 wGA, 3-<6 months group, from 112.2 days to 106.6 days in the commercial population and from 112.0 days to 104.9 days in the Medicaid population. The proportion of infants admitted to the ICU during an RSVH was greater for all groups in the 2014–2017 period compared with the 2011–2014 period (). Average costs for RSVH rose among all wGA and chronologic age subgroups following the policy change; this was also true of median costs, although the median cost differences across seasons tended to be smaller than mean costs (). Among infants aged <3 months at the time of RSVH, the mean RSVH costs for preterm infants were more than double those of term infants (Commercial: 45,891 USD [median 19,747 USD] vs. 18,963 USD [median 10,722 USD]; Medicaid: 27,063 USD [median 9,291 USD] vs. 10,652 USD [median 4,436 USD]). For infants aged 3-<6 months at the time of RSVH, hospitalization costs were greater among preterm infants compared with term infants. Across both gestational age groups and insurers, costs were greater among infants aged <3 months during the RSV seasons than among infants aged 3-<6 months.
Table 6. RSV hospitalization characteristics among preterm and term infants aged <6 months
Discussion
In this retrospective analysis of commercially insured and Medicaid-insured infants aged <6 months during an RSV season, the utilization of outpatient RSV IP decreased significantly among preterm infants during the 3 RSV seasons following the more restrictive AAP policy issued in 2014 when compared with utilization in the 3 preceding RSV seasons. After the policy change, the relative risk of RSVH increased significantly among preterm infants compared with term infants. Among preterm infants, risk varied by wGA; however, even as the absolute rate of RSVH decreased among term infants, it was stable or increased among the subpopulations of preterm infants included in this analysis, thus widening the risk disparity between preterm and term infants. Among infants hospitalized for RSV, costs were higher for preterm infants than term infants across all wGA and chronologic age subgroups examined.
This study extends our previous analyses conducted using the MarketScan database and other data sets that have examined the impact of decreased RSV IP in the first and second RSV seasons following the policy change.Citation20,Citation23 Using the methodology in Goldstein et al.Citation23 this study demonstrates that the increased risk of RSVH and all-cause bronchiolitis hospitalization observed in Goldstein et al.’s 2-year pre-/post-study of the impact of the 2014 AAP policy change remained present when the observation window was expanded to include 3 years in the pre- and post-change periods.Citation23 All trends demonstrated in the Goldstein et al. study were replicated here with a larger population and a longer study period, providing further support that the observed increase in RSVH risk is related to reduced RSV IP among infants born at 29 to 34 wGA and not reflective of annual variations in RSV circulation levels or attack rates (both were removed by comparing preterm to term infants before vs. after the policy change). There is likely considerable patient overlap between the Goldstein et al. study and the present study because both studies used the MarketScan Commercial and Medicaid databases. However, the Goldstein et al. study had a 2-year pre-/post-AAP policy change window and occurred earlier with possibly a different set of data providers than the present study with a 3-year pre-/post-AAP policy change window.
Our findings are consistent with the limited literature on this topic. An observational study of infants born at 29 to 35 wGA who did not receive IP and were hospitalized for RSV found that both gestational age and chronologic age were inversely related to indicators of RSV severity, including hospitalization frequency, ICU admission, and mechanical intervention.Citation18 Studies that have examined the pooled rate of RSVH among children aged <2 years have generally reported no change in the hospitalization rate following the implementation of the revised policy.Citation26,Citation27 However, these studies did not examine or adjust for seasonal variations in RSV severity or separately examine the experience of preterm infants, who were the focus of the policy change. In studies that have examined the specific subpopulation of infants for whom the AAP policy no longer recommended RSV IP, outcomes such as RSVH rate, mean length of stay, ICU admission rate, mechanical ventilation use, and hospitalization costs have worsened following the implementation of the updated RSV IP policy.Citation19,Citation21,Citation23,Citation28,Citation29
Limitations
This analysis is subject to many of the limitations common to claims-based studies. First, claims data are collected for administrative purposes and are not held to the same rigorous standard as data collected explicitly for research purposes. Miscoding and under-coding may introduce bias. As specifically relates to this study, because the AAP does not recommend routine virologic testing, the true incidence of RSVH may be underestimated by exclusively using RSV-coded hospitalizations. To account for this possibility, we repeated the analysis with all-cause bronchiolitis hospitalizations (excluding hospitalizations with a recent diagnosis of other bronchiolitis-causing pathogens) and found the same trends as observed when the outcome was limited to RSVH. Second, the databases used in this analysis do not capture information on inpatient pharmacy utilization; therefore, this study underestimates the proportion of preterm infants who received palivizumab, particularly those who were born during the RSV season and may have received a dose during their birth hospitalization. In addition, we captured only whether palivizumab was received in the outpatient setting and did not report the number of doses of palivizumab received in this study. However, there is no evidence that either limitation biases one time frame more than the other. Third, in some of the preterm subgroups (e.g., 29–30 wGA), the number of hospitalizations while aged <6 months was quite small, as many infants born at this gestational age remain hospitalized after birth for weeks to months. We also noted a smaller decrease in outpatient RSV IP among the 29–30 wGA preterm subgroup. Both of these factors may have contributed to the result from modeling nearing, but not meeting, statistical significance. To ensure a robust analysis, we also analyzed the data by combining all preterm infants affected by the change in the AAP policy.
In the 2014–2017 RSV seasons, outpatient use of RSV IP decreased significantly among infants born at 29 to 34 wGA compared with use during the 2011–2014 seasons. Multiple US studies of the 2014–2015 and 2015–2016 RSV seasons have demonstrated an increased risk of RSVH after the AAP’s 2014 change to the RSV IP policy among otherwise healthy infants born at 29 to 34 wGA and <6 months chronologic age; this study demonstrates that this significantly elevated risk continued in the 2016–2017 season and is clearly visible in comparisons of RSVH in the 3 years before and after the policy change. These data and the increased risk of RSVH in 29 to 34 wGA infants suggest that the policy for RSV IP should be realigned with the original FDA indication for palivizumab. Future studies should continue to assess the impact of the AAP policy change among otherwise healthy 29–34 wGA infants who remain susceptible to severe RSV disease.
Disclosure of potential conflicts of interest
Lance Brannman is an employee of AstraZeneca; Amanda M. Kong is an employee of IBM Watson Health, and Sally W. Wade is a consultant to IBM Watson Health, which was contracted by AstraZeneca for data analyses and writing support; Leonard R. Krilov has received grant/research support from AstraZeneca/MedImmune, and Mitchell Goldstein and Jaime Fergie have received grant/research support from AstraZeneca/MedImmune and were members of the AstraZeneca Speakers’ Bureau.
Acknowledgments
The authors would like to acknowledge David Diakun, Lorena Lopez-Gonzalez, and Isabelle Winer for analytic support. Medical writing support was provided by Jessamine Winer-Jones. David Diakun, Lorena Lopez-Gonzalez, Isabelle Winer, and Jessamine Winer-Jones are employees of IBM Watson Health, and AstraZeneca funded their services. Editorial support was provided by PRECISIONscientia and was funded by Sobi, Inc.
Additional information
Funding
References
- Hall CB. Respiratory syncytial virus and parainfluenza virus. N Engl J Med. 2001;344(25):1917–28. doi:10.1056/NEJM200106213442507.
- Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, Staat MA, Auinger P, Griffin MR, Poehling KA, Erdman D, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360(6):588–98. doi:10.1056/NEJMoa0804877.
- Leader S, Kohlhase K. Recent trends in severe respiratory syncytial virus (RSV) among US infants, 1997 to 2000. J Pediatr. 2003;143(5):127–32. doi:10.1067/S0022-3476(03)00510-9.
- Rose EB, Wheatley A, Langley G, Gerber S, Haynes A. Respiratory syncytial virus seasonality - United States, 2014-2017. MMWR Morb Mortal Wkly Rep. 2018;67(2):71–76. doi:10.15585/mmwr.mm6702a4.
- Boyce TG, Mellen BG, Mitchel EF, Wright PF, Griffin MR. Rates of hospitalization for respiratory syncytial virus infection among children in Medicaid. J Pediatr. 2000;137(6):865–70. doi:10.1067/mpd.2000.110531.
- Rossi GA, Medici MC, Arcangeletti MC, Lanari M, Merolla R, Paparatti UDL, Silvestri M, Pistorio A, Chezzi C. Risk factors for severe RSV-induced lower respiratory tract infection over four consecutive epidemics. Eur J Pediatr. 2007;166(12):1267–72. doi:10.1007/s00431-007-0418-y.
- Straňák Z, Saliba E, Kosma P, Posfay-Barbe K, Yunis K, Farstad T, Unnebrink K, van Wyk J, Wegzyn C, Notario G, et al. Predictors of RSV LRTI hospitalization in infants born at 33 to 35 weeks gestational age: A large multinational study (PONI). PLoS One. 2016;11(6):e0157446. doi:10.1371/journal.pone.0157446.
- Krilov LR, Noor A Respiratory syncytial virus infection treatment and management. WebMD, LLC; 2019 Feb 25 [accessed 2020 Mar 17]. https://emedicine.medscape.com/article/971488-treatment.
- IMpact-RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics. 1998;102(3 Pt 1):531–37. doi:10.1542/peds.102.3.531.
- Synagis [package insert]. Gaithersburg (MD): MedImmune LLC; 2017.
- Anderson EJ, Carosone-Link P, Yogev R, Yi J, Simões EAF. Effectiveness of palivizumab in high-risk infants and children: A propensity score weighted regression analysis. Pediatr Infect Dis J. 2017;36(8):699–704. doi:10.1097/INF.0000000000001533.
- American Academy of Pediatrics Committee on Infectious Diseases. Prevention of respiratory syncytial virus infections: indications for the use of palivizumab and update on the use of RSV-IGIV. Pediatrics. 1998;102(5):1211–16. doi:10.1542/peds.102.5.1211.
- American Academy of Pediatrics Committee on Infectious Diseases. Modified recommendations for use of palivizumab for prevention of respiratory syncytial virus infections. Pediatrics. 2009;124(6):1694–701. doi:10.1542/peds.2009-2345.
- American Academy of Pediatrics Committee on Infectious Diseases; American Academy of Pediatrics Bronchiolitis Guidelines Committee. Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics. 2014;134(2):415–20. doi:10.1542/peds.2014-1665.
- American Academy of Pediatrics. Respiratory syncytial virus. In: Pickering LK, Baker CJ, Kimberlin DW, Long SS, editors. Red book: 2012 report of the committee on infectious diseases. Elk Grove Village (IL): American Academy of Pediatrics; 2012. p. 609–18.
- American Academy of Pediatrics Committee on Infectious Diseases. Revised indications for the use of palivizumab and respiratory syncytial virus immune globulin intravenous for the prevention of respiratory syncytial virus infections. Pediatrics. 2003;112(6 Pt 1):1442–46. doi:10.1542/peds.112.6.1442.
- Ralston SL, Lieberthal AS, Me issnerHC, Alverson BK, Baley JE, Gadomski AM, Johnson DW, Light MJ, Maraqa NF, Mendonca EA, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474–e1502. doi:10.1542/peds.2014-2742.
- Anderson EJ, Krilov LR, DeVincenzo JP, Checchia P, Halasa N, Simões E, Domachowske J, Forbes M, Pannaraj P, McBride S, et al. SENTINEL1: an observational study of respiratory syncytial virus hospitalizations among US infants born at 29 to 35 weeks’ gestational age not receiving immunoprophylaxis. Am J Perinatol. 2017;34(1):51–61. doi:10.1055/s-0036-1584147.
- Krilov LR, Fergie J, Goldstein M, Brannman L. Impact of the 2014 American academy of pediatrics immunoprophylaxis policy on the rate, severity, and cost of respiratory syncytial virus hospitalizations among preterm infants. Am J Perinatol. 2020;37(2):174–83. doi:10.1055/s-0039-1694008.
- Kong AM, Krilov LR, Fergie J, Goldstein M, Diakun D, Wade S, Pavilack M, McLaurin K. The 2014-2015 national impact of the 2014 American academy of pediatrics guidance for respiratory syncytial virus immunoprophylaxis on preterm infants born in the United States. Am J Perinatol. 2018;35(2):192–200. doi:10.1055/s-0037-1606352.
- Rajah B, Sánchez PJ, Garcia-Maurino C, Leber A, Ramilo O, Mejias A. Impact of the updated guidance for palivizumab prophylaxis against respiratory syncytial virus infection: a single center experience. J Pediatr. 2017;181:183–188.e1. doi:10.1016/j.jpeds.2016.10.074.
- Anderson EJ, DeVincenzo JP, Simões EAF, Krilov LR, Forbes ML, Pannaraj PS, Espinosa CM, Welliver RC, Wolkoff LI, Yogev R, et al. SENTINEL1: two-season study of respiratory syncytial virus hospitalizations among U.S. infants born at 29 to 35 weeks‘ gestational age not receiving immunoprophylaxis. Am J Perinatol. 2020;37(4):421–29. doi:10.1055/s-0039-1681014.
- Goldstein M, Krilov LR, Fergie J, McLaurin K, Wade S, Diakun D, Lenhart G, Bloomfield A, Kong A. Respiratory syncytial virus hospitalizations among U.S. preterm infants compared with term infants before and after the 2014 American Academy of Pediatrics guidance on immunoprophylaxis: 2012–2016. Am J Perinatol. 2018;35(14):1433–42. doi:10.1055/s-0038-1660466.
- US Bureau of Labor Statistics. Consumer price index detailed reports, annual average 2017. 2018 [accessed 2020 Mar 17] https://www.bls.gov/cpi/tables/home.htm.
- Dimick JB, Ryan AM. Methods for evaluating changes in health care policy: the difference-in-differences approach. JAMA. 2014;312(22):2401–02. doi:10.1001/jama.2014.16153.
- Belleudi V, Trotta F, Pinnarelli L, Davoli M, Addis A. Neonatal outcomes following new reimbursement limitations on palivizumab in Italy. Arch Dis Child. 2018;103(12):1163–67. doi:10.1136/archdischild-2018-315349.
- Grindeland CJ, Mauriello CT, Leedahl DD, Richter LM, Meyer AC. Association between updated guideline-based palivizumab administration and hospitalizations for respiratory syncytial virus infections. Pediatr Infect Dis J. 2016;35(7):728–32. doi:10.1097/INF.0000000000001150.
- Capizzi A, Silvestri M, Orsi A, Cutrera R, Rossi GA, Sacco O. The impact of the recent AAP changes in palivizumab authorization on RSV-induced bronchiolitis severity and incidence. Ital J Pediatr. 2017;43(1):71. doi:10.1186/s13052-017-0390-8.
- Zuccotti G, Fabiano V. Indications to respiratory syncytial virus immunoprophylaxis in the 29-32 wGA group: is there still room for debating? Ital J Pediatr. 2017;43(1):17. doi:10.1186/s13052-017-0341-4.