ABSTRACT
Operation Warp Speed and global vaccine research efforts have succeeded in rapidly launching three vaccine candidates for coronavirus disease 2019 (COVID-19) into Phase III clinical trials. A recent letter from Centers for Disease Control and Prevention (CDC) Director Redfield underscored the possibility of “large-scale” distribution of a coronavirus vaccine as early as November 1, 2020. However, recent polling reveals that the majority of Americans remain skeptical of both the safety and efficacy of a potential Covid-19 vaccine. Even more troublesome is the fact that a comprehensive, collaborative vaccine marketing campaign has not been initiated to educate the U.S. public on and encourage widespread Covid-19 vaccination. Accordingly, this article lays out a plan of action, utilizing proven immunization marketing strategies and novel approaches, that could be used to combat vaccine hesitancy toward Covid-19. A vaccine may indeed be our ticket out of this pandemic, but targeted marketing is needed to increase public optimism toward that fact.
Under Operation Warp Speed, a partnership between the Department of Health and Human Services (HHS), Department of Defense (DoD), private firms, and other federal agencies, the federal government plans to produce and distribute an effective vaccine for coronavirus disease 2019 (Covid-19) by January 2021. To date, the program has invested in six vaccine candidates with three of those candidates (from AstraZeneca PLC, Pfizer Inc., and Moderna, Inc.) currently undergoing Phase III clinical trials. While original estimates predicted an approval date between March 2021 and December 2021, a recent letter from Dr. Robert Redfield, the Director of the Centers for Disease Control and Prevention (CDC), informed governors to prepare for “large-scale” distribution of a coronavirus vaccine by November 1, 2020.Citation1 While this unprecedented timeline is certainly in the public’s best interest, assuming all candidate approvals meet safety and efficacy standards, little has been done concerning educating the public on the benefits and overarching need for a vaccine in generating antibody-mediated immunity. In fact, a recent poll of 1,506 adults revealed that only 42% of Americans reported that they plan to get the vaccine if and when it becomes available. This poll also found that Black and Hispanic Americans, as well as lower-income individuals (making under 50 USD K/year), were less likely to report that they will get the vaccine than their White and higher-income counterparts.Citation2 This is especially troubling considering the disproportionate effect Covid-19 has had on these racial groups as seen in both hospitalization and mortality rates.Citation3
These results beg an important question: are the Covid-19 vaccine trials moving faster than public perception? And if so, what can be done to both increase public awareness and trust in the fast-approaching Food and Drug Administration (FDA)-authorized vaccine? To answer this, a multi-dimensional model is proposed that would build on the existing framework for seasonal influenza vaccination programs with targeted approaches for those most affected by the coronavirus (). Additionally, the model aims to combat safety concerns, efficacy concerns, and misinformation surrounding potential Covid-19 vaccines via both novel and mainstream marketing strategies.
Figure 1. A multi-dimensional approach to educating the U.S. public on and encouraging Covid-19 vaccination
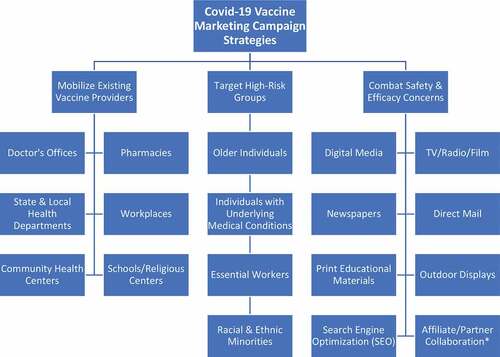
Multiple independent analyses have found that successful immunization marketing campaigns center around the mobilization of existing vaccine providers (mainly clinics and pharmacies).Citation4 Thus, it is essential that the Covid-19 vaccine campaign focuses on supplying these providers with the information and resources needed to properly educate and prepare patients for when the Covid-19 vaccine becomes available. These are the so-called “boots on the ground” when it comes to vaccine distribution and thus are in a unique position to relay information and guidelines to their patients. Additionally, the strong collaboration seen between public and private entities in addressing the pandemic provides a unique opportunity to make vaccines more accessible than ever to the public by targeting non-traditional distribution sites such as the workplace, schools, and social hubs (e.g., grocery stores, restaurants, etc.).
As mentioned before, Covid-19 has had a disproportionate impact across certain demographic groups. Current figures estimate that approximately 80% of deaths linked to Covid-19 have occurred in adults aged 65 and over, with the highest percentage of severe outcomes among individuals aged 85 and over. Additionally, the CDC has stated that individuals at any age with certain underlying medical conditions are at increased risk of developing severe illness from Covid-19. Finally, it is believed that essential workers have the highest occupational risk of developing the coronavirus – although recent trends suggest that this may be due to personal protective equipment (PPE) shortages.Citation5 Thus, a successful vaccine marketing campaign must strategically educate individuals in these groups regarding the potential benefit of a Covid-19 vaccine in securing antibody-mediated immunity and its importance in these individuals due to their risk factor(s). For instance, a targeted campaign for older individuals could concentrate educational resources and advertising media in nursing homes, senior centers, and high-traffic periodicals such as the AARP Bulletin. Similarly, marketing for individuals with underlying medical conditions could focus on providing educational content to dialysis centers and via the American Diabetes Association. To date, the CDC has made hundreds of infographics, guideline posters, and fact sheets available for workplaces depicting proper social distancing, handwashing, and PPE protocols. Workplace adoption of these materials provides an excellent avenue to expand vaccine education and promotion articles into both the mainstream and occupations at heightened risk of exposure. An organization could further incentivize vaccine promotion by providing vaccine booths, paid time off, and vaccine subsidies/mandates.
It is well understood that there are a multitude of factors inhibiting wide-scale vaccination in the U.S. population. The unexpected announcement of a potential “large-scale” distribution of a Covid-19 vaccine on November 1, 2020, may exacerbate existing concerns regarding the safety and effectiveness of a coronavirus vaccine. Vaccine hesitancy, in particular, has been observed in both social marketing and immunization participation studies. Various social marketing frameworks have been proposed to counter this notion, including the World Health Organization (WHO) initiative Tailoring Immunization Programs (TIP) and the CDC’s CDCynergy Social Marketing platform. While both have their merits, it may prove more beneficial to address the root cause(s) of vaccine hesitancy: general insufficient knowledge and misinformation.
Arguably, the most direct way to solve these issues is through increased vaccine marketing exposure and repetition. Positive results have been reported in previous immunization programs utilizing such goal measures.Citation4 As shown in , this can be achieved by presenting immunization facts and benefits to potential consumers in areas where they live, work, and play. For example, let’s assume that there is a fictitious mother, Jane, who is suffering from diabetes. Jane also has a school-aged child and is skeptical of a Covid-19 vaccine for both her and her child. At work, Jane encounters CDC infographics depicting that individuals with diabetes are at an increased risk of severe illness due to Covid-19. Next to this sign is a QR code, which leads to a website listing vaccination centers in her area. Jane takes a look but is worried about the cost and safety for her or her child. On the way home from work, Jane stops to pick up her child from school. Her child gets in the car and presents Jane with a flyer distributed to all students (through HHS-school partnerships) with a list of local health centers providing free Covid-19 vaccines and displaying the same QR code from the workplace poster. Later on, Jane decides to scroll through her favorite social media app for daily updates. As she opens the app, there is a pop-up stating, “Protect Your Child from Covid-19!”. Having been repeatedly exposed to the benefits, affordability, and safety of the vaccine, Jane decides to click the QR code and make an appointment for her and her child to get vaccinated. While this is an ideal scenario, it casts into light the level of preparation and collaboration needed to successfully educate the public and encourage widespread vaccination.
In conclusion, it is imperative that federal, state, and local governments, health advocacy groups, immunization initiatives, community clinics, and pharmacies launch a targeted and cohesive marketing campaign alongside Operation Warp Speed to increase public awareness and optimism regarding Covid-19 vaccination. The coming FDA-authorized vaccine(s) may offer a path out of the Covid-19 pandemic, but much work remains to be done in convincing the public of that fact.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The author thanks Ms. Kathryn Wells for her helpful comments on earlier drafts of the manuscript.
References
- Fact Sheet: Explaining Operation Warp Speed. Department of health & human services (HHS) documents/FIND. Department of Health & Human Services (HHS) Documents/FIND; 2020 Aug 07.
- Yahoo! News, & YouGov. Coronavirus poll; 2020 Jul 30. [accessed 2020 Sep 8]. https://docs.cdn.yougov.com/l9txxcxdi3/20200730_yahoo_coronavirus_crosstabs.pdf
- Tai D, Geno B, Shah A, Doubeni CA, Sia IG, Wieland ML. The disproportionate impact of COVID-19 on racial and ethnic minorities in the United States. Clin Infect Dis. 2020 Jul 20. doi:10.1093/cid/ciaa815.
- Nowak GJ, Gellin BG, MacDonald NE, Butler R. Addressing vaccine hesitancy: the potential value of commercial and social marketing principles and practices. Vaccine. 2015;33(34):4204–11. doi:10.1016/j.vaccine.2015.04.039.
- Centers for Disease Control Prevention, issuing body. Key updates for week 35, ending August 29, 2020. [accessed 2020 Sep 8]. COVIDView: A Weekly Surveillance Summary of U.S. Covid-19 Activity; 2020. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covidview/index.html
