ABSTRACT
There is an urgent need for a safe, efficacious, and cost-effective vaccine for the coronavirus disease 2019 (COVID-19) pandemic caused by novel coronavirus strain, severe acute respiratory syndrome-2 (SARS-CoV-2). The protective immunity of certain types of vaccines can be enhanced by the addition of adjuvants. Many diverse classes of compounds have been identified as adjuvants, including mineral salts, microbial products, emulsions, saponins, cytokines, polymers, microparticles, and liposomes. Several saponins have been shown to stimulate both the Th1-type immune response and the production of cytotoxic T lymphocytes against endogenous antigens, making them very useful for subunit vaccines, especially those for intracellular pathogens. In this review, we discuss the structural characteristics, mechanisms of action, structure–activity relationship of saponins, biological activities, and use of saponins in various viral vaccines and their applicability to a SARS-CoV-2 vaccine.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic caused by the novel coronavirus strain, severe acute respiratory syndrome CoV-2 (SARS-CoV-2), has created widespread global health concerns by rapidly spreading to more than 215 countries. To date, >30 million have been infected and >1 million are dead. Currently, there is no widely available specific treatment for this virus and the researchers face a challenge to develop a safe, efficacious, and cost-effective vaccine.1,Citation2 The protective immunity of vaccines can be enhanced by the addition of adjuvants. The addition of an adjuvant in the COVID-19 vaccine besides boosting immunogenicity would also reduce the requirement of vaccine protein per dosage. Many diverse classes of compounds have been used as adjuvants, including mineral salts, microbials products, emulsions, saponins, cytokines, polymers, microparticles, and liposomes. Based on their proposed mechanisms of action, vaccine adjuvants have been broadly divided in two different groups: (1) immunostimulants (e.g., saponins, Toll-like receptor (TLR) agonists, cytokines) and (2) delivery agents (e.g., emulsions, microparticles, mineral salts).Citation3 Immunostimulants activate the antigen-presenting cells (APCs) and promote the secretion of various cytokines. On the contrary, the delivery agents help to preserve the conformation of antigens (Ag) for proper presentation to the APCs and to provide a slow release for continuing immune stimulation. For instance, TLR agonists and other immunostimulatory substances enhance immune cell recruitment and cytokine secretion, whereas emulsions and mineral salts produce a depot effect at the injection site, resulting in a prolonged release of the antigen and continued stimulation of immune cells.Citation4 Saponins, the steroid or triterpenoid glycosides found in wild or cultivated plants, lower marine animals, and some bacteria, have immunostimulatory properties. Several saponins play critical role in stimulating both the Th1 immune response and the production of cytotoxic T lymphocytes against the exogenous antigens, thus have high potential to be used as ideal adjuvants in subunit vaccines and vaccines for the intracellular pathogens, as well as with therapeutic cancer vaccines.
Saponins are a diverse group of naturally occurring active compounds widely found in the plant kingdom and are active constituents in over 100 families of fungi, including endophytic terrestrial and marine fungi.Citation5 Structurally, saponins contain a triterpene or steroid aglycone called sapogenin, with one or more sugar chains attached to it. Steroidal saponins are present mainly in monocotyledons, while triterpenoid saponins occur in dicotyledon plants. Saponins exhibit emulsifying and foaming properties due to the presence of hydrophobic aglycone and hydrophilic sugar chains in their structure (amphiphilic nature).Citation6 Saponin foaming capacity is attributed to the combination of a hydrophobic (fat-soluble) sapogenin with a hydrophilic (water-soluble) sugar base.Citation7
There is one saponin adjuvanted licensed vaccine approved by the FDA in the year 2017. It is the recombinant zoster vaccine (RZV, Shingrix, GlaxoSmithKline) containing AS01B which is a saponin-based adjuvant. It is a subunit vaccine that contains recombinant varicella herpes zoster virus glycoprotein E. The AS01 adjuvant system, consists of two immunostimulants, monophosphoryl lipid A (MPL) and QS-21 saponin. The QS-21 saponin is purified from the bark of the Quillaja saponaria Molina tree. It induces antigen-specific antibody as well as cell-mediated immune response. The MPL signals through Toll-like receptor-4 (TLR4), which results in the activation of APCs and the production of cytokines and interferons (IFNs).Citation8 This adjuvant system has been used in recently developed RTS, S/AS01 malaria vaccine, Mosquirix (phase 3 trial completed in 2019, vaccine approved in three pilot countries of South Africa, viz., Ghana, Malawi and Kenya, WHO), polyprotein HIV-1 candidate vaccineCitation9 and tuberculosis (Mtb72F/AS02 candidate) vaccine.Citation10 These vaccines have been administered to the susceptible population and the safety and efficacy evaluation of AS01 adjuvant is underway.Citation8
Recently, saponin-based microemulsion adjuvant has also been studied in the vaccine for COVID-19. SARS-CoV-2 S1-Fc vaccine candidate with saponin microemulsion adjuvant developed high titers of S1 (recombinant protein)-specific neutralizing antibodies in cynomolgus monkeys.Citation11 The AS01 adjuvant system when co-administered with recombinant SARS-CoV S protein, induced high titers of antigen-specific serum antibodies and protected from viral infection.Citation12 Keeping in view the robust immune response induced by saponin adjuvants, it becomes important that saponin-based adjuvants be further explored for use in a subunit vaccine against COVID-19. Nevertheless, in the current situation, a proven safe and efficacious adjuvant should be used in the vaccine for SARS-CoV-2 to get rapid approval of the regulatory agencies. Therefore, saponin might not be preferred over other adjuvants, but definitely represents a viable alternative for SARS-CoV-2 subunit vaccine for long-term future use. In this review, we discuss the structural characteristics, mechanisms of action, structure–activity relationship of saponins, biological activities, use of saponins in various antiviral vaccines, and the possible use in a vaccine for SARS-CoV-2.
Structural characteristics of saponins
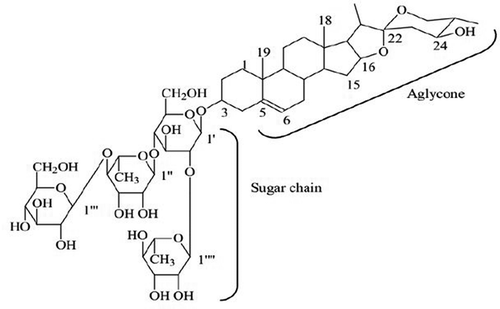
Saponins belong to a class of compounds that contain a rigid skeleton of at least four hydrocarbon rings to which sugar chains are attached in groups of one or two (normally no more than 10 units) ().Citation13,Citation14 They may be classified as triterpenoid (C30) or steroid (C27) based on the number of carbon atoms present in the core (aglycone).Citation15 There are 11 major saponin classes and saponins containing the oleanane skeleton are the most common in the plant kingdom.Citation16,Citation17 Saponins with the carbohydrate or oligosaccharide chains attached at position C‐3 are monodesmosidic, while carbohydrate chains attached at two positions C‐3 and C‐26 or C‐28 are bidesmosidic. Numerous forms of saponins can be derived from a variety of aglycones, carbohydrates, and different attachment positions. Both steroidal and triterpene saponins may contain other functional groups: – OH, – COOH, – CH3, which further add to their diversity.Citation18
Figure 1. Chemical structure of steroid saponin.Citation13
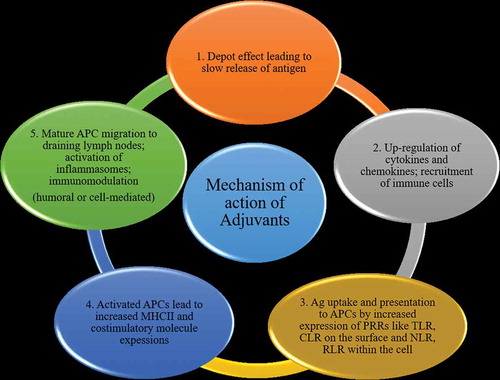
Mechanism of action of adjuvants
Several specific groups of compounds, including mineral salts, microbial products, emulsions, saponins, cytokines, polymers, microparticles, and liposomes, have been classified as adjuvants.Citation19 The vaccine adjuvants have been narrowly divided into delivery systems and immunostimulatory adjuvants based on their suggested modes of action.Citation4 Nevertheless, recent progress in immunobiological research has revealed several mechanisms ().Citation20
Depot effect
This is the most widely recognized mechanism of adjuvant action where the antigens are entrapped and then released slowly at the injection site. This slow delivery of antigens can enhance the continuous stimulation of the immune system to produce high antibody titers.Citation21 Depot formation was first observed with the alum adjuvantsCitation22 and the antigens were detected for 2–3 weeks inside the granulomas produced by these alum compounds.Citation23 Various other adjuvants, such as water-in-oil emulsions [e.g., Complete Freund’s Adjuvant (CFA)] and biodegradable micro- and nano-particles, have also been shown to act via the depot effect to produce prolonged and sustained high antibody titers.Citation24,Citation25
Up-regulation of cytokines and chemokines
Recent studies of adjuvant mechanisms have focused on the recruitment of innate immune cells at the injection site. It has been shown that certain adjuvants establish a local pro-inflammatory environment necessary to recruit the immune cells.Citation26 At the injection site, adjuvant core response genes expressed by the immune cells are strongly upregulated and modulated by alum, CpG-ODN, and MF59 adjuvants. Activation of these core response genes promotes upregulation of cytokine and chemokine synthesis.Citation27 The activity exhibited by some of the common adjuvants have been shown in .
Table 1. Activity exhibited by licensed adjuvants
Antigen cross-presentation
Some adjuvants such as alum, oil-based emulsions, and microparticles work by “targeting” antigens to the APCs, resulting in increased MHC antigen presentation.Citation36,Citation37 Alum plays an important role by enhancing the antigen internalization by the APCs and limiting the rate of internalized antigen degradation.Citation38 QS-21, a purified saponin adjuvant from Quillaja saponaria, can activate both Th1 and CD8+ T cells to produce a robust antibody and cell-mediated immune response.Citation39 One study showed that QS-21 can stimulate the production of IL-1β and IL-18 via activation of NLRP3 inflammasome in murine dendritic cells (DCs).Citation40 However, in the presence of QS-21, NLRP3-deficient mice showed higher levels of Th1 and Th2 antigen-specific T-cell responses, and increased IgG1 and IgG2c, suggesting a more complex regulatory role for NLRP3. Duewell and coworkersCitation41 demonstrated that subcutaneous administration of saponin-based adjuvant vaccines in mice resulted in mobilization and activation of immune cells in vaccine site-draining lymph nodes. This group also demonstrated an efficient antigen uptake by dendritic cells (DCs), DC maturation induction, and in vivo production of IL-12.
Activation of inflammasomes
Inflammasomes are innate immune system receptors and sensors that control caspase-1 activation and induce inflammation in response to infectious microbes and host protein-derived molecules.Citation42 Innate immune cells express pattern-recognition receptors (PRRs) to identify various pathogens. The family of PRRs includes TLRs, C-type lectin-like receptors (CLRs), nucleotide oligomerization domain (NOD)-like receptors (NLRs), and retinoic acid-inducible gene-1 (RIG-1)-like receptors (RLR). NLRP3 inflammasome activation induces caspase-1, which, in turn, cleaves inactive proforms of IL-1β, IL-18, and IL-33 into their active forms.Citation43 Alum is one of the most widely used vaccine adjuvants; however, it is a poor inducer of cell-mediated immunity.Citation44,Citation45 In vitro, alum and other inflammatory activators, such as lipopolysaccharide (LPS), upregulate the expression pro-IL-1β and NLRP3.Citation46,Citation47
QS-21, a highly purified and soluble saponin adjuvant currently used in licensed and exploratory vaccines, induces caspase-1-dependent release of IL-1β and IL-18 in APCs, such as macrophages and DCs, when co-stimulated with the TLR4-agonist monophosphoryl lipid A adjuvant.Citation48 QS-21 elicits a strong antibody and cell-mediated immune response and is also a potent Th1 and CD8+ T cell activator.Citation49,Citation50 It is an adjuvant candidate for many current trials of vaccines, such as HIV-1,Citation51,Citation52 cancer,Citation53,Citation54 hepatitis B,Citation55 malaria.,Citation56,Citation57 tuberculosisCitation58 and Alzeihmer’s disease.Citation59 Due to the promising immunopotentiator effects exhibited by QS-21, it should be studied and evaluated as an adjuvant in a COVID 19 subunit vaccine as the SARS-CoV-2 could reemerge every year and new vaccines may be needed to combat it.
Mechanisms of action of saponin adjuvants
Saponins exert their adjuvant activity via the immunostimulatory effects and by activation of cytokine production, e.g., interferons and interleukins.Citation60,Citation61 It was proposed that saponins interact with APCs, activate intracellular signaling, and, therefore, enhance the cytokines release.Citation62 Saponins promote the entry of antigens via the endogenous pathway of antigen presentation and by enhancing cytotoxic T-lymphocyte mediated immune responses.Citation63 Adjuvant activity of saponin compounds is mainly due to the presence of aldehyde group and sugar side chainCitation64 or acyl residues in the aglycone portion of saponin.Citation61 Several saponins, such as lablabosides and soyasaponins, lack acyl group in their chemical structure and produce immunostimulatory activity with the help of sugar moiety.Citation65
The lipophilic acyl side chain present in saponins can favor strong cytotoxic T lymphocyte (CTL) activation by the exogenous antigens and can promote hemolysis. Deacylation of this lipophilic side chain decreases the toxic hemolytic effect and activates Th2 immune response with simultaneous inactivation of Th1 response.Citation39 Saponin contains an aldehyde group at its C-4 position and acylated fucopyranosyl residue at its C-28 position.Citation66,Citation67 Arabinose present at the terminal of acyl chain strongly elicits Th1/Th2 immunity with the CTL production. Furthermore, deacylation of these fucosyl pyranose molecules can solely trigger Th2 immunity.Citation68 The ɛ-amino group of the T-cell receptor forms an imine with the aldehyde group of the saponin. Activated receptors then trigger signals via tyrosyl phosphorylation leading to stimulation of mitogen-activated protein (MAP) kinase along with changes noticed in K+ and Na+ transport channels. Consequently, activated T-cells are biased toward Th1 immunity with increased production of Th1 cytokines.Citation62
Whereas the Quillaja saponins adjuvants such as QS-21 act on dendritic cells (DCs) in a non-receptor-mediated manner. The exogenous protein antigens (ag) and QS-21 enter DCs by endocytosis, where QS-21 disrupts the endosomal membrane, allowing early escape of the antigens for further processing inside the cell into peptides. Properly degraded antigens are loaded into MHCI by the vacuolar pathway, while antigens that need more processing are transported to the cytosol to be cleaved by the proteasome (cytosolic pathway). These peptides are then transported to the endoplasmic reticulum (ER), which after additional processing are loaded into MHCI. Peptides derived from either the vacuolar or cytosolic pathways after binding to MHCI are presented on the DC surface to naive CD8 + T cells in a process called cross-presentation to yield CTLs.Citation69
Structure-activity relationship of saponins
Aldehyde
The mechanism of saponin adjuvant activity was first correlated with the aldehyde group attached to the core aglycone. Formation of bond (Schiff base) between the aldehyde group of saponin and free amino groups of APCs was the first stage of immune-activating mechanism.Citation39 The proportion and conformation of the aldehyde group in the saponin molecule plays a crucial role in maintaining the integrity and strength of Th1 response. Axial aldehyde shifts the immune system toward the stimulation of humoral immune responses, whereas equatorial aldehyde produces cell-mediated immune responses.Citation70
Lipophilic acyl groups
Acyl residues of saponin can enhance the activation of CTL against exogenous antigens. Deacylation of saponins, such as QS-18 and QS-21, shows reduced antibody production and Th1 response compared to the acylated saponin, suggesting that the acyl residues are important for the activation of CTL-mediated immune response.Citation39
Sugar chains
The sugar chain linked at the C-28 position is essential for the initiation of immune-activating mechanism, as well as for the toxic hemolytic effect.Citation71 The amphipathic nature of saponins is the result of sapogenin (hydrophobic) and sugar chain (hydrophilic) presence in their chemical structure. The balance between these sapogenin (hydrophobic) and sugar chain (hydrophilic) properties is important for maintaining the adjuvanticity of saponins.Citation39
Biological activities
Saponins are a diverse group of glycosides with a wide range of biological properties. They are thought to be the main constituents of many plant-based drugs and traditional medicines, responsible for various pharmacological properties.Citation72
Hemolytic activity
Saponins have the ability to lyse the erythrocyte membrane. This property has led to the development of hemolytic assays to detect the presence of saponins in drugs or plant extracts. Hemolytic properties are generally attributed to the interaction between the saponins and the sterols in the erythrocyte membrane.Citation73 The undesirable hemolytic activity of the saponin molecules is mainly due to the presence of saccharide side chain and the acyl residues in the aglycone.Citation39 Cell membrane cholesterol enhances saponin attachment and induces pore formation. The level of cell permeabilization is highly influenced by the concentration and structure of saponin molecules.Citation74–77 As a result, formation of membrane pores allows ionic transportationCitation74 and protein mobilityCitation78 between the inter- and intra-cellular spaces.
Anti-inflammatory activity
Many saponins isolated from plant sources have an inhibitory effect on inflammation. Aescin, a triterpenoid saponin mixture isolated from Aesculus hippocastanum L. (Hippocastanaceae), is known to have anti-inflammatory, anti-edematous, and venotonic properties.Citation79 The loniceroside C, a triterpenoid saponin isolated from the Lonicera japonica Thunb. aerial parts (Caprifoliaceae), the medicinal plant known for centuries as an anti-inflammatory agent, demonstrated its anti-inflammatory activity in vivo when tested with croton oil in the mouse ear edema model.Citation80 A novel steroidal saponin isolated from the Agave attenuate Salm-Dyck (Agavaceae) leaves was evaluated for its anti-inflammatory activity using the capillary permeability assay.Citation81 The steroidal saponin inhibited the increase in vascular permeability caused by acetic acid, a standard model for the inflammatory reaction at first stage. Kim and coworkersCitation82 investigated the ginseng (Panax ginseng C.A. Mey., Araliaceae) saponins for their anti-complementary activity. Ginsenoside Ro and oleanolic acid showed that these saponins have the highest anti-complementary activity. They suggested that the anti-inflammatory activity of these saponins is mediated through the classical pathway of inflammation to anti-complementary action. A good correlation between the radical scavenging activity and a weak cytotoxicity against the murine monocytic macrophage cell line was produced in vitro by the hydroalcoholic extract of Silene vulgaris.Citation83 The saponin-enriched fraction of S. vulgaris showed lower in vitro hemolytic activity and cell cytotoxicity in the VERO cells and this could be studied further as a newer source of saponin adjuvant.Citation84
Antibacterial/antimicrobial activity
Saponins have also been shown to possess antimicrobial activity. Three butanol-extractable 5β-spirostan-3β-ol saponins have been shown to have antimicrobial activity against both prokaryotic and eukaryotic organisms at low cell densities. However, these saponins did not inhibit dense populations of microbial growth.Citation85 Saponins with tetraglycoside have stronger activity compared to the saponins with triglycoside. A new saponin jujubogenin, isolated from Colubrina retusa L. (Rhamnaceae), had antimycobacterial activity at minimum inhibitory concentration (MIC) of 10 μg/ml when tested against Mycobacterium intracellulare.Citation86 It has been documented that triterpenoid saponins from other sources, such as Maesalanceolata, Maesachisia, and Maesaindica, display direct virucidal activity against Newcastle disease virus, vaccinia virus, and herpes simplex virus.Citation87 Several saponins, for example aescine from Aesculus hippocastanum, primula saponin from Primula veris, saikosaponin A from Bupleurum falcatum, theasaponin from Thea sinensis, and gymnemic acid from Gymnema sylvestre, showed antagonist activity against influenza A2 virus.Citation88,Citation89Arganine C, the saponin isolated from the fruit of Tieghemella heckelii Pierre ex A. Chev. (Sapotaceae), showed antiviral activity against HIV virus.Citation90 Arganine C saponin strongly inhibited HIV entry into the cells during the cell fusion test, and did not show substantial cytotoxicity to HeLa-CD4+ cells. Mixture of maesasaponin isolated from Maesa lanceolata Forssk. (Myrsinaceae) has been reported to have anti-herpes simplex type 1 virus (HSV-1) and type 1 poliovirusCitation91 properties. Triterpenoid saponin isolated from the family Fabaceae also showed antiviral activity against herpes viruses. Activity of anti-herpes simplex virus has been found to be related to the chemical structure of fabaceous saponins. The sugar moiety of this saponin has a glucosyl unit in the central part, exerting a better antiviral activity.Citation92 Simoes et al.Citation93 tested two triterpenoid saponins, oleanane and ursane from Brazilian and Chinese plants, for their antiviral activity. The oleanane-type saponins inhibited the DNA synthesis of herpes simplex virus type 1, whereas the ursane-type saponin inhibited the capsid protein synthesis of HSV type 1.
Immunomodulatory activity
Bushneva and coworkersCitation94 showed that pectic polysaccharide named silenan, isolated from the aerial parts of S. vulgaris, possessed immunomodulatory activity. Ghonime and coworkersCitation95 confirmed the immunomodulatory activity of the Silene species. In a study conducted by Rivera and coworkers,Citation96 porcine parvovirus (PPV) vaccines containing Rb1 fraction of ginseng was evaluated for inducing Th1 or Th2 type of immunity in mice. The study revealed the production of large amounts of cytokines, including IFN-γ, IL-2, IL-4, IL-10, and TNF-α, and stimulated titers of antigen-specific IgG1, IgG(2a), and IgG(2b).
Antitumor activity
A number of pharmacological properties have been attributed to saponins, such as immunomodulative potential by the cytokine interplayCitation39 and cytotoxic effects on cells from the malignant tumors.Citation97 Saponins have surface-active properties due to the amphiphilic nature of their chemical structure. The mechanisms suggested for saponin’s anticarcinogenic properties include direct cytotoxicity, immune-modulatory activity, bile acid binding, and cancer-induced cell proliferation normalization.Citation98 Saponins have been shown to not only increase antibody responses but also induce helper and cytotoxic T-cell responses.Citation99 QS-21 saponin adjuvants show strong Th1 reactions by stimulating cytokine production (IL-2 and IFN-γ) as well as specific CTL via MHCI against the exogenous antigens and cancer cells.Citation39 The conformation of aldehyde in the QS-21 saponin determines the integrity of Th1 immune responses. Cellular immune responses are highly stimulated by the equatorial conformation of triterpene aldehyde.Citation70
Saponins as adjuvant candidates for COVID-19 vaccine
COVID-19, a disease caused by the novel SARS-CoV-2 coronavirus, based on genetic and clinical evidence, appears to follow a mechanism similar to SARS and MERS. The outbreak of this disease has led to a pandemic endangering global public health and posed high challenges to contain it.Citation100–103 The production of vaccines is the most promising method for prevention and elimination of this highly contagious respiratory disease. Coronaviruses (CoVs) are large enveloped viruses that carry single-stranded positive-sense RNA genome. The viral membrane is studded with spikes of glycoproteins which give coronaviruses a crown-like appearance. Four types of coronaviruses are identified and they include alpha, beta, gamma, and delta. Severe acute respiratory syndrome (SARS) virus (SARS-CoV), Middle East respiratory syndrome (MERS) virus (MERS-CoV), and SARS-CoV-2 belong to the betacoronavirus class. The genomic sequence of SARS-CoV-2 demonstrated a similar but distinct composition compared to the SARS-CoV and MERS-CoV genomes. SARS-CoV-2 binds to target cells located in the lower respiratory system to cause viral pneumonia, similar to SARS-CoV and MERS-CoV, but can also affect the GI tract, CNS, heart, kidneys, and liver and lead to multiple organ failure.Citation104,Citation105
Currently, scientists are racing toward the development of safe and effective vaccines to prevent COVID-19. According to the WHO (as on September 17th, 2020), 36 candidate vaccines are in different phases of clinical evaluation whereas, 146 candidate vaccines are in the pre-clinical evaluation stage. Several platforms, including non-replicating viral vector vaccine, inactivated vaccine, RNA or DNA vaccine, protein subunit vaccine and virus-like particle vaccine, with (like Matrix M, Advax, MF59, CpG 1018, GlaxoSmithKline adjuvants) or without adjuvants are being investigated. Nine candidate vaccines are in the phase 3 of the clinical trial. These include the ChAdOx1-S (University of Oxford/AstraZeneca), Adenovirus Type 5 Vector (CanSino Biological Inc./Beijing Institute of Biotechnology), Adeno-based (rAd26-S+ rAd5-S) (Gamaleya Research Institute), Ad26COVS1 (Janssen Pharmaceutical Companies), three inactivated vaccines (Sinovac, Wuhan Institute of Biological Products/Sinopharm, Beijing Institute of Biological Products/Sinopharm), LNP-encapsulated mRNA (Moderna/NIAID), and 3 LNP-mRNAs (BioNTech/Fosun Pharma/Pfizer). Spike protein and its fragments, including S1, S2, RBD, and N were the prime targets for MERS and SARS vaccine development. Similarly, SARS-CoV-2 regions are expected to be considered for COVID-19 vaccines as important targets.Citation106,Citation107 Spike protein-based vaccines would induce antibodies to block not only viral receptor binding, but also virus genome uncoating. The S protein has a major role in the induction of protective immunity during the infection with SARS-CoV-2 by generating neutralizing antibodies and T-cell responses. Thus, full-length or functional domains of S glycoprotein are believed to be the most promising candidates for SARS-CoV-2 vaccine composition.Citation1 The identification of immunodominant region among the subunits and domains of S protein is critical for developing an effective vaccine against the coronavirus. Further investigations are needed to determine the immunodominant regions of SARS-CoV-2 to facilitate the vaccine development.Citation100
Several animal-testing research and human trials have shown promising trends, focusing on achieving high rates of neutralizing antibodies. Until now, high titers of S-protein neutralizing antibodies in pre-clinical models have been achieved for two vaccines developed by the traditional methods. Efforts are being made to use novel adjuvants that can potentiate humoral, cellular, and memory immune responses to prevent COVID-19 infection and subsequent disease from being established. Both triterpenoid and steroidal saponins show antiviral activity against different viral groups. Aqueous extracts from the Chilean soapbark tree (Quillaja saponaria Molina) produce many physiologically active triterpenoid saponinsCitation108 and demonstrate high adjuvant activity for use in animal and human vaccines.Citation61,Citation109 As earlier discussed, AS01, a novel adjuvant, contains liposomes and two immunostimulants, 3-O-desacyl-4 incl-monophosphoryl lipid A and distilled saponin QS-21. Both immunostimulant compounds in this adjuvant appear to be critical for the stimulation or activation of antigen-specific cellular and humoral immune responses.Citation110
Saponin purified from Quillaja saponaria alone or introduced as part of the immunostimulating complexes (ISCOMs), proved to be a powerful adjuvant in human cytomegalovirus vaccines,Citation111 influenza vaccines,Citation112 or polysaccharide vaccines.Citation113 Platycodin D (PD) has been evaluated as an adjuvant in vaccine formulations for the recombinant hepatitis B surface antigenCitation114 and Newcastle disease virus-based recombinant avian influenza vaccineCitation115 in mice. Con A-, LPS-, and antigen-induced splenocyte proliferation and serum antigen-specific IgG, IgG1, IgG2a, and IgG2b antibodies titers were significantly enhanced by formulations containing PD. The mRNA expression of Th1 and Th2 cytokines in splenocytes was also up-regulated by PD, which remarkably increased the killing activities of natural killer cells from splenocytes in the immunized mice. Thus, PD showed adjuvant activity in both formulations. Platycodin D2 (PD2) improved both cellular and humoral responses to hepatitis B surface antigen (HBsAg) in mice. PD2 also significantly increased the Con A-, LPS-, and HBsAg-induced splenocyte proliferation, as well as enhanced HBsAg-specific IgG, IgG1, IgG2a, and IgG2b antibody levels in HBsAg-immunized mice. Moreover, PD2 promoted the production of Th1 (IL-2 and INF-gamma) and Th2 (IL-4 and IL-10) cytokines from splenocytes in HBsAg-immunized mice.Citation115
Recently, the effectiveness of Quillaja brasiliensis saponins has also been confirmed in experimental vaccines against bovine herpesvirus type 1 and 5 (BoHV), human poliovirus, and rabies in mice.Citation116–120 These saponins are able to form micellar nanometric ISCOM-type structures which are even more effective as vaccine adjuvants, generating both humoral and cellular immune responses.Citation117
Conclusions
At present, vaccine development for SARS-CoV-2 causing COVID-19 is the highest priority of the global medical research community. For a vaccine to be safe, effective and of increased immunogenicity, adequate adjuvants are required. QS-21, the most active saponin fraction from Quil A, possesses high potent adjuvant activity with minimal toxicity. Saponin-based adjuvants selectively stimulate Th1 and cytotoxic T cell responses because they direct antigens into endogenous processing pathways and enhance IFN-γ release by dendritic cells. As a result, a robust antibody and cell-mediated immune response is activated. Therefore, more research is needed to develop saponin adjuvanted recombinant spike or RBD protein subunit vaccine. Development of a saponin adjuvanted subunit vaccine for SARS-COV-2 would also help us in tackling future pandemics associated with other novel coronaviruses.
Author contributions
RS conceptualized and designed the review. AP and RS wrote the manuscript. KD and RS critically edited the manuscript. GM, BS and KPS collected literature and reviewed the manuscript. All the authors read and approved the final manuscript.
Disclosure of potential conflicts of interest
All authors declare that there exist no commercial or financial relationships that could, in any way, lead to a potential conflict of interest.
Acknowledgments
The mini-review paper was supported by the research project, IVRI/PALAM/17-19/013 and for this we gratefully acknowledge the Director, ICAR-IVRI, Izatnagar, Bareilly, Uttar Pradesh, India.
Additional information
Funding
References
- Dhama K, Sharun K, Tiwari R, Dadar M, Malik YS, Singh KP, Chaicumpa W. COVID-19, an emerging coronavirus infection: advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics. Hum Vaccin Immunother. 2020a Mar 18:1–7. doi:https://doi.org/10.1080/21645515.2020.1735227.
- Yatoo MI, Hamid Z, Parray OR, Wani AH, Wani A, Saxena A, Patel SK, Pathak M, Tiwari R, Malik YS, et al..COVID-19 - Recent advancements in identifying novel vaccine candidates and current status of upcoming SARS-CoV-2 vaccines. Human Vaccin Immunother. 2020. doi:https://doi.org/10.1080/21645515.2020.1788310.
- Cox JC, Coulter AR. Adjuvants - a classification and review of their modes of action. Vaccin. 1997;15:248–56. doi:https://doi.org/10.1016/S0264-410X(96)00183-1.
- Singh M, O’Hagan D T. Recent advances in veterinary vaccine adjuvants. Internat J Parasitol. 2003;33:469–78.
- Van Dyck S, Flammang P, Meriaux C, Bonnel D, Salzet M, Fournier I, Wisztorski M. Localization of secondary metabolites in marine invertebrates: contribution of MALDI MSI for tH. Study of saponins in cuvierian tubules of H. forskali. PLoS One. 2010;5:e13923. doi:https://doi.org/10.1371/journal.pone.0013923.
- Hostettmann KA, Marston A. Chemistry and Pharmacology of Natural Products: Saponins. Cambridge, New York: Cambridge Univ. Press;1995.
- Kregiel D, Berlowska J, Witonska I, Antolak H, Proestos C, Babic M, Babic L, Zhang B. Saponin-based, biological-active surfactants from plants. Applicn Charactn Surfact. 2017;183–205. http://dx.doi.org/10.5772/68062
- Didierlaurent AM, Laupèze B, Pasquale AD, Hergli N, Collignon C, Garçon N. Adjuvant system AS01: helping to overcome the challenges of modern vaccines. Expert Rev Vaccines. 2017;16(1):55–63. doi:https://doi.org/10.1080/14760584.2016.1213632.
- van Braeckel E, Buorgognon P, Koutsokos M, Clement F, Jensens M, Carletti I, Collard A, Demoitie MA, Voss G, Leroux-Roels G, McNally L. An adjuvanted polyprotein HIV-1 vaccine induces polyfunctional cross-reactive CD4+ T cell responses in seronegative volunteers. Clin Inf Dis. 2011;54:522–31. doi:https://doi.org/10.1093/cid/ciq160.
- Leroux-Roels I, Forgus S, De Boever F, Clement F, Demoitié MA, Mettens P, Moris P, Ledent E, Leroux-Roels G, Ofori-Anyinam O, M72 Study Group. Improved CD4+ T cell responses to Mycobacterium tuberculosis in PPD-negative adults by M72/AS01 as compared to the M72/AS02 and Mtb72F/AS02 tuberculosis candidate vaccine formulations: a randomized trial. Vaccine. 2013;31(17):2196–206. doi:https://doi.org/10.1016/j.vaccine.2012.05.035.
- Ren W, Sun H, Gao GF, Chen J, Sun S, Zhao R, Gao G, Hu Y, Zhao G, Chen Y, et al.. Recombinant SARS-CoV-2 spike S1-Fc fusion protein induced high levels of neutralizing responses in nonhuman primates. Vaccine. 2020;38(35):5653–58. doi:https://doi.org/10.1016/j.vaccine.2020.06.066.
- Bisht H, Roberts A, Vogel L, Subbarao K, Moss B. Neutralizing antibody and protective immunity to SARS coronavirus infection of mice induced by a soluble recombinant polypeptide containing an N-terminal segment of the spike glycoprotein. Virology. 2005;334:160–65. doi:https://doi.org/10.1016/j.virol.2005.01.042.
- El Aziz MMA, Ashour AS, Melad ASG. A review on saponins from medicinal plants: chemistry, isolation, and determination. J Nanomed Res. 2019;7(4):282 ‒288. doi:https://doi.org/10.15406/jnmr.2019.07.00199.
- Saxena M, Saxena J, Nema R, Singh D, Gupta A. Phytochemistry of medicinal plants. J Pharmacog Phytochem. 2013;1:168–82.
- Springob K, Kutchan TM. Introduction to the different classes of natural products. In: Plant-derived natural products. Editor(s): Osbourn AE, Lanzotti V. New York (NY): Springer; 2009. p. 3–50.
- Szakiel A, Ruszkowski D, Janiszowska W. Saponins in Calendula officinalis L. -structure, biosynthesis, transport and biological activity. Phytochem Rev. 2005;4:151–58. doi:https://doi.org/10.1007/s11101‐005‐4053‐9.
- Vincken JP, Heng L, de Groot A, Gruppen H. Saponins, classification and occurrence in the plant kingdom.. Phytochem. 2007;68(3):275–97. doi:https://doi.org/10.1016/j.phytochem.2006.10.008.
- Oleszek W, Hamed A. Saponin‐based surfactants. In: Kjellin M, Johansson I, editors. Surfactants from renewable sources resources. Chichester (UK): John Wiley & Sons Ltd; 2010. p. 239–49. doi:https://doi.org/10.1002/9780470686607.ch12.
- Guy B. The perfect mix: recent progress in adjuvant research. Nat Rev Microbiol. 2007;5(7):396–97. doi:https://doi.org/10.1038/nrmicro1681.
- Awate S, Babiuk LA, Mutwiri G. Mechanisms of action of adjuvants. Front Immunol. 2013;4:114. doi:https://doi.org/10.3389/fimmu.2013.00114.
- Siskind GW, Benacerraf B. Cell selection by antigen in the immune response. Adv Immunol. 1969;10:1–50.
- Glenny AT, Pope CG, Waddington H, Wallace V. The antigenic value of toxoid precipitated by potassium-alum. J Pathol Bacteriol. 1926;29:38–45.
- Osebold JW. Mechanisms of action by immunologic adjuvants. J Am Vet Med Assoc. 1982;181:983–87.
- Herbert WJ. The mode of action of mineral-oil emulsion adjuvants on antibody production in mice. Immunol. 1968;14:301–18.
- Kreuter J. Possibilities of using nanoparticles as carriers for drugs and vaccines. J Microencapn. 1988;5(2):115–27. doi:https://doi.org/10.3109/02652048809056475.
- Goto N, Akama K. Histopathological studies of reactions in mice injected with aluminum-adsorbed tetanus toxoid. Microbiol Immunol. 1982;26:1121–32. doi:https://doi.org/10.1111/j.1348-0421.1982.tb00261.x.
- Mosca F, Tritto E, Muzzi A, Monaci E, Bagnoli F, Iavarone C, O’Hagan D, Rappuoli R, De Gregorio E. Molecular and cellular signatures of human vaccine adjuvants. Proc Natl Acad Sci. U.S.A. 2008;105:10501–06. doi:https://doi.org/10.1073/pnas.0804699105.
- He P, Zou Y, Hu Z. Advances in aluminum hydroxide-based adjuvant research and its mechanism. Hum Vaccin Immunother. 2015;11(2):477–88. doi:https://doi.org/10.1080/21645515.2014.1004026.
- Calabro S, Tortoli M, Baudner BC, Pacitto A, Cortese M, O’Hagan DT, De Gregorio E, Seubert A, Wack A. Vaccine adjuvants alum and MF59 induce rapid recruitment of neutrophils and monocytes that participate in antigen transport to draining lymph nodes. Vaccin. 2011;29:1812–23. doi:https://doi.org/10.1016/j.vaccine.2010.12.090.
- Seubert A, Monaci E, Pizza M, O’Hagan DT, Wack A. The adjuvants aluminum hydroxide and MF59 induce monocyte and granulocyte chemoattractants and enhance monocyte differentiation toward dendritic cells. J Immunol. 2008;180:5402–12. doi:https://doi.org/10.4049/jimmunol.180.8.5402.
- Morel S, Didierlaurent A, Bourguignon P, Delhaye S, Baras B, Jacob V, Planty C, Elouahabi A, Harvengt P, Carlsen H. Adjuvant system AS03 containing a-tocopherol modulates innate immune response and leads to improved adaptive immunity. Vaccin. 2011;29:2461–73. doi:https://doi.org/10.1016/j.vaccine.2011.01.011.
- Didierlaurent AM, Morel S, Lockman L, Giannini SL, Bisteau M, Carlsen H, Kielland A, Vosters O, Vanderheyde N, Schiavetti F, et al.. AS04, an aluminum salt- and TLR4 agonist based adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. J Immunol. 2009;183:6186–97. doi:https://doi.org/10.4049/jimmunol.0901474.
- Klinman DM, Yi AK, Beaucage SL, Conover J, Krieg AM. CpG motifs present in bacteria DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon gamma. Proc Natl Acad Sci. U.S.A. 1996;93:2879–83.
- Klaschik S, Tross D, Klinman DM. Inductive and suppressive networks regulate TLR9- dependent gene expression in vivo. J Leukoc Biol. 2009;85:788–95. doi:https://doi.org/10.1189/jlb.1008671.
- Nanishi E, Dowling DJ, levy O. Towards precision adjuvants: optimizing science and safety. Curr Opin Pediatr. 2020;32(1):125–38. doi:https://doi.org/10.1097/MOP.0000000000000868.
- Guery JC, Ria F, Adorini L. Dendritic cells but not B cells present antigenic complexes to class II-restricted T cells after administration of protein in adjuvant. J Exp Med. 1996;183:751–57.
- Schijns VEJC, Lavelle EC. Trends in vaccine adjuvants. Expert Rev Vaccin. 2011;10:539–50. doi:https://doi.org/10.1586/erv.11.21.
- Ghimire TR, Benson RA, Garside P, Brewer JM. Alum increases antigen uptake, reduces antigen degradation and sustains antigen presentation by DCs in vitro. Immunol Lett. 2012;147:55–62. doi:https://doi.org/10.1016/j.imlet.2012.06.002.
- Sun HX, Xie Y, Ye YP. Advances in saponin-based adjuvants. Vaccine. 2009;27(12):1787–96. doi:https://doi.org/10.1016/j.vaccine.2009.01.091.
- Marty-Roix R, Vladimer GI, Pouliot K, Weng D, Buglione-Corbett R, West K, MacMicking JD, Chee JD, Wang S, Lu S, et al.. Identification of QS-21 as an inflammasome-activating molecular component of saponin adjuvants. J Biol Chem. 2016;291(3):1123–36. doi:https://doi.org/10.1074/jbc.M115.683011.
- Duewell P, Kisser U, Heckelsmiller K, Hoves S, Stoitzner P, Koernig S, Morelli AB, Clausen BE, Dauer M, Eigler A, et al.. ISCOMATRIX adjuvant combines immune activation with antigen delivery to dendritic cells in vivo leading to effective cross-priming of CD8+ T cells. J Immunol. 2011;187(1):55–63. doi:https://doi.org/10.4049/jimmunol.1004114.
- Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21(7):677. doi:https://doi.org/10.1038/nm.3893.
- Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–65. doi:https://doi.org/10.1146/annurev.immunol.021908.132715.
- Oleszycka E, Lavelle EC. Immunomodulatory properties of the vaccine adjuvant alum. Curr Opin Immunol. 2014;28C:1–5. doi:https://doi.org/10.1016/j.coi.2013.12.007.
- Didierlaurent AM, Morel S, Lockman L, Giannini SL, Bisteau M, Carlsen H, Kielland A, Vosters O, Vanderheyde N, Schiavetti F, et al.. AS04, an aluminum salt- and TLR4 agonist-based adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. J Immunol. 2009;183:6186–97. doi:https://doi.org/10.4049/jimmunol.0901474.
- Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847–56. doi:https://doi.org/10.1038/ni.1631.
- Li H, Willingham SB, Ting JP, Re F. Cutting edge: inflammasome activation by alum and alum’s adjuvant effect are mediated by NLRP3. J Immunol. 2008;181:17–21. doi:https://doi.org/10.4049/jimmunol.181.1.17.
- Marty-Roix R, Vladimer GI, Pouliot K, Weng D, Buglione-Corbett R, West K, MacMicking JD, Chee JD, Wang S, Lu S, et al.. Identification of QS-21 as an inflammasome-activating molecular component of saponin adjuvants. J Biol Chem. 2015;291:1123-1136.
- Kashala O, Amador R, Valero MV, Moreno A, Barbosa A, Nickel B, Daubenberger CA, Guzman F, Pluschke G, Patarroyo ME. Safety, tolerability and immunogenicity of new formulations of the Plasmodium falciparum malaria peptide vaccine SPf66 combined with the immunological adjuvant QS-21. Vaccin. 2002;20:2263–77. doi:https://doi.org/10.1016/S0264-410X(02)00115-9.
- Kim SK, Ragupathi G, Musselli C, Choi SJ, Park YS, Livingston PO. Comparison of the effect of different immunological adjuvants on the antibody and T-cell response to immunization with MUC1-KLH and GD3-KLH conjugate cancer vaccines. Vaccin. 1999;18:597–603. doi:https://doi.org/10.1016/S0264-410X(99)00316-3.
- Kennedy JS, Co M, Green S, Longtine K, Longtine J, O’Neill MA, Adams JP, Rothman AL, Yu Q, Johnson-Leva R, et al.. The safety and tolerability of an HIV-1 DNA prime-protein boost vaccine (DP6-001) in healthy adult volunteers. Vaccin. 2008;26:4420–24. doi:https://doi.org/10.1016/j.vaccine.2008.05.090.
- Evans TG, McElrath MJ, Matthews T, Montefiori D, Weinhold K, Wolff M, Keefer MC, Kallas EG, Corey L, Gorse GJ, et al.. QS-21 adjuvant activates NLRP3 inflammasome promotes an adjuvant effect allowing for reduced antigen dose during HIV-1 envelope subunit immunization in humans. Vaccin. 2001;19:2080–91. doi:https://doi.org/10.1016/S0264-410X(00)00415-1.
- Gin DY, Slovin SF. Enhancing immunogenicity of cancer vaccines: QS-21 as an immune adjuvant. Curr Drug Ther. 2011;6:207–12. doi:https://doi.org/10.2174/157488511796391988.
- Krug LM, Ragupathi G, Hood C, George C, Hong F, Shen R, Abrey L, Jennings HJ, Kris MG, Livingston PO. Immunization with N-propionyl polysialic acid-KLH conjugate in patients with small cell lung cancer is safe and induces IgM antibodies reactive with SCLC cells and bactericidal against group B meningococci. Cancer Immunol Immunother. 2011;61:9–18. doi:https://doi.org/10.1007/s00262-011-1083-6.
- Vandepapeliere P, Horsmans Y, Moris P, Van Mechelen M, Janssens M, Koutsoukos M, Van Belle P, Clement F, Hanon E, Wettendorff M, et al.. Vaccine adjuvant systems containing monophosphoryl lipid A and QS21 induce strong and persistent humoral and T cell responses against hepatitis B surface antigen in healthy adult volunteers. Vaccin. 2008;26:1375–86. doi:https://doi.org/10.1016/j.vaccine.2007.12.038.
- Partnership RCT. Efficacy and safety of RTS, S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet. 2015;386:31–45.
- Tin MM, Cho CH, Chan K, James AE, Ko JK. Astragalus saponins induce growth inhibition and apoptosis in human colon cancer cells and tumor xenograft. Carcinogenesis. 2007;28(6):1347–55. doi:https://doi.org/10.1093/carcin/bgl238.
- Von Eschen K, Morrison R, Braun M, Ofori-Anyinam O, De Kock E, Pavithran P, Koutsoukos M, Moris P, Cain D, Dubois M-C, et al.. The candidate tuberculosis vaccine Mtb72F/AS02A: tolerability and immunogenicity in humans. Hum Vaccin. 2009;5(7):475–82. doi:https://doi.org/10.4161/hv.8570.
- Vellas B, Black R, Thal L, Fox N, Daniels M, McLennan G, Tompkins C, Leibman C, Pomfret M, Grundman M, et al.. Long-term follow-up of patients immunized with AN1792: reduced functional decline in antibody responders. Curr Alzheimer Res. 2009;6(2):144–51. doi:https://doi.org/10.2174/156720509787602852.
- Jie YH, Cammisuli S, Baggiolini M. Immunomodulatory effects of Panax ginseng C. A. Meyer in the mouse. Agents Actions. 1984;15:386–91. doi:https://doi.org/10.1007/BF01972376.
- Kensil CR. Saponins as vaccine adjuvants. Crit Rev Ther Drug Carrier Syst. 1996;13:1–55.
- Marciani DJ. Vaccine adjuvants: role and mechanisms of action in vaccine immunogenicity. Drug Discov Today. 2003;8:934–43. doi:https://doi.org/10.1016/S1359-6446(03)02864-2.
- Sjolander A, Drane D, Maraskovsky E, Scheerlinck JP, Suhrbier A, Tennent J, Pearse M. Immune responses to ISCOM® formulations in animal and primate models. Vaccin. 2001;19(17–19):2661–65. doi:https://doi.org/10.1016/S0264-410X(00)00497-7.
- Bomford R, Stapleton M, Winsor S, Beesley JE, Jessup EA, Price KR, Fenwick GR. Adjuvanticity and ISCOM formation by structurally diverse saponins. Vaccin. 1992;10(9):572–77. doi:https://doi.org/10.1016/0264-410X(92)90435-M.
- Oda K, Matsuda H, Murakami T, Katayama S, Ohgitani T, Yoshikawa M. Adjuvant and haemolytic activities of 47 saponins derived from medicinal and food plants. Biolog Chem. 2000;381:67–74.
- Soltysik S, Wu JY, Recchia J, Wheeler DA, Newma MJ, Coughlin RT, Kensil CR. Structure/function studies of QS-21 adjuvant: assessment of triterpene aldehyde and glucuronic acid roles in adjuvant function. Vaccin. 1995;13(15):1403–10. doi:https://doi.org/10.1016/0264-410X(95)00077-E.
- Press JB, Reynolds RC, May RD, Marciani DJ. Structure/function relationships of immunostimulating saponins. Studies in Nat Prod Chem. 2000;24:131–74.
- Marciani DJ. New Th2 adjuvants for preventive and active immunotherapy of neurodegenerative proteinopathies. Drug Discov Today. 2014;19:912–20. doi:https://doi.org/10.1016/j.drudis.2014.02.015.
- Marciani DJ, Reynolds RC, Pathak AK, Finley-Woodman K, May RD. Fractionation, structural studies, and immunological characterization of the semi-synthetic Quillaja saponins derivative GPI-0100. Vaccin. 2003;21:3961–71. doi:https://doi.org/10.1016/S0264-410X(03)00298-6.
- Nico D, Santos FN, Borja-Cabrera GP, Palatnik M, de Sousa CP. Assessment of the monoterpene, glycidic and triterpene-moieties’ contributions to the adjuvant function of the CP05 saponin of Calliandra pulcherrima Benth during vaccination against experimental visceral leishmaniasis. Vaccin. 2007;25(4):649–58. doi:https://doi.org/10.1016/j.vaccine.2006.08.035.
- Oda K, Matsuda H, Murakami T, Katayama S, Ohgitani T, Yoshikawa M. Relationship between adjuvant activity and amphipathic structure of soyasaponins. Vaccin. 2003;21(17–18):2145–51. doi:https://doi.org/10.1016/S0264-410X(02)00739-9.
- Estrada A, Katselis GS, Laarveld B, Barl B. Isolation and evaluation of immunological adjuvant activities of saponins from Polygala senega L. Comp Immunol Microbiol Infect Dis. 2000;23(1):27–43. doi:https://doi.org/10.1016/S0147-9571(99)00020-X.
- Baumann E, Stoya G, Volkner A, Richter W, Lemke C, Linss W. Hemolysis of human erythrocytes with saponin affects the membrane structure. Acta Histochem. 2000;102(1):21–35. doi:https://doi.org/10.1078/0065-1281-00534.
- Armah CN, Mackie AR, Roy C, Price K, Osbourn AE, Bowyer P, Ladha S. The membrane-permeabilizing effect of avenacin A-1 involves the reorganization of bilayer cholesterol. Biophys J. 1999;76(1):281–90. doi:https://doi.org/10.1016/S0006-3495(99)77196-1.
- Gogelein H, Huby A. Interaction of saponin and digitonin with black lipid membranes and lipid monolayers. BBA-Biomembranes. 1984;773(1):32–38. doi:https://doi.org/10.1016/0005-2736(84)90547-9.
- Keukens EA, de Vrije T, van den Boom C, de Waard P, Plasman HH, Thiel F, Chupin V, Jongen WM, de Kruijff B. Molecular basis of glycoalkaloid induced membrane disruption. BBA-Biomembranes. 1995;1240(2):216–28. doi:https://doi.org/10.1016/0005-2736(95)00186-7.
- Nishikawa M, Nojima S, Akiyama T, Sankawa U, Inoue K. Interaction of digitonin and its analogs with membrane cholesterol. J Biochem. 1984;96(4):1231–39. doi:https://doi.org/10.1093/oxfordjournals.jbchem.a134941.
- Mazzucchelli GD, Cellier NA, Mshviladzade V, Elias R, Shim YH, Touboul D, Quinton L, Brunelle A, Laprevote O, De Pauw EA, et al.. Pores formation on cell membranes by hederacolchiside A1 leads to a rapid release of proteins for cytosolic subproteome analysis. J Proteom Res. 2008;7(4):1683–92. doi:https://doi.org/10.1021/pr7006973.
- Sirtori CR. Aescin: pharmacology, pharmacokinetics and therapeutic profile. Pharmacol Res. 2001;44:183–93. doi:https://doi.org/10.1006/phrs.2001.0847.
- Kwak WJ, Han CK, Chang HW, Kim HP, Kang SS, Son KH, Loniceroside C. An anti-inflammatory saponin from Lonicera japonica. Chem Pharmaceut Bull. 2003;51:333–35. doi:https://doi.org/10.1248/cpb.51.333.
- da Silva BP, De Sousa AC, Silva GM, Mendes TP, Parente JP. A new bioactive steroidal saponin from Agave attenuata. J Biosci Sec C. 2002;57:423–28.
- Kim DS, Oh SR, Lee IS, Jung KY, Park JD, Kim SI, Lee HK. Anti-complementary activity of Ginseng saponins and their degradation products. Phytochem. 1998;47:397–99. doi:https://doi.org/10.1016/S0031-9422(97)00580-3.
- Conforti F, Marrelli M, Carmela C, Menichini F, Valentina P, Uzunov D, Statti GA, Duez P, Menichini F. Bioactive phytonutrients (omega fatty acids, tocopherols, polyphenols), in vitro inhibition of nitric oxide production and free radical scavenging activity of non-cultivated Mediterranean vegetables. Food Chemi. 2011;129(4):1413–19. doi:https://doi.org/10.1016/j.foodchem.2011.05.085.
- Singh R, Sharma R, Mal G, Varshney R. A comparative analysis of saponin-enriched fraction from Silene vulgaris (Moench) Garcke, Sapindus mukorossi (Gaertn) and Chlorophytum borivilianum (Santapau and Fernandes): an in vitro hemolytic and cytotoxicity evaluation. Ani Biotech. 2020. doi:https://doi.org/10.1080/10495398.2020.1775627.
- Killeen GF, Madigan CA, Connolly CR, Walsh GA, Clark C, Hynes MJ, Timmins BF, James P, Headon DR, Power RF. Antimicrobial saponins of Yucca schidigera and the implications of their in vitro properties for their in vivo impact. J Agricul Food Chem. 1998;46:3178–86. doi:https://doi.org/10.1021/jf970928j.
- ElSohly HN, Danner S, Li XC, Nimrod AC, Clark AM. New anti-mycobacterial saponin from Colubrina retusa. J Nat Prod. 1999;62:1341–42. doi:https://doi.org/10.1021/np9901940.
- Jassim SA, Naji MA. Novel antiviral agents: a medicinal plant perspective. J Appl Microbiol. 2003;95:412–27. doi:https://doi.org/10.1046/j.1365-2672.2003.02026.x.
- Subba Rao G, Sinsheimer JE, Cochran KW. Antiviral activity of triterpenoid saponins containing acylated-amyrin aglycones. J Pharm Sci. 1974;63:471–73. doi:https://doi.org/10.1002/jps.2600630341.
- Sinsheimer JE, Rao GS, Mc Ilhenny HM, Smith RV, Maassab HF, Cochran KW. Isolation and antiviral activity of the gymnemic acids. Experientia. 1968;24(302–303):89. doi:https://doi.org/10.1007/BF02152834.
- Gosse B, Gnabre J, Bates RB, Dicus CW, Nakkiew P, Huang RCC. Antiviral saponins from Tieghemella heckelii. J Nat Prod. 2002;65:1942–44. doi:https://doi.org/10.1021/np020165g.
- Sindambiwe JB, Calomme M, Geerts S, Pieters L, Vlietinck AJ, VandenBerghe DA. Evaluation of biological activities of triterpenoid saponins from Maesa lanceolata. J Nat Prod. 1998;61:585–90. doi:https://doi.org/10.1021/np9705165.
- Kinjo J, Yokomizo K, Hirakawa T, Shii Y, Nohara T, Uyeda M. Anti-herpes virus activity of fabaceous triterpenoidal saponins. Biolog Pharmaceut Bull. 2000;23:887–89. doi:https://doi.org/10.1248/bpb.23.887.
- Simoes CMO, Amoros M, Girre L. Mechanism of antiviral activity of triterpenoid saponins. Phytother Res. 1999;13:323–28. doi:https://doi.org/10.1002/(SICI)1099-1573(199906)13:4<323::AID-PTR448>3.0.CO;2-C.
- Bushneva OA, Ovodova RG, Shashkov AS, Chizhov AO, Ovodov YS. Structure of silenan, a pectic polysaccharide from campion Silene vulgaris (Moench) Garcke. Biochem. (Moscow). 2003;68:1360–68.
- Ghonime M, Eldomany R, Abdelaziz A, Soliman H. Evaluation of immunomodulatory effect of three herbal plants growing in Egypt. Immunopharmacol Immunotoxicol. 2011;33(1):141–45. doi:https://doi.org/10.3109/08923973.2010.487490.
- Rivera E, Ekholm Pettersson F, Inganas M, Paulie S, Gronvik KO. The Rb1 fraction of ginseng elicits a balanced Th1 and Th2 immune response. Vaccin. 2005;23(46–47):5411–19. doi:https://doi.org/10.1016/j.vaccine.2005.04.007.
- Bachran C, Bachran S, Sutherland M, Bachran D, Fuchs H. Saponins in tumor therapy. Mini Rev Med Chem. 2008;8(6):575–84. doi:https://doi.org/10.2174/138955708784534445.
- Sung MK, Kendall CWC, Rao AV. Effect of soybean saponins and gypsophila saponin on morphology of colon carcinoma cells in culture. Food Chem Toxicol. 1995;33(5):357–66. doi:https://doi.org/10.1016/0278-6915(95)00007-O.
- Ragupathi G, Gardner JR, Livingston PO, Gin DY. Natural and synthetic saponin adjuvant QS-21 for vaccines against cancer. Expert Rev Vaccin. 2011;10(4):463–70. doi:https://doi.org/10.1586/erv.11.18.
- Dhama K, Khan S, Tiwari R, Sircar S, Bhat S, Malik YS, Singh KP, Chaicumpa W, Bonilla-Aldana DK, Rodriguez-Morales AJ. Coronavirus disease 2019–COVID-19. Clin Microbiol Rev. 2020b;33:e00028–20. doi:https://doi.org/10.1128/CMR.00028-20.
- Gorbalenya AE, Baker SC, Baric RS, deGroot RJ, Drosten C, Gulyaeva AA, Haagmans BL, Lauber C, Leontovich AM, Neuman BW, Penzar D, Perlman S, Poon LLM, Samborskiy DV, Sidorov IA, Sola I, Ziebuhr J. The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020. doi:https://doi.org/10.1038/s41564-020-0695-z.
- Kupferschmidt K, Cohen J. Will novel virus go pandemic or be contained? Sci. 2020;367:610–11.
- Malik YS, Kumar N, Sircar S, Kaushik R, Bhat S, Dhama K, Gupta P, Goyal K, Singh MP, Ghoshal U, et al.. Coronavirus disease pandemic (COVID-19): challenges and a global perspective. Pathog. 2020 Jun 28;9(7):E519. doi:https://doi.org/10.3390/pathogens9070519.
- Su S, Wong G, Shi W, Liu J, Lai ACK, Zhou J, Liu W, Bi Y, Gao GF. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trend Microbiol. 2016;24(6):490–502. doi:https://doi.org/10.1016/j.tim.2016.03.003.
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, et al.. A novel coronavirus from patients with pneumonia in China. N Engl J Med. 2020;382(8):727–33. doi:https://doi.org/10.1056/NEJMoa2001017.
- Wang N, Shang J, Jiang S, Du L. Subunit vaccines against emerging pathogenic human coronaviruses. Front Microbiol. 2020;11:298.
- Jiang S, Du L, Shi Z. An emerging coronavirus causing pneumonia outbreak in Wuhan, China: calling for developing therapeutic and prophylactic strategies. Emerg Microbes Infect. 2020;9:275–77. doi:https://doi.org/10.1080/22221751.2020.1723441.
- Guo S, Kenne L. Structural studies of triterpenoid saponins with new acyl components from Quillaja saponaria Molina. Phytochem. 2000;55(5):419–28. doi:https://doi.org/10.1016/S0031-9422(00)00340-X.
- Dalsgaard K. Saponin adjuvants. Archiv Virol. 1974;44:243–54.
- Didierlaurent AM, Berger A, Heineman TC, Henderickx V, Da Silva FT, Vekemans J, Voss G, Garçon N. The development of the adjuvant system AS01: a combination of two immunostimulants MPL and QS-21 in liposomes. In Immunopotentiators Mod Vaccin. (Second Edition) 2017; 265–85. Academic Press, Elsevier.
- Britt W, Fay J, Seals J, Kensil C. Formulation of immunogenic human cytomegalovirus vaccine: responses in mice. J Infect Dis. 1995;171:18–25. doi:https://doi.org/10.1093/infdis/171.1.18.
- Lovgren K. The serum antibody response distributed in subclasses and isotypes after intranasal and subcutaneous immunization with influenza virus immunostimulating complex. Stand J Lmmunol. 1988;27:241–45. doi:https://doi.org/10.1111/j.1365-3083.1988.tb02343.x.
- Coughlin RT, Fattom A, Chu C, White AC, Winston S. Adjuvant activity of QS-21 for experimental E. co/i018 polysaccharide vaccines. Vaccin. 1995;13:17–21. doi:https://doi.org/10.1016/0264-410X(95)80005-X.
- Xie Y, Sun HX, Platycodin LD. D is a potent adjuvant of specific cellular and humoral immune responses against recombinant hepatitis B antigen. Vaccin. 2009;27(5):757–64. doi:https://doi.org/10.1016/j.vaccine.2008.11.029.
- Xie Y, Sun HX, Platycodin LD. D improves the immunogenicity of Newcastle Disease virus‐based recombinant Avian Influenza vaccine in mice. Chem Biodiv. 2010;7(3):677–89. doi:https://doi.org/10.1002/cbdv.200900183.
- Yendo ACA, de Costa F, Cibulski SP, Teixeira TF, Colling LC, Mastrogiovanni M, Soule S, Roehe PM, Gosmann G, Ferreira FA, et al.. A rabies vaccine adjuvanted with saponins from leaves of the soap tree (Quillaja brasiliensis) induces specific immune responses and protects against lethal challenge. Vaccin. 2016;34(20):2305–11. doi:https://doi.org/10.1016/j.vaccine.2016.03.070.
- Cibulski SP, Silveira F, Mourglia-Ettlin G, Teixeira TF, Dos Santos HF, Yendo AC, de Costa F, Fett-Neto AG, Gosmann G, Roehe PM. Quillaja brasiliensis saponins induce robust humoral and cellular responses in a bovine viral diarrhea virus vaccine in mice. Comp Immun Microbiol. 2016;45:1–8. doi:https://doi.org/10.1016/j.cimid.2016.01.004.
- de Costa F, Yendo ACA, Cibulski SP, Fleck JD, Roehe PM, Spilki FR, Gosmann G, Fett-Neto AG. Alternative inactivated poliovirus vaccines adjuvanted with Quillaja brasiliensis or Quil-A saponins are equally effective in inducing specific immune responses. PLoS One. 2014;9:e105374.
- Silveira F, Cibulski SP, Varela AP, Marques JM, Chabalgoity A, De Costa F, Yendo ACA, Gosmann G, Roehe PM, Fernández C, et al.. Quillaja brasiliensis saponins are less toxic than Quil A and have similar properties when used as an adjuvant for a viral antigen preparation. Vaccin. 2011;29(49):9177–82. doi:https://doi.org/10.1016/j.vaccine.2011.09.137.
- Fleck JD, Kauffmann C, Spilki F, Lencina CL, Roehe PM, Gosmann G. Adjuvant activity of Quillaja brasiliensis saponins on the immune responses to bovine herpesvirus type 1 in mice. Vaccin. 2006;24(49–50):7129–34. doi:https://doi.org/10.1016/j.vaccine.2006.06.09.