ABSTRACT
VGX-3100 is an investigational DNA-based immunotherapy being developed as an alternative to surgery and ablation for cervical High-Grade Squamous Intraepithelial Lesion (HSIL) with the aim of preserving reproductive health while treating precancerous disease. Response durability up to 1.5 y following dosing is now reported.
Histologic regression and HPV16 and/or HPV 18 (HPV16/18) clearance were previously demonstrated in a randomized, placebo-controlled, double-blind trial and reported for 6 months after the last dose of VGX-3100 or placebo. The presence of HPV16/18, Pap smear diagnoses, and immunogenicity longer-term responses were assessed at 18 months after the last dose.
91% (32/35) VGX-3100-treated women, whose cervical HSIL regressed and avoided excision at 6 months following study treatment completion, had no detectable HPV16/18 at 18 months following treatment completion. These results were comparable to those for women who received placebo and then later underwent surgery. For VGX-3100 recipients who regressed at 6 months following study treatment completion and avoided excision during the trial, Pap testing showed no HSIL recurrence at 18 months following VGX-3100 treatment. VGX-3100-induced cellular immune responses specific for HPV 16/18 E6/E7 remained higher than for placebo control recipients at 18 months.
In women with cervical HSIL who responded to VGX-3100 and were able to avoid surgery, clinical outcomes were comparable to the placebo control group which underwent conventional surgical treatment. These findings extend the understanding of the durability of the treatment effect of VGX-3100 up to 1.5 y and support that VGX-3100 could be used as an alternative to surgery.
Introduction
Approximately 70% of cervical cancers are derived from the high-grade squamous intraepithelial lesions (HSILs) caused by HPV16 or 18 (HPV 16/18).Citation1 HPV also causes squamous cancers at other anatomic sites, including the vagina, vulva, anus, and oropharynx: of these, the great majority are caused by HPV16.Citation1 While prophylactic HPV vaccines confer virtually 100% protection against infections by genotypes covered by the vaccine formulations, they confer no therapeutic effect upon those infections, HSIL, or cancers, particularly in the absence of any biopsy or surgical manipulation.Citation2 Therefore, the standard care of cervical HSIL requires either surgical excision or in some circumstances watchful waiting.
VGX-3100, which is a DNA-medicine administered intramuscularly using the CELLECTRA™ electroporation device, is being developed as an immune-mediated alternative to surgery for women with HPV16 or 18- associated cervical HSIL. Proof-of-concept efficacy and safety for VGX-3100 treatment has been previously reported for VGX-3100 from a Phase 2b randomized, placebo-controlled, double-blind clinical trial.Citation3 From that trial, in the mITT population, concomitant histopathological regression and virologic clearance occurred in 40% (46/114) of the VGX-3100 recipients and 15% (6/40) of the placebo recipients (strata adjusted percentage point difference, 23.2; 95% CI, 8.7–37.8; p = .001). The mITT population was defined as all subjects who received at least one administration of VGX3100 or placebo and had histology and virologic clearance results. We now report the durability of HPV clearance, cervical HSIL regression as assessed by cytology, and antigen-specific cellular immune responses through 18 months following completion of VGX-3100 treatment. An immune-mediated approach to treat HSIL is desirable in order to preserve normal cervical anatomy and consequently reproductive health.Citation4
Methods
Adult women 18 to 55 y of age who had histologically confirmed cervical HSIL and who were cervical HPV 16/18 positive at entry were evaluated at 18 months following completion of VGX-3100 or placebo treatment in the proof-of-concept treatment trial of VGX-3100 (ClinicalTrials.gov Identifier: NCT01304524), where subjects were randomized 3:1 either to treatment with VGX-3100 (6 mg/dose) or with placebo, administered at d 0, week 4, and week 12 using the CELLECTRA™ 2000 electroporation device, prior to planned therapeutic resection at 6 months following completion of VGX-3100 or placebo dosing (study week 36). VGX-3100 drug product contains DNA plasmids (6 mg) which express HPV16 and HPV18 E6 and E7 antigens. Placebo was comprised of sterile water. The primary endpoint was histologic regression to either CIN1 or normal at study week 36. Participants who had either a tissue diagnosis of cervical HSIL, as determined by a blinded pathology adjudication panel, at this time point, or who had undergone a prior cervical biopsy based on suspicion of disease progression were classified as non-regressors. Subjects who showed colposcopic evidence of residual cervical disease were to undergo resection of the lesion via a loop electrosurgical excision procedure (LEEP) or cold-knife conization (CKC). Subjects without evidence of cervical disease underwent biopsy at the site of the original cervical lesion.
As a secondary analysis of the population from this randomized, controlled clinical trial, long-term virology, and Pap testing assessment was conducted based upon the data of subjects who had evaluable primary endpoint HSIL regression results at 6 months following completion of treatment with either VGX-3100 or placebo. ThinPrep® (Hologic, Inc., Bedford, MA) specimens were used for HPV genotyping using the Linear Array HPV assay (Roche, Basel, Switzerland) and were collected at baseline and at weeks 6, 14, 24, 36, 62, and 88. Cytology was evaluated at weeks 14, 62, and 88. There were 102 subjects (72, VGX-3100; and 30, Placebo) with ELISpot data 18 months following completion of treatment. The ELISpot assay was performed as previously described on whole blood samples to test antigen-specific cellular immune responses.Citation5 Two sets of pooled peptides were used, each containing 2.5 μg/mL of 15-amino acid residues that overlapped by 11 amino acids and spanned the full-length consensus sequence of either HPV16 E6 and E7 or HPV18 E6 and E7 antigens. The average number of spot-forming units (SFU) counted in R10 wells (media alone) was subtracted from the average in HPV peptide pool-stimulated wells and then adjusted to SFU per 1 × 106 peripheral blood mononuclear cells (PBMCs).
Protocols for this clinical study received institutional review board or ethics committee approval (at each center) and adhered to the ethical guidelines set forth by Good Clinical Practice and the Declaration of Helsinki. All study participants provided written informed consent.
Results
Clinical durability and outcomes were measured using virology testing and Pap tests, and immunologic durability was measured using ELISpot at the end of the trial, 18 months following treatment with VGX-3100 or placebo. The derivation of the populations of interest is displayed in .
Figure 1. Long-term follow-up populations
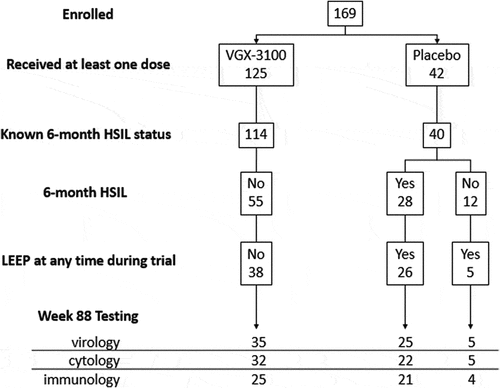
For the groups which received surgery, the procedure was done within 6 months of the evaluation of virologic and cytologic status. All groups received a biopsy to evaluate disease status within 6 months prior to virologic and cytologic evaluation. Thirty-one of the 169 (18%) patients randomized in the trial did not have evaluable endpoint data at the 18-month evaluation. The reasons for missing data in order of frequency were lost to follow-up (17), withdrawal by subject not associated with an adverse event (5), adverse event (5), unspecified (2), investigator decision (1), and pregnancy (1). The five adverse events which led to subject withdrawal were: an unintended pregnancy (subject received placebo with electroporation), injection site pain that resolved in less than 24 hours (two subjects received VGX-3100 with electroporation), one occurrence of maculopapular rash (subject received VGX-3100 with electroporation) that resolved in 7 d with oral chlorpheniramine and hydrocortisone cream, and one occurrence of itching and facial rash (subject received placebo with electroporation) that resolved without treatment in 6 d.
Virologic clearance
A summary of virologic clearance at 18 months for VGX-3100-treated women who resolved their HSIL and avoided surgery and women who had received placebo control and underwent surgery (i.e. LEEP or CKC) is presented in . For the VGX-3100 treated group who had HSIL regression at week 36, 35 women avoided surgery based upon investigator judgment and patient choice and had 18-month virology results. Of those 35 women, 32 (91%) were clear of detectable HPV16/18 at the 18-month post treatment completion evaluation. For the VGX-3100 treated group who had HSIL at week 36, 48 women had surgery based upon investigator judgment and patient choice and had 18-month virology results. Of those 48 women, 40 (83%) were clear of detectable HPV16/18 at the 18-month post treatment completion evaluation. The placebo recipients who had surgery had non-detection of HPV 16/18 was 88% (for those who had HSIL at Week 36) and 100% (for those who had spontaneous regression at Week 36) at 18 months. Of these 30 participants, five had spontaneously resolved their HSIL at the 6-month follow up and all five had no detectable HPV 16/18 at the 18-month evaluation.
Table 1. Viral clearance outcomes at 18 months following VGX-3100 or placebo
Pap test assessments
The clinical response of study participants was assessed using Pap tests at 18 months, after the last dose of VGX-3100 or placebo control. Pap test results were categorized according to the Bethesda system and each result was classified as atypical squamous cells of undetermined significance (ASCUS); low-grade squamous intraepithelial lesions (LSIL); high-grade squamous intraepithelial lesions (HSIL), which included CIN2 and CIN3; and atypical squamous cells-cannot exclude HSIL (ASC-H). Pap test results by previous history of LEEP/CKC and biopsy are provided in .
Table 2. Pap smear results at 18 months post-treatment completion with VGX-3100 or placebo
Among recipients of VGX-3100, at the 18-month follow up, cytologic improvement was observed in 100% (32/32) of those who had HSIL regression at the 6-month follow up and then avoided surgery, and in 98% (45/46) of those who had HSIL at the 6-month follow up and then underwent surgery. None of the VGX-3100-treated subjects were noted to have worsening of their Pap test findings at the long-term follow-up time point.
For placebo recipients who underwent a LEEP or CKC cytologic improvement at 18 months was 80% (for those who had HSIL at Week 36) and 100% (for those who had spontaneous regression at Week 36). Of these 27 subjects, five had spontaneously resolved their HSIL at the 6-month follow-up, and four of those five had cytologic improvement at 18 months. Worsening of Pap results to atypical glandular cells, occurred in 1 subject at the 18-month post-treatment completion evaluation who had received placebo, undergone LEEP/CKC, and had spontaneous regression of HSIL prior to surgery.
Cellular immune responses
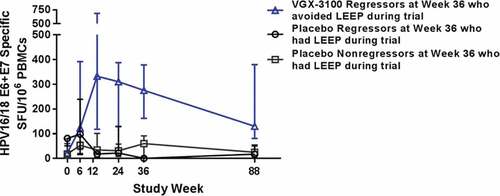
Cellular immune responses were measured by HPV 16/18 E6 and E7-specific interferon gamma production from peripheral blood mononuclear cells ELISpot. For the VGX-3100 recipients with no HSIL at the 6-month follow-up and who avoided LEEP, the median response was 130.0 (n = 25) at 18 months. For the VGX-3100 recipients with HSIL at the 6-month follow-up and who underwent LEEP, the median response was 69.2 (n = 32) at 18 months. For the placebo recipients without (n = 4) and with (n = 21) HSIL at the 6-month follow-up and who had LEEP, the median responses were 17.4 and 25.0 at 18 months, respectively ().
Figure 2. HPV16/18 E6/E7 IFN-gamma ELISpot results (SFU/106 PBMC) for follow-up groups by visit
Safety
The safety information for the HPV-003 trial has been previously reported through the 6-month follow-up period (week 36).Citation3 Beyond the 6-month follow-up period, 51% of VGX-3100 recipients experienced an adverse event versus 48% of placebo recipients, there were no related SAEs, and no deaths. Seven subjects had findings related to squamous cell carcinoma or the premalignant condition of adenocarcinoma in situ at the primary endpoint (i.e. Week 36) assessment, using surgical excision which represented advancement of the CIN2 or CIN3 initially diagnosed by biopsy. These cases were evaluated by a trial-specific central pathology group of laboratories, i.e. the Pathology Adjudication Panel, and in some instances also by a local pathologist. Three of the cases were reported as adenocarcinoma in situ (AIS), (2 VGX-3100, 1 placebo), out of which two cases (1 VGX-3100, 1 placebo) were confirmed as AIS by the Pathology Adjudication Panel. Adenocarcinoma in situ is a pre-invasive glandular lesion which can be difficult to capture on standard of care screening with initial punch biopsy and is more commonly identified by full excision (e.g. LEEP, conization). There were four reports that included the term squamous cell carcinoma, and two of those (VGX-3100 group) were confirmed by the Pathology Adjudication Panel. The other two cases (1 VGX 3100, 1 placebo) were diagnosed as CIN3 by the Pathology Adjudication Panel. None of the findings were considered related to treatment, and all subjects were able to receive appropriate care (i.e. excisional therapy or referral to an oncologist) for their condition as assessed through the 18-month (i.e. Week 88) safety follow-up period. There were no additional cases identified through the follow-up period.
Discussion
The aim of VGX-3100 therapy is to reduce the need for surgery for women with cervical HSIL. Therefore, long-term clinical durability and immunology data were reviewed for two populations of interest: women who were able to avoid surgery following VGX-3100 treatment as compared to those who were untreated (i.e. received placebo) and underwent conventional surgery. These clinical outcome data support that VGX-3100 is a viable alternative to surgery for the treatment of cervical HSIL and indicates that appropriate follow up could be managed within the typical visit intervals that are used in standard of care. As additional context, recurrent disease as an indicator of durability has been reported following LEEP at a cumulative rate of 14% over a 6-y period for women with CIN3 and 9% for women with CIN2 with an annual incidence rate of 1%.Citation6
Women who had participated in a Phase 2b study of VGX-3100 treatment of cervical HSIL were followed for 18 months after the last dose of VGX-3100 or placebo. Demographics for the Phase 2b study have been previously describedCitation3 and are comparable between the VGX-3100 recipients who had surgery and those who avoided surgery (data not shown). The establishment of efficacy based on histopathologic evidence required the removal of tissue at week 36 by either punch biopsy(ies) or more extensive surgical resection (i.e. LEEP, CKC). Subjects with colposcopic evidence of residual disease were to undergo LEEP or CKC. In a few cases, patients and their treating physicians elected to have excisional surgery irrespective of the trial results. Cytology based upon Pap tests and HPV-16/18 clearance based upon cervical swab testing was assessed at 18 months after VGX-3100 or placebo dosing completion, and immunogenicity was assessed at the 18-month follow-up visit to evaluate for durability of response to VGX-3100 and data are presented for all subjects who had testable samples with valid results.
Virologic clearance at 18 months was comparable for the VGX-3100 treated group who regressed at the 6-month follow-up and avoided surgery and the placebo group who had surgery. Pap tests that were conducted at the long-term evaluation time point support that both VGX-3100 treatment and surgical excision results in elimination of the underlying cervical HSIL which can be long-lasting. Cellular immune responses to VGX-3100, which are thought to represent the key mechanism of action, were significantly higher than the placebo control through 18 months after completion of dosing and suggests that longer term durability of protection against recurrence of cervical viral infection and HSIL is possible.
In this trial, the proportion of microinvasive cancer found in larger surgical excision samples in subjects who had no evidence of invasive cancer by initial biopsy was 1.8% (2/114 with specimens), which is consistent with and even lower than the proportion (6.7%) that has been reported under standard of care settings.Citation7 All subjects received definitive care without complications which supports that VGX-3100 treatment can be safely completed in a period of time that does not put subjects at increased risk for progression of disease. For the subgroup of women who did not respond to VGX-3100 treatment and then received usual care excisional surgery, there was no adverse influence on long-term medical outcomes as assessed by virologic clearance and Pap testing.
There were a number of limitations to the interpretation of these data. The overall completion rate at the end of the study (18 months) was 82%. The most common reason for non-completion was loss to follow up, which was not unexpected after 18 months. Another related limitation was that not all subjects who followed up at 18 months had all samples evaluable due to the relatively small but expected limitations in diagnostic samples. The populations of interest were chosen to represent what might happen in the medical use of VGX-3100 but had relatively small sample sizes. There was also an unavoidable potential impact of tissue manipulation (i.e. biopsy) at the 6-month time point which is associated with local viral clearance and potential impacts upon histopathology. Furthermore, Pap tests have a significant screening and diagnostic insensitivity,Citation8–11 making their results challenging to interpret without the additional context of virology. Lastly, the final visit for this trial was completed 18 months following the blinded administration of VGX-3100 or placebo; however, longer follow-up would be needed to inform upon the full extent to which durability of response lasts.
The key findings of these longer term follow-up data – virology, cytology, and immune responses – from the Phase 2b study are that the effect of VGX-3100 appears to be long lasting at least through 1.5 y following the last dose, and efficacy measures were comparable to those of conventional surgery. The durability of viral clearance and Pap smear findings support that VGX-3100 recipients who avoid surgery could be safely followed up to 18 months later using standard diagnostic tools. Also, a durable effect is desirable because of the significant psychosocial and other quality of life impacts on patients with outright cervical HSIL, or even only abnormal Pap results and/or HPV infection per HPV test results.Citation12 The presence of a persistent detectable HPV16/18 specific cellular response in the blood of VGX-3100-treated patients at 18 months following treatment that was greater in magnitude than placebo recipients suggests that extended protection is possible, although such a statement would require confirmation with greater sample sizes. The overall safety profile of VGX-3100 shows no increased risk beyond current standard of care. In fact, a non-surgical treatment alternative avoids the alteration of cervical anatomy, thereby enabling a therapeutic approach that preserves reproductive health for young womenCitation4 and also avoids the adverse events specifically associated with surgical treatment for cervical HSIL.Citation4,Citation13–17
Disclosure of potential conflicts of interest
All authors are currently employees of or otherwise affiliated with Inovio Pharmaceuticals. Author affiliations are: PKB, MD, KK, TH, MM, JB, SD and JK, Inovio Pharmaceuticals, Plymouth Meeting, PA, USA. DBW, The Wistar Institute, Philadelphia, PA, USA
Acknowledgments
The authors thank the HPV-003 trial investigators site staff, and study participants who conducted the long-term follow-up activities. A full listing of investigators, sites, and institutions can be found on clinicaltrials.gov, NCT01304524. The authors also than the supporting laboratories and clinical trial logistics managers of Inovio Pharmaceuticals, Inc.
Additional information
Funding
References
- Bruni LAG, Serrano B, Mena M, Gómez D, Muñoz J, Bosch FX, de Sanjosé S Human papillomavirus and related diseases in the world. Summary Report 17 June 2019. 2019.
- Group TFIS. Supplement to: The FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007;356:SupplementalMaterial.
- Trimble CL, Morrow MP, Kraynyak KA, Shen X, Dallas M, Yan J, Edwards L, Parker RL, Denny L, Giffear M, et al. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: a randomised, double-blind, placebo-controlled phase 2b trial. Lancet (London, England). 2015;386(10008):2078–88. doi:10.1016/S0140-6736(15)00239-1.
- Kyrgiou M, Athanasiou A, Paraskevaidi M, Mitra A, Kalliala I, Martin-Hirsch P, Arbyn M, Bennett P, Paraskevaidis E. Adverse obstetric outcomes after local treatment for cervical preinvasive and early invasive disease according to cone depth: systematic review and meta-analysis. BMJ (Clinical Research Ed). 2016;354:i3633.
- Hollenberg RK, Krieger DR, Samuels R, Kraynyak K, Sylvester A, Morrow M, Boyer J, Dallas M, Bhuyan PK. Safety and immunogenicity of VGX-3100 formulations in a healthy young adult population. Hum Vaccin Immunother. 2020;16(6):1404–1412.
- Melnikow J, McGahan C, Sawaya GF, Ehlen T, Coldman A. Cervical intraepithelial neoplasia outcomes after treatment: long-term follow-up from the British Columbia Cohort Study. J Natl Cancer Inst. 2009;101:721–28. doi:10.1093/jnci/djp089.
- Xiang L, Li J, Yang W, Xu X, Wu X, Wang H, Li Z, Yang H. Conization using an electrosurgical knife for cervical intraepithelial neoplasia and microinvasive carcinoma. PloS One. 2015;10:e0131790. doi:10.1371/journal.pone.0131790.
- Koliopoulos G, Nyaga VN, Santesso N, Bryant A, Martin-Hirsch PP, Mustafa RA, Schünemann H, Paraskevaidis E, Arbyn M. Cytology versus HPV testing for cervical cancer screening in the general population. Cochrane Database Syst Rev. 2017;8:Cd008587.
- Wright TC, Stoler MH, Behrens CM, Sharma A, Zhang G, Wright TL. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136(2):189–97. doi:10.1016/j.ygyno.2014.11.076.
- Renshaw AA, DiNisco SA, Minter LJ, Cibas ES. A more accurate measure of the false-negative rate of Papanicolaou smear screening is obtained by determining the false-negative rate of the rescreening process. Cancer. 1997;81:272–76. doi:10.1002/(SICI)1097-0142(19971025)81:5<272::AID-CNCR3>3.0.CO;2-P.
- Fahey MT, Irwig L, Macaskill P. Meta-analysis of Pap test accuracy. Am J Epidemiol. 1995;141:680–89. doi:10.1093/oxfordjournals.aje.a117485.
- Mast TC, Zhu X, Demuro-Mercon C, Cummings HW, Sings HL, Ferris DG. Development and psychometric properties of the HPV Impact Profile (HIP) to assess the psychosocial burden of HPV. Curr Med Res Opin. 2009;25:2609–19. doi:10.1185/03007990903238786.
- Long S, Leeman L. Treatment options for high-grade squamous intraepithelial lesions. Obstet Gynecol Clin North Am. 2013;40:291–316. doi:10.1016/j.ogc.2013.03.004.
- Armarnik S, Sheiner E, Piura B, Meirovitz M, Zlotnik A, Levy A. Obstetric outcome following cervical conization. Arch Gynecol Obstet. 2011;283:765–69. doi:10.1007/s00404-011-1848-3.
- Leimbacher B, Samartzis N, Imesch P, Dedes KJ, Fink D, Canonica C. Inpatient and outpatient loop electrosurgery excision procedure for cervical intraepithelial neoplasia: a retrospective analysis. Arch Gynecol Obstet. 2012;285:1441–45. doi:10.1007/s00404-011-2148-7.
- Martirosian TE, Smith SC, Baras AS, Darracott MM. Depot medroxyprogesterone acetate: a risk factor for cervical stenosis after loop electrosurgical excisional procedure management of cervical intraepithelial neoplasia? J Low Genit Tract Dis. 2010;14:37–42. doi:10.1097/LGT.0b013e3181b0f73f.
- ACOG. American College of Obstetricians and Gynecologists 2017. Frequently asked questions, FAQ100. Loop Electrosurgical Excision Procedure (LEEP). 2017.
