ABSTRACT
The poor uptake and limited effectiveness of seasonal influenza vaccines mean that influenza continues to create a significant burden of disease. It has been hypothesized that sex differences are present in responses to seasonal influenza vaccines, and that these differences may contribute to this poor vaccine success. This has led to the suggestion that vaccines should be tailored to an individual’s biological sex. However, studies in this field are often low quality. Comprehensive analysis of the available literature reveals that there is insufficient evidence to support sex differences in vaccine immunogenicity, effectiveness, or efficacy. Nonetheless, differences in vaccine safety are consistently observed, with females reporting adverse events following immunization more frequently than males. Bias introduced by gender differences in passive reporting of adverse effects may underlie this phenomenon. Highly controlled studies are required in future before any conclusions can be made about potential sex differences in response to seasonal influenza vaccines.
Introduction
Despite biannual reevaluation and reformulation, seasonal influenza vaccines remain unsuccessful in preventing disease, with influenza accounting for between 291 243 and 645 832 deaths annually worldwideCitation1. This is a result of both poor uptake and low vaccine effectiveness. Estimates of influenza vaccine effectiveness range from 10% to 60%,Citation2 in contrast to vaccines for other common viral diseases. For instance, the measles vaccine is effective in 97%.Citation3 Mismatch due to antigenic drift is often the main barrier to vaccine success, which reformulation often fails to circumvent. This is highlighted in . Different types of vaccines have been trialed to improve outcomes. Age is an important determinant for their success; this has been useful in informing vaccine recommendations, as shown in . For example, the quadrivalent influenza vaccine (QIV) was recommended in children and young adults since it was more protective against influenza B subtypes than the trivalent influenza vaccine (TIV).Citation6 However, no benefit of the QIV was seen in older adults, who are less susceptible to influenza B infection, thus the TIV is still recommended for this age group.Citation7 Age-specific differences have also been reflected in vaccine design. For example, an adjuvanted TIV was introduced in the 2018–19 season for those over 65 following observations of poor effectiveness of the standard TIV in this age group. This has resulted in better prevention of flu.Citation8
Figure 1. Antigenic mismatch decreases effectiveness of seasonal influenza vaccinesCitation4. The prevalent circulating strains are selected 6 months before the beginning of the flu season, to allow adequate time for vaccine production. These strains are grown in eggs. Mutations may occur in the vaccine strains to increase their ability to grow in eggs (egg-adaptation). Mutations may occur in the circulating strains between selection and vaccine administration (genetic drift). Both types of mutations may lead to antigenic mismatch, so vaccinated populations do not have protection against the influenza strains circulating at the time. This leads to low vaccine effectiveness for that season
Table 1. NHS influenza vaccine recommendations for the 2019–2020 season.Citation5
While age-specific differences in outcomes have been accounted for in vaccine strategy, sex differences have been largely ignored. Sexual dimorphism has been observed following immunization against numerous pathogens.Citation9 The mechanisms underlying these differences are not well understood and have been speculated elsewhere.Citation10,Citation11 Recently, certain research groups have hypothesized that responses to seasonal influenza vaccination differ between males and females.Citation9,Citation12,Citation13 This has led to the suggestion that vaccine design should be tailored to sex. If true, these differences could contribute to the poor effectiveness of seasonal influenza vaccination. This mini-review aims to explore whether sexual dimorphism exists in seasonal influenza vaccination, through focusing on the available literature from model animal and human studies. Possible implications of any differences will be discussed, including whether sex-specific vaccines should be introduced.
Sex differences in immunogenicity are present in mouse models of influenza vaccination
Although the presence of sex differences in immune responses is widely acknowledged,Citation11 preclinical vaccine studies rarely report the sex of animals used, let alone investigate differences in responses between sexes. Biannual reformulations of seasonal vaccines do not require preclinical testing for approval. Consequently, there are few preclinical studies of seasonal influenza vaccination, and even fewer investigating sex differences. The studies that have been performed have all used mice, which are the most widely used model animals in pre-clinical vaccine studies.Citation14 A number of reagents allowing measurement of immunogenicity can be used in mice,Citation14 including functional antibody assays (viral microneutralization (MN) and hemagglutinin inhibition (HAI) assays) which are commonly used to assess vaccine-mediated protection against influenza in humans. Titers of IgG are also measured due to its important role in vaccine-mediated protection.Citation15 These measures of immunogenicity are relative correlates of protection in humans.Citation16
Studies investigating vaccine immunogenicity have shown that female mice develop greater neutralizing antibody and IgG titers against immunized inactivated H1N1Citation17–21 and H3N2.Citation17–20 For example, in a study by Živković et al. (2015), total IgG, HAI, and MN titers against H1N1 in male mice were on average half of that of females at both 3- and 6-weeks post-immunization. However, this study only found differences between males and females for total IgG titers in response to H3N2 or B influenza strains, with no significant differences found in MN or HAI titers.Citation17 There is no clear consensus on sex differences in antibody quality. IgG avidity was shown to be greater in females than males in one study,Citation21 while another study showed no difference.Citation19 Recent studies have implied IgG avidity changes with age, yet data here are conflicting too. One study shows no sex difference in young mice, with strain-specific IgG showing around 40% avidity, but a greater avidity of 60% or more in aged females with no such increase seen in aged male mice.Citation20 Another study shows the opposite effect, with young female mice showing an avidity index of 0.3 compared to 0.15 in young males, while aged mice of both sexes show an index of around 0.15.Citation22 This study investigated responses to an inactivated 2009 H1N1 influenza vaccine only. Further studies are required to understand why these data are conflicting.
Sex differences have also been observed in response to live attenuated influenza vaccines (LAIV). Neutralizing and total antibody responses to H1N1 or H3N2 influenza strains were higher in female mice following immunization than in age-matched male mice.Citation23 These differences were not consistent across all time points measured. For example, females showed almost double the total anti-H1N1 IgG of males at 14 and 21 d post-immunization but no difference was observed at 28 d. A similar difference was seen between males and females for total anti-H3N2 IgG at 28 d but not at 14 or 21 d. No significant differences in morbidity or mortality were observed between females and males upon challenge with a homologous virus.Citation23 Female mice showed slightly lower morbidity on challenge with a heterosubtypic strain of influenza (e.g. H3N2 challenge following initial vaccination with H1N1),Citation23 however, cross-protection has rarely been observed in human studies so it is unlikely that this sex difference is present in humans. This highlights one aspect in which mouse models poorly replicate human influenza pathophysiology.
A further factor that makes these immunogenicity studies poorly representative of human vaccination is the use of anesthesia during vaccination.Citation21,Citation23 Anesthesia alters the immune response to influenza in mice, with infection under anesthesia resulting in a viral pneumonia which is unrelated to upper airway influenza infection observed in awake mice and humans.Citation24 It is likely that vaccination under anesthesia also alters immune responses. This makes these studies unsuitable for understanding sex differences that may be present in human immune responses to vaccination.
The use of inbred commercial mice strains in these studies may also interfere with our understanding of human sex differences following influenza vaccination.Citation19–21,Citation23 X chromosome inactivation has been shown to have no effect on immune responses in these inbred strains, unlike in humans and outbred animals. This implies sex differences observed in these mice models may be very different to sex differences present in humans. Using outbred mice may be more suitable to modeling human sex-differences in vaccine immune responses.Citation17,Citation18
Few studies have linked immunogenicity with protection by challenging mice with influenza following vaccination. Two studies have associated greater antibody responses in vaccinated females to faster viral clearance and lower morbidity following challenge with a H1N1 drift variant.Citation21,Citation22 In a study by Fink et al. (2018) female mice had twice the IgG titers and triple the neutralizing antibody titers of males.Citation21 These female mice exhibited an average 3% loss in body mass compared to 8% loss of body mass in males.Citation21 While the body mass of females had returned to normal 4 d post-challenge, male body mass was still lower than normal 14 d following challenge.Citation21 Antibody titers from vaccinated females were also better at protecting naive mice than antibodies from vaccinated males.Citation21 This increased protection was seen in young mice only, highlighting that potential sex differences may be dependent on age. More challenge studies such as these are required in future to determine whether sex differences in immunogenicity correspond to differences in protection. However, a major issue is encountered when challenging vaccinated mice with influenza strains. Most human influenza strains do not replicate efficiently in mice without prior virus adaptation through serial passages in the lungs. Some well-adapted strains, such as the Influenza A/Puerto Rico/8/34 H1N1 used by Lorenzo et al. (2011),Citation23 have undergone numerous mutations altering their antigenicity and replication kinetics in order to cause disease in mice.Citation24 Consequently, challenge of vaccinated mice with these strains poorly replicates human challenge with circulating influenza strains. It is thus unlikely that the differences in vaccine-induced protection observed between sexes in this study reflects human differences.
Selection of animal models that accurately reflect human immune responses remains one of the major challenges facing influenza vaccination research.Citation14 Thus, far only mice have been used to investigate sex differences. These studies have shown that females generate greater humoral responses to influenza vaccines than males. However, dissimilarities in influenza vaccination between mouse models and humans means that it is impossible to extrapolate these sex differences onto human populations. Directly investigating responses in humans to influenza vaccination will most accurately reveal any sex differences. Nonetheless, pre-clinical studies are much easier to conduct under highly controlled conditions than human trials, and so are less effected by confounding factors. There is an immediate need for more preclinical vaccine studies to be designed with equal numbers of males and females, and with the aim of investigating sex-dependant differences in outcome measures. Ferrets should be used to continue preclinical investigation into the sex differences observed in mice, as they model human influenza pathophysiology more representatively.Citation24
Clear sex differences are not present in human responses to influenza vaccination
Immunogenicity studies
As is the case for preclinical studies, clinical trials are not required for seasonal influenza vaccine updates. Furthermore, clinical trials rarely evaluate sex differences, so few human studies have investigated the sex differences in seasonal influenza vaccine outcomes. The studies that have been done mainly assess vaccine immunogenicity. These usually measure humoral immune response to vaccination using a hemagglutinin inhibition assay (HAI), in which a 1:40 antibody titer corresponds to strain-specific protection following immunization with an inactivated influenza vaccine. There is no suitable correlate of protection for the LAIV, so few studies investigating LAIV immunogenicity have been carried out. A few studies observed antibody responses to be greater in females than males following immunization.Citation25–29 For example, post-hoc analyses carried out by Falsey et al. (2009) in a randomized controlled trial of the TIV found females to have greater HAI geometric mean titers.Citation27 This difference was most pronounced for H3N2-specific titers, which had a mean value of 382.8 in females compared to 280.4 in males following immunization with a standard TIV dose.Citation27 These findings have led to some research groups hypothesizing immunogenicity to be greater in females following vaccination than males.Citation21,Citation30–32 However, observed differences are rarely clear cut. For example, Cook et al. reported a higher immunogenicity in females than males for all influenza strains following intramuscular TIV immunization, but observed no differences at all following subcutaneous TIV immunization.Citation26 Furthermore, the study by Falsey et al. (2009) found no significant sex differences for other influenza strains, with geometric mean titers for males and females registering at 52.1 and 52.5, respectively, for B-specific titers.Citation27
Numerous studies reveal either a higher immunogenicity in males,Citation31,Citation32 or no sex differences in immunogenicity following influenza vaccination.Citation26,Citation33–43 The latter by far outnumber those studies showing the presence of sex differences. A systematic review by Tadount et al. (2019) highlighted that of the published studies investigating sex differences in influenza vaccine immunogenicity, the majority found no sex differences.Citation44 Only studies investigating adult participants were included, so whether this is the case for children remains unknown. Meta-analyses using individual participant data from the original trial data sets would be a more reliable approach to investigating sex differences than a systematic review. However, a meta-analysis could not be conducted due to the heterogeneity of study populations and the lack of precision in reported measures. Nonetheless, this review implies that this hypothesis is unsupported by the available evidence.
Efficacy and effectiveness studies
Immunogenicity does not provide a measure of vaccine success; for this, disease prevention among vaccinated populations must be assessed. The gold standard measurement for disease prevention is vaccine efficacy, measured in randomized control trials (RCTs) and defined as the percentage reduction in disease incidence in a vaccinated group compared to an unvaccinated group under optimal conditions.Citation30 These studies usually use laboratory diagnostic tests to assess influenza status. Nonetheless, RCTs investigating laboratory-confirmed infection are affected by confounding factors, such as the sensitivity and specificity of the diagnostic tests.Citation45
No RCTs have been carried out with the primary aim of investigating sex differences in influenza vaccine efficacy. A few studies separate males and females, allowing efficacy values for each sex to be estimated. Rarely were sex differences in efficacy evaluated in these studies, as it is poor practice to assess subgroup comparisons not prespecified in the trial protocol.Citation46 A single efficacy study stratified by sex met eligibility criteria to be included by Tadount et al. (2019) in their systematic review.Citation44 This phase III RCT found a significantly higher TIV efficacy in healthy young males compared to age-matched females.Citation32 This result is likely to be affected by interference of confounding factors such as vaccination history. The female group of this study had higher previous immunization rates, thus increased exposure to viral antigens. This could underlie the weaker immune response observed when compared to males.Citation47
Carrying out placebo-controlled trials in populations for which vaccination is recommended is unethical,Citation48 and so RCTs are often unsuitable. Instead, observational studies are often used to estimate vaccine effectiveness, defined as the ability of vaccines to prevent outcomes of interest in the “realworld”Citation49 Effectiveness studies adjust for confounding factors. Sex is often considered a confounder,Citation50 and its effect is eliminated through multivariate analysis. Some studies use stratification instead to provide an effective measure for each sex stratum. This enables comparison of effectiveness between sexes. Unfortunately, influenza vaccine data is rarely stratified by sex. Sex-stratified studies of influenza vaccine effectiveness are reviewed by Tadount et al. (2019).Citation44 Most effectiveness studies are not designed to investigate sex differences, and sex-stratified effectiveness values had to be adjusted for age, health status, and vaccination history, in order for the risk of bias to be deemed low enough for inclusion in the review.Citation44 Studies with a high risk of bias were excluded.Citation44
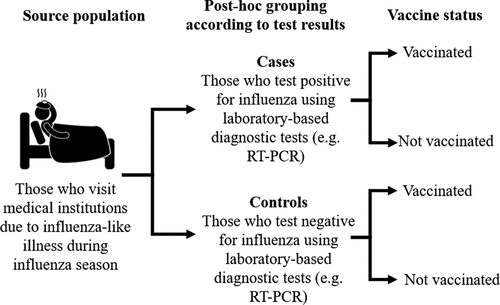
Many effectiveness studies use clinically relevant health outcomes rather than laboratory-based outcomes. All-cause mortality from influenza-like illness is often used as an outcome. Some studies have used this outcome to imply that influenza vaccine effectiveness in greater in females than in males.Citation51–53 However, the use of such outcomes compromises the reliability of these findings, as the criteria used to define influenza-like illnesses lacks specificity for influenza diagnosis.Citation54 Test-negative designs minimize this issue by using laboratory-based diagnostic tests, as illustrated in . Tadount et al. (2019) only considered studies using TNDs in their review.Citation44 Two effectiveness estimates showed no differences between older males and females.Citation55,Citation56 A further four low-quality studies gave more crude estimates of effectiveness, and also showed no differences.Citation57–60 While TNDs aim to minimize bias in these studies, gender differences in health-seeking behaviors may introduce selection biases that interfere with sex-specific estimates.Citation49 Evidence implies that men show delayed health-seeking behavior and women do not.Citation61 Men with may be less likely to seek care for influenza-like illness than women when symptoms are mild. However, men with severe symptoms will seek help and be tested for influenza. These men are more ill and more likely to test positive for influenza. Therefore, while fewer men get tested for influenza, a higher proportion will test positive. This may affect sex-specific effectiveness estimates. The low quality of these studies meant that generation of pooled sex-stratified effectiveness estimates was not possible, and this data could not reliably be used to evaluate sex differences in vaccine effectiveness.
Figure 2. Test-negative design studies. A form of observational study commonly used in effectiveness studies of influenza vaccination to reduce disease misclassification and confounding by health care-seeking behaviors. However, gender differences in health-seeking behavior may introduce selection bias
Safety studies
Vaccine safety is crucial and is strictly monitored prior to authorization. Currently licensed influenza vaccines display good safety profiles across the age groups in which they are used.Citation62 However, as influenza vaccines are administered to large proportions of the population, adverse effects following immunization (AEFI) may affect a significant number of individuals. AEFIs can be local (e.g. site injection pain/redness/swelling) or systemic (e.g. fever). Changes in vaccine formulations and host or environmental factors may affect the prevalence or severity of AEFIs. Consequently, constant monitoring of safety is essential.Citation63 This is achieved through the routine use of surveillance systems which rely on detection by health professionals or passive reporting by the recipient.
Unlike other vaccine responses, vaccine safety does appear to differ between sexes. Overall, 21 of the 35 investigated study groups included in the review by Tadount et al. (2019) revealed females experienced AEFIs more frequently than males,Citation44 such as in the study by Govaert et al. (1994) in which females showed twice the rate of AEFIs (30%) than males (15%).Citation41 This was most pronounced in studies into local reactions, with 9 of the 10 studies showing significantly higher rates in females. Again, there is a risk that gender differences in health-seeking behavior may affect the data of these studies; men experiencing AEFIs may be less likely to report this to a physician than women, which could lead to underreporting in male AEFIs.Citation61 This bias may be more prevalent in local reactions than systemic reactions, as these are less severe. This may reflect why only 5 of 13 studies showed higher incidence of systemic reactions in females, with the other 8 studies finding no sex differences. While meta-analysis of this data was not possible due to heterogeneity of effect measures, there appears to be a definite trend toward females reporting more AEFIs than males. It is yet unclear whether this is because of this reporting bias. There is a need for collection of AEFI data through methods other than passive reporting to investigate whether this trend is a true biological phenomenon.
Conclusions and future perspectives
While animal studies and a few human studies support the hypothesis that influenza vaccine immunogenicity is greater in females, much of this data is unreliable. Mouse studies poorly model sex differences that may occur in humans, and there is a need for more physiological models of human influenza vaccination need to be used in future. Human studies reporting no sex differences vastly outnumber those that report sex differences. Consequently, the suggestion that male-specific vaccines designed to potentiate immunogenicity should be introduced is unsupported by the available evidence.Citation12
Evaluation of effectiveness and efficacy studies into influenza vaccines does not support the existence of sex differences in vaccination outcomes.Citation12 There is a distinct lack of high-quality research investigating sex differences, which makes this evaluation difficult. Large-scale efficacy studies must be carried out in future, with the aim of investigating sex differences. These should provide more reliable insights into whether sex differences are present in vaccine-mediated disease prevention.
Females have been consistently observed to report AEFIs more frequently than males in safety studies. This has been suggested to contribute to the increased vaccine hesitancy observed among women.Citation10,Citation64,Citation65 However, willingness to report AEFIs may differ between men and women, and this is likely to play a role in this observed difference. Some have suggested vaccines with lower reactogenicity should be designed for females to reduce AEFI frequency while retaining protective antibody responses.Citation12 This could be achieved through lowering the vaccine dosage. A large phase II trial conducted by Engler et al. (2008) demonstrated that the antibody response of females to a half dose of TIV was equivalent to that of a full dose in males,Citation66 implying that half the usual TIV dose may be sufficient in females. However, there is so-far insufficient evidence to support the proposal that AEFIs are indeed higher in females, or that sex-specific vaccines should be designed given the logistical complexity in managing different influenza vaccines for both different age and sex populations. It is crucial that highly controlled trials are carried out investigating this trend for more frequently reported AEFIs in females. If this phenomenon is indeed biological, it may underly the increased vaccine hesitancy observed in females.Citation64,Citation65 By reducing the rate of these AEFIs in females, more females may choose to receive a seasonal influenza vaccine each year. Thus, a greater proportion of the population would be protected from the predicted circulating influenza strains. This could be crucial in reducing the number of seasonal influenza deaths in future.
This mini-review focuses on the available literature and hopes to provide a balanced view of the present data. Nonetheless, the limitations of the literature mean biases may have impacted any conclusions drawn. Sex differences are rarely considered in any study and are never the main purpose of a clinical vaccine trial; therefore, the published literature is full of reporting and publication bias. Re-analysis of the existing data, including those unpublished on sex differences, may help to reduce these biases in the literature. It is hoped that the increased availability of individual participant data in clinical trials in recent years will allow more meta-analyses of sex differences to be carried out in future, therefore providing high-quality data on sex differences which are currently not available.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Iuliano AD, Roguski KM, Chang HH, Muscatello DJ, Palekar R, Tempia S, Choen C, Gran JM, Schanzer D, Cowling BJ, Wu P, Kyncl J, Ang LW, Park M, Redlberger-Fritz M, Yu H, Espenhain L, Krishnan A, Emukule G, van Asten L, Pereira da Silva S, Aungkulanon S, Buchholz U, Widdowsen MA, Bresee JS. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet. 2018;391(10127):1285–300. doi:10.1016/S0140-6736(17)33293-2.
- CDC Seasonal Flu Vaccine Effectiveness Studies. CDC. Available at: https://www.cdc.gov/flu/vaccines-work/effectiveness-studies.htm. (Accessed 16 March 2020)
- MMR Vaccination. What You Should Know | measles, Mumps, Rubella. CDC. Available at: https://www.cdc.gov/vaccines/vpd/mmr/public/index.html. (Accessed 16 March 2020)
- Paules CI, Fauci AS. Influenza vaccines: good, but we can do better. J. Infect. Dis. 2019;219(Supplement_1):S1–S4. doi:10.1093/infdis/jiy633.
- Edwards A. Annual National Flu programme letter 2019 to 2020. 2019.
- Baxter D. Evaluating the case for trivalent or quadrivalent influenza vaccines. Hum Vaccin Immunother. 2016;12(10):2712–17. doi:10.1080/21645515.2015.1091130.
- Public Health England (PHE). Guidance. Summary of data to support the choice of influenza vaccination for adults in primary care. 2018.
- Van Buynder PG, Konrad S, Van Buynder JL, Brodkin E, Krajden M, Ramler G, Bigham M. The comparative effectiveness of adjuvanted and unadjuvanted trivalent inactivated influenza vaccine (TIV) in the elderly. Vaccine. 2013;31(51):6122–28. doi:10.1016/j.vaccine.2013.07.059.
- Flanagan KL, Fink AL, Plebanski M, Klein SL. Sex and gender differences in the outcomes of vaccination over the life course. Annu. Rev. Cell Dev. Biol. 2017;33(1):577–99. doi:10.1146/annurev-cellbio-100616-060718.
- Klein SL, Jedlicka A, Pekosz A. The Xs and Y of immune responses to viral vaccines. Lancet Infect Dis. 2010;10(5):338–49. doi:10.1016/S1473-3099(10)70049-9.
- Markle JG, Fish EN. SeXX matters in immunity. Trends Immunol. 2014;35(3):97–104. doi:10.1016/j.it.2013.10.006.
- Klein SL, Pekosz A. Sex-based biology and the rational design of influenza vaccination strategies. J. Infect. Dis. 2014;209(suppl 3):S114–S119. doi:10.1093/infdis/jiu066.
- Klein SL, Marriott I, Fish EN. Sex-based differences in immune function and responses to vaccination. Trans. R. Soc. Trop. Med. Hyg. 2014;109:9–15.
- Van Der Laan JW, Herberts C, Lambkin-Williams R, Boyers A, Mann AJ, Oxford J. Animal models in influenza vaccine testing. Expert Rev Vaccines. 2008;7(6):783–93. doi:10.1586/14760584.7.6.783.
- Huber VC, McKeon RM, Brackin MN, Miller LA, Keating R, Brown SA, Makarova N, Perez DR, MacDonald GH, McCullers JA, et al. Distinct contributions of vaccine-induced immunoglobulin G1 (IgG1) and IgG2a antibodies to protective immunity against influenza. Clin. Vaccine Immunol. 2006;13(9):981–90. doi:10.1128/CVI.00156-06.
- Plotkin SA. Immunologic correlates of protection induced by vaccination. Pediatric Infect Dis J. 2001;20(1):63–75. doi:10.1097/00006454-200101000-00013.
- Živković I, Bufan B, Petrušić V, Minić R, Arsenović-Ranin N, Petrović R, Leposavić G. Sexual diergism in antibody response to whole virus trivalent inactivated influenza vaccine in outbred mice. Vaccine. 2015;33(42):5546–52. doi:10.1016/j.vaccine.2015.09.006.
- Živković I, Petrović R, Arsenović-Ranin N, Petrušić V, Minić R, Bufan B, Popović O, Leposavić G. Sex bias in mouse humoral immune response to influenza vaccine depends on the vaccine type. Biologicals. 2018;52:18–24. doi:10.1016/j.biologicals.2018.01.007.
- Petrović R, Bufan B, Arsenović-Ranin N, Živković I, Minić R, Radojević K, Leposavić G. Mouse strain and sex as determinants of immune response to trivalent influenza vaccine. Life Sci. 2018;207:117–26. doi:10.1016/j.lfs.2018.05.056.
- Arsenović-Ranin N, Petrović R, Živković I, Bufan B, Stoiljković V, Leposavić G. Influence of aging on germinal centre reaction and antibody response to inactivated influenza virus antigens in mice: sex-based differences. Biogerontology. 2019;20(4):475–96. doi:10.1007/s10522-019-09811-8.
- Fink AL, Engle K, Ursin RL, Tang WY, Klein SL. Biological sex affects vaccine efficacy and protection against influenza in mice. Proc. Natl. Acad. Sci. U. S. A. 2018;115(49):12477–82. doi:10.1073/pnas.1805268115.
- Potluri T, Fink AL, Sylvia KE, Dhakal S, Vermillion MS, vom Steeg L, Deshpande S, Narasimhan H, Klein SL. Age-associated changes in the impact of sex steroids on influenza vaccine responses in males and females. Npj Vaccines. 2019;4.
- Lorenzo ME, Hodgson A, Robinson DP, Kaplan JB, Pekosz A, Klein SL. Antibody responses and cross protection against lethal influenza A viruses differ between the sexes in C57BL/6 mice. Vaccine. 2011;29(49):9246–55. doi:10.1016/j.vaccine.2011.09.110.
- Luke CJ, Subbarao K. The role of animal models in influenza vaccine research. Influenza vaccines for the future, 161–202. Birkhäuser Basel. 2008. 10.1007/978-3-7643-8371-8_8
- Furman D, Hejblum BP, Simon N, Jojic V, Dekker CL, Thiebaut R, Tibshirani RJ, Davis MM. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc. Natl. Acad. Sci. U. S. A. 2014;111(2):869–74. doi:10.1073/pnas.1321060111.
- Cook IF, Barr I, Hartel G, Pond D, Hampson AW. Reactogenicity and immunogenicity of an inactivated influenza vaccine administered by intramuscular or subcutaneous injection in elderly adults. Vaccine. 2006;24(13):2395–402. doi:10.1016/j.vaccine.2005.11.057.
- Falsey AR, Treanor JJ, Tornieporth N, Capellan J, Gorse GJ. Randomized, double‐blind controlled phase 3 trial comparing the immunogenicity of high‐dose and standard‐dose influenza vaccine in adults 65 years of age and older. J. Infect. Dis. 2009;200(2):172–80. doi:10.1086/599790.
- Hara M, Hanaoka T, Maeda K, Kase T, Ohfuji S, Fukushima W, Hirota Y. Immunogenicity and efficacy of A/H1N1pdm vaccine among subjects with severe motor and intellectual disability in the 2010/11 influenza season. J. Epidemiol. 2016;26(6):300–06. doi:10.2188/jea.JE20150036.
- Talaat KR, Greenberg M, Lai M, Hartel G, Wichems C, Rockman S, Jeanfreau R, Ghosh M, Kabongo M, Gittleson C, et al. A single dose of unadjuvanted novel 2009 H1N1 vaccine is immunogenic and well tolerated in young and elderly adults. J. Infect. Dis. 2010;202(9):1327–37. doi:10.1086/656601.
- Fink AL, Klein SL. Sex and gender impact immune responses to vaccines among the elderly. Physiology. 2015;30(6):408–16. doi:10.1152/physiol.00035.2015.
- Looker C, Luster MI, Calafat AM, Johnson VJ, Burleson GR, Burleson FG, Fletcher T. Influenza vaccine response in adults exposed to perfluorooctanoate and perfluorooctanesulfonate. Toxicol. Sci. 2014;138(1):76–88. doi:10.1093/toxsci/kft269.
- Jackson LA, Gaglani MJ, Keyserling HL, Balser J, Bouveret N, Fries L, Treanor JJ. Safety, efficacy, and immunogenicity of an inactivated influenza vaccine in healthy adults: A randomized, placebo-controlled trial over two influenza seasons. BMC Infect. Dis. 2010;10(1). doi:10.1186/1471-2334-10-71.
- Olafsdottir TA, Alexandersson KF, Sveinbjornsson G, Lapini G, Palladino L, Montomoli E, Del Giudice G, Gudbjartsson DF, Jonsdottir I. Age and influenza-specific pre-vaccination antibodies strongly affect influenza vaccine responses in the icelandic population whereas disease and medication have small effects. Front. Immunol. 2018;8:1872. doi:10.3389/fimmu.2017.01872.
- Hagihara Y, Ohfuji S, Watanabe K, Yamagami H, Fukushima, W, Maeda K, Kamata N, Sogawa M, Shiba M, Tanigawa T, Tominaga K, Watanabe T, Fujiwara Y, Hirota Y, Arakawa T. Infliximab and/or immunomodulators inhibit immune responses to trivalent influenza vaccination in adults with inflammatory bowel disease. J. Crohn’s Colitis. 2014;8(3):223–33. doi:10.1016/j.crohns.2013.08.008.
- Rapezzi D, Sticchi L, Racchi O, Mangerini R, Ferraris AM, Gaetani GF. Influenza vaccine in chronic lymphoproliferative disorders and multiple myeloma. Eur. J. Haematol. 2003;70(4):225–30. doi:10.1034/j.1600-0609.2003.00028.x.
- Gorse GJ, Falsey AR, Ozol-Godfrey A, Landolfi V, Tsang PH. Safety and immunogenicity of a quadrivalent intradermal influenza vaccine in adults. Vaccine. 2015;33(9):1151–59. doi:10.1016/j.vaccine.2015.01.025.
- Coleman BL, McGeer AJ, Halperin SA, Langley JM, Shamout Y, Taddio A, Shah V, McNeil SA. A randomized control trial comparing immunogenicity, safety, and preference for self- versus nurse-administered intradermal influenza vaccine. Vaccine. 2012;30(44):6287–93. doi:10.1016/j.vaccine.2012.08.006.
- Nakaya HI, Hagan T, Duraisingham S, Lee E, Kwissa M, Rouphael N, Frasca D, Gersten M, Mehta A, Gaujoux R, et al. Systems analysis of immunity to influenza vaccination across multiple years and in diverse populations reveals shared molecular signatures. Immunity. 2015;43(6):1186–98. doi:10.1016/j.immuni.2015.11.012.
- Moynihan JA, Larson MR, Treanor J, Duberstein PR, Power A, Shore B, Ader R. Psychosocial factors and the response to influenza vaccination in older adults. Psychosom. Med. 2004;66(6):950–53. doi:10.1097/01.psy.0000140001.49208.2d.
- Strindhall J, Ernerudh J, Mörner A, Waalen K, Löfgren S, Matussek A, Bengner M. Humoral response to influenza vaccination in relation to pre-vaccination antibody titres, vaccination history, cytomegalovirus serostatus and CD4/CD8 ratio. Infect. Dis. (Auckl). 2016;48(6):436–42. doi:10.3109/23744235.2015.1135252.
- Govaert TME, Sprenger MJW, Dinant GJ, Aretz K, Masurel N, Knottnerus JA. Immune response to influenza vaccination of elderly people. A randomized double-blind placebo-controlled trial. Vaccine. 1994;12(13):1185–89. doi:10.1016/0264-410X(94)90241-0.
- Barrett PN, Berezuk G, Fritsch S, Aichinger G, Hart MK, El-Amin W, Kistner O, Ehrlich HJ. Efficacy, safety, and immunogenicity of a Vero-cell-culture-derived trivalent influenza vaccine: A multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2011;377(9767):751–59. doi:10.1016/S0140-6736(10)62228-3.
- Rivera L, Mazara S, Vargas M, Fragapane E, Casula D, Groth N. Phase III, randomized controlled trial to evaluate lot consistency of a trivalent subunit egg-based influenza vaccine in adults. Vaccine. 2012;30(35):5285–92. doi:10.1016/j.vaccine.2012.05.021.
- Tadount F, Doyon-Plourde P, Rafferty E, MacDonald S, Sadarangani M, Quach C. Is there a difference in the immune response, efficacy, effectiveness and safety of seasonal influenza vaccine in males and females? – A systematic review. Vaccine. 2019. doi:10.1016/j.vaccine.2019.10.091.
- Ferdinands JM, Shay DK. Magnitude of potential biases in a simulated case-control study of the effectiveness of influenza vaccination. Clin. Infect. Dis. 2012;54(1):25–32. doi:10.1093/cid/cir750.
- Voysey M, Pollard AJ, Perera R, Fanshawe TR. Assessing sex-differences and the effect of timing of vaccination on immunogenicity, reactogenicity and efficacy of vaccines in young children: study protocol for an individual participant data meta-analysis of randomised controlled trials. BMJ Open. 2016;6(7):e011680. doi:10.1136/bmjopen-2016-011680.
- Mosterín Höpping A, McElhaney J, Fonville JM, Powers DC, Beyer WEP, Smith DJ. The confounded effects of age and exposure history in response to influenza vaccination. Vaccine. 2016;34(4):540–46. doi:10.1016/j.vaccine.2015.11.058.
- How Flu Vaccine Effectiveness and Efficacy are Measured. CDC. Available at: https://www.cdc.gov/flu/vaccines-work/effectivenessqa.htm. (Accessed 1 January 2020)
- Mcneil S. Overview of vaccine efficacy and vaccine effectiveness.
- Mori M, Oura A, Ohnishi H, Washio M. Confounding in evaluating the effectiveness of influenza vaccine. Vaccine. 2008;26(50):6459–61. doi:10.1016/j.vaccine.2008.06.040.
- Vila-Córcoles A, Rodriguez T, de Diego C, Ochoa O, Valdivieso A, Salsench E, Ansa X, Badía W, Saún N. Effect of influenza vaccine status on winter mortality in Spanish community-dwelling elderly people during 2002-2005 influenza periods. Vaccine. 2007;25(37–38):6699–707. doi:10.1016/j.vaccine.2007.07.015.
- Fleming DM, Watson JM, Nicholas S, Smith GE, Swan AV. Study of the effectiveness of influenza vaccination in the elderly in the epidemic of 1989–90 using a general practice database. Epidemiol. Infect. 1995;115:581–89.
- Nichol KL, Nordin JD, Nelson DB, Mullooly JP, Hak E. Effectiveness of influenza vaccine in the community-dwelling elderly. N. Engl. J. Med. 2007;357:1373–81.
- Nichol KL. Heterogeneity of influenza case definitions and implications for interpreting and comparing study results. Vaccine. 2006;24(44–46):6726–28. doi:10.1016/j.vaccine.2006.05.064.
- Kwong JC, Campitelli MA, Gubbay JB, Peci A, Winter A-L, Olsha R, Turner R, Rosella LC, Crowcroft NS. Vaccine effectiveness against laboratory-confirmed influenza hospitalizations among elderly adults during the 2010-2011 season. Clin. Infect. Dis. 2013;57(6):820–27. doi:10.1093/cid/cit404.
- Talbot HK, Griffin MR, Chen Q, Zhu Y, Williams JV, Edwards KM. Effectiveness of seasonal vaccine in preventing confirmed influenza-associated hospitalizations in community dwelling older adults. J. Infect. Dis. 2011;203(4):500–08. doi:10.1093/infdis/jiq076.
- Trebbien R, Fischer TK, Krause TG, Nielsen L, Nielsen XC, Weinreich LS, Lis-Tønder J, Skov MN, Christiansen CB, Emborg H-D, et al. Changes in genetically drifted H3N2 influenza A viruses and vaccine effectiveness in adults 65 years and older during the 2016/17 season in Denmark. J. Clin. Virol. 2017;94:1–7. doi:10.1016/j.jcv.2017.06.007.
- Gilca R, Skowronski DM, Douville-Fradet M, Amini R, Boulianne N, Rouleau I, Martineau C, Charest H, De Serres G. Mid-Season estimates of influenza vaccine effectiveness against influenza A(H3N2) hospitalization in the elderly in Quebec, Canada, January 2015. PLoS One. 2015;10(7):e0132195. doi:10.1371/journal.pone.0132195.
- Bragstad K, Emborg HD, Fischer TK, Voldstedlund M, Gubbels S, Andersen B, Molbak K, Krause TG. Low vaccine effectiveness against influenza A(H3N2) virus among elderly people in Denmark in 2012/13 - A rapid epidemiological and virological assessment. Eurosurveillance. 2013;18:20397.
- Emborg HD, Krause TG, Nielsen L, Thomsen MK, Christiansen CB, Skov MN, Nielsen XC, Weinreich LS, Fischer TK, Rønn J, et al. Influenza vaccine effectiveness in adults 65 years and older, Denmark, 2015/16 – a rapid epidemiological and virological assessment. Eurosurveillance. 2016;21(14):30189. doi:10.2807/1560-7917.ES.2016.21.14.30189.
- Galdas PM, Cheater F, Marshall P. Men and health help-seeking behaviour: literature review. J Adv Nurs. 2005;49(6):616–23. doi:10.1111/j.1365-2648.2004.03331.x.
- Trombetta CM, Gianchecchi E, Montomoli E. Influenza vaccines: evaluation of the safety profile. Hum Vaccin Immunother. 2018;14(3):657–70. doi:10.1080/21645515.2017.1423153.
- Li R, Stewart B, McNeil MM, Duffy J, Nelson J, Kawai AT, Baxter R, Belongia EA, Weintraub E. Post licensure surveillance of influenza vaccines in the vaccine safety datalink in the 2013–2014 and 2014–2015 seasons. Pharmacoepidemiol. Drug Saf. 2016;25(8):928–34. doi:10.1002/pds.3996.
- Schmid P, Rauber D, Betsch C, Lidolt G, Denker ML. Barriers of influenza vaccination intention and behavior - A systematic review of influenza vaccine hesitancy, 2005-2016. PLoS ONE. 2017;12.
- Marti M, de Cola M, MacDonald NE, Dumolard L, Duclos P. Assessments of global drivers of vaccine hesitancy in 2014—Looking beyond safety concerns. PLoS One. 2017;12:e0172310.
- Engler RJM, Nelson MR, Klote MM, VanRaden MJ, Huang C, Cox NJ, Klimov A, Keitel WA, Nichol KL, Carr WW, Treanor JT. Half- vs full-dose trivalent inactivated influenza vaccine (2004-2005): age, dose, and sex effects on immune responses. Arch. Intern. Med. 2008;168(22):2405–14. doi:10.1001/archinternmed.2008.513.
