ABSTRACT
Rotaviruses (RVs) are the leading cause of acute gastroenteritis in children, while histo-blood group antigens (HBGAs) are believed to be host attachment and susceptibility factors of RVs. A large case–control study nested in a population-based diarrhea surveillance targeting children <5 y of age was performed in rural Hebei province, north China. Saliva and serum samples were collected from all participants to determine HBGA phenotyping, FUT2 mutations, and RV IgG antibody titers. A logistic model was employed to assess the association between host HBGA secretor status and risk of RV infection. Among 235 RV cases and 680 non-diarrhea controls studied, 82.4% of participants were IgG positive by an average age of 77 months. Out of the 235 RV cases, 216 (91.9%) were secretors, whereas the secretor rate was 76.3% in the non-diarrhea controls, resulted in an adjusted OR of 3.0 (95%CI: 1.9–4.7, P < .0001) between the two groups. Our population-based case–control study indicated a strong association between host HBGA secretor status and risk of RV infection in Chinese children. The high prevalence of Lewis-positive secretor status strongly suggests that Chinese children may be genetically susceptible to current co-circulating RV strains, and thus, a universal childhood immunization program against RV disease should be successful in China.
Introduction
Rotavirus (RV) is the leading cause of severe acute gastroenteritis in infants worldwide.Citation1,Citation2 The known transmission kinetics derived from several birth cohort studies indicated that nearly 100% of children were infected once or several times at the age of 36 months.Citation3,Citation4 Approximately 60–70% of the RV infection manifested as clinical illnesses.Citation3 Worldwide, RV infection is associated with 110 million cases of diarrhea in children less than 5 y of age, of which ~25 million were outpatients and 2 million were hospitalized, resulting in hundreds of thousands death annually, mostly in developing countries.Citation1,Citation5,Citation6 In China, an annual incidence of ~5 cases per 100 children per year was measured from population-based surveillances, and eventually, 60% of children were attacked by RVs with clinical diseases by 36 months.Citation7,Citation8
Following the recognition of histo-blood group antigens (HBGA) as a host attachment factor or receptor for human norovirus infection, Citation9,Citation10 similar scenario has been found for RVs.Citation11–14 Later, RV-HBGAs interaction was further demonstrated by structural analyses of several VP8-glycan complexes.Citation15–21 However, the role of host HBGA phenotypes in the risk of RV infection remains to be validated by large cohort studies. In this study, an epidemiological association between the host HBGA polymorphism and risk of RV infection was searched through a large longitudinal cohort in north China.
Methods
Study cohort
The population- and health-care facility-based surveillance targeting children <5 y of age in Zhengding County, Hebei Province, China was conducted from October 1, 2011 through March 31, 2012, covering a typical peak season of viral diarrheal illness in children. The study design has been described elsewhere.Citation8,Citation22 Briefly, children less than age five with acute onset of diarrhea residing in the catchment area were eligible for enrollment with informed consent. Diarrhea was defined as three or more loose bowel movements during a 24-hour period. Recovery was defined as 3 consecutive days free of diarrhea. To quantify the severity of gastroenteritis, a modified Vesikari Clinical Severity Scoring System was applied.Citation23 RVs in the stool samples were screened for the presence of RVs using an enzyme immunoassay kit (Oxoid, Basingstoke, UK), and RV G and P types were determined by multiplex PCR, followed by sequencing using the standard protocol from WHO.Citation24 An RV diarrhea episode was defined as an episode of diarrhea with laboratory-confirmed RV infection.
To study the association between host HBGA types and risk of RV infection, a 1:3 matched case–control study nested in the aforementioned population-based surveillance was implemented. An RV diarrhea case was defined as a laboratory-confirmed RV diarrhea, and a control was defined as eligible children without diarrhea during the surveillance period, and met the following conditions: (i) living in the same township as the case; (ii) being same gender as the case; and (iii) being born within 90 d from the birthday of the matched case. Most children might start to be infected around month 6, and experienced at least one episode of infection by month 36.Citation3 Thus, theoretically, an eligible control might develop RV infection or even RV diarrhea before or after the 6-month surveillance. To eliminate the potential misclassification bias, all RV diarrhea cases and matched non-diarrhea controls were followed and required for a saliva and serum samples in May 2016, which was 4 y after the completion of surveillance, to ensure every participated child was over 36 months of age. An RV infection was defined as RV-specific IgG positive 4 y after completion of surveillance, regardless of the status of RV diarrhea during surveillance.
Detection and genotyping of rotaviruses
All bulk stools were stored at −20°C until batch testing at the end of the surveillance period. All stool samples were tested for RV antigens with a commercial enzyme-linked immunosorbent assay (DAKO Diagnostics Ltd, United Kingdom) according to the manufacturer’s instructions. For the antigen-positive samples, nucleic acid was extracted from stool supernatant by an automated bead-beating step using TianLong Stool DNA/RNA Extraction Kit (TianLong Science & Technology, China). G- and P-types were determined using a hemi-nested multiplex RT-PCR assay according to the WHO’s protocol, Citation24 the details were described previously.Citation8
HBGA phenotyping
The HBGA phenotyping of the saliva samples was measured by EIA assays using monoclonal antibodies against corresponding HBGAs, according to established protocol.Citation25 Used MAbs (Biolegend, San Diego, CA) included anti-A (BG-2, SIG-3311), anti-B (BG-3, SIG-3312), anti-H type 1 (BG-4, SIG-3313), anti-Lea (BG-5, SIG-3314), anti-Leb (BG-6, SIG-3315), anti-Lex (BG-7, SIG-3339), and anti-Ley (BG-8, SIG-3317). Saliva samples were boiled at 100°C for 10 min and diluted 1:1000 in PBS. Microtiter plates (CORNING, New York, USA) were coated with 100 µl pre-treated saliva specimens overnight at 4°C in a duplicated well manner. After blocking with 5% dried nonfat milk (BD, New York, USA), 100 µl monoclonal antibodies (MAbs) against A, B, H1, Lex, Ley, Lea, and Leb antigens were added to each well, respectively, at a dilution of 1:100 and incubated for 1 hr at 37°C. Then, 100 µl horseradish peroxidase (HRP) conjugated goat anti-mouse IgG or IgM antibodies, respectively, were added and incubated for 1 hr at 37°C. One hundred microlitres of the enzyme substrate (TMB) were then added and the mixture was kept at 37°C for 10 min at room temperature in dark. The reaction was stopped with 100 µl of 0.1 M phosphoric acid, and the optical density (OD) was measured at 450 nm. Saliva samples with known HBGA phenotypes (from Cincinnati Children’s Hospital Medical Center), as well as PBS, were applied as positive-, negative-controls, and blank, respectively. After deducting the OD value of blank, an average OD value of ≥0.2 was defined as positive.
Saliva samples without A and B antigen were considered as O type; samples with A and B antigens simultaneously were AB types but they were counted into A type and B type to emphasize the role of A or B antigen, and samples with H1 antigen were defined as H1 type regardless of A/B antigen to emphasize the role of H1 antigen. Secretor status was defined as Lea−b+, Lex−y+, or H+; weak secretor was defined as Lea+b+ or Lex+y+; and non-secretor was defined as Lea+b− and/or Lex+y−, Lea−b−/Lex−y−, and H antigens negative.Citation26,Citation27
FUT2 genotyping
To verify the HBGA phenotypes derived from EIA assay, 15% of saliva samples that had ODs near the cutoff value were further tested for the three most common mutations (A358T, G428A, and C571T) in the FUT2 gene encoding α 1,2-fucosyltransferase (FUT2), which is a key enzyme to catalyze the formation of H antigens.Citation28,Citation29 Genomic DNA was extracted from saliva samples using the FineGene purification of DNA from Buccal cell kit (FineGene, Shanghai, DS02), according to the manufacturer’s instructions. As described previously, Citation30 specific primer-directed PCR (SSP-PCR) was performed to amplify the corresponding gene fragments, using antisense primer paired with wild-type and mutated sense primers (). The gene encoding human growth hormone (hGH) was served as an internal PCR control.
Table 1. Primer sequences of SSP-PCR and the expected product sizes
Measuring rotavirus G type-specific IgG antibodies
RV-specific IgG in serum samples was determined using a modified enzyme-linked immunosorbent assay (ELISA) described elsewhere.Citation31 Microtiter plates were coated with 100 µl inactivated G1-G4 RV particles (6.5 lg CCID50/ml, provided by Lanzhou Institute of Biological Products) pre-mixed equally at 37°C for 2 hr and then at 4°C overnight. After blocked with 200 µl 20% fetal bovine serum (FBS, Royacel technology, Lanzhou) at 37°C for 2 hr and then 4°C overnight, 1:20 diluted (specimen diluent, GILSON, Middleton, USA) serum samples were added to the microtiter plate and incubated at 37°C for 1 hr. One hundred microlitres of horseradish peroxidase (HRP) conjugated goat anti–human IgG (1:10000 diluted, Lanzhou Institute of Biological Products) were added. After incubation, 50 µl of the enzyme substrate A (TMB-A) and enzyme substrate B (TMB-B) were added successively and kept for 10 min at room temperature. The reaction was stopped with 50 µl of 0.1 M phosphoric acid. OD values were determined at 450 nm. A positive RV IgG was defined as a signal of sample to noise ratio (OD sample/OD control) ≥2.1.
Data management and analysis
All case report forms (CRFs) were double entered into a custom-made data entry program (the EpiData program, version 3.1). The data management program included checks for error and consistency. The SAS program (SAS Institute Inc., Cary, NC, USA) was employed for statistical analysis. For binary data, the Chi-square test was used, or Fisher’s exact test was used when data were sparse. Due to the OD values were used to analyze the association between RV-IgG titer and secretor status, Kruskal–Wallis Test was applied. To assess the potential associations between HBGAs and risk of RV infection, odds ratios (ORs) and their 95% confidence intervals (95% CIs) were calculated. Based on previous experience, Citation22 the age at which eligible children enter the study, and RV vaccination status would be the potential confounding biasing the association between secretor status and RV infection, an unconditional logistic model was employed to pursue adjusted ORs. All P values and 95% CIs were interpreted in a two-tailed fashion. Statistical significance was designated as a P value less than 0.05.
Based on the published HBGA phenotype data, an OR of 2, and a proportion of secretor status of 80% in general population was assumed, a total of 252 cases and 756 controls were required to satisfy a 1:3 matched case–control design, with a statistical power of 90%.Citation32 Considering that lost to follow-up might break the matching, leading to a reduction of design efficiency, the data were analyzed ultimately in a grouped case–control fashion, and thus, a total of 236 cases and 708 controls were required to ensure a statistical power of 90%.Citation33
Ethics
This study was reviewed and approved by the Institutional Review Board of Hebei Center for Disease Control and Prevention and Institutes of Biomedical Sciences, Fudan University. Written informed consent was obtained from the parent/guardian of each child. The study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Results
Summary of the study population and incidence of RV gastroenteritis
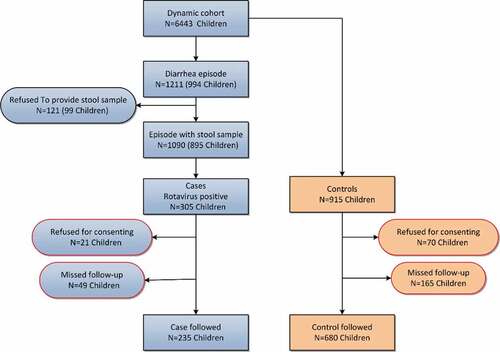
A total of 6,443 children less than 5 y of age were registered, of which, 63 did not consent to participate. During the 6-month surveillance period, 1,211 diarrhea episodes were reported, and 1090 individuals provided a stool sample for laboratory tests. Of these, 305 children were confirmed as RV diarrhea, resulted in an incidence rate of 54.7/1000 children/year. Four years after completion of surveillance, out of 305 RV cases and 915 non-diarrhea controls, 235 cases and 680 controls (average age: 77.6 months, SD: 8.5) were followed, and saliva and serum samples were obtained, with a drop-out rate of 23% and 26%, respectively (). The predominant G type was G3 (63.4%), followed by G1 (17.4%), and G9 (8.9%), while the most detected P type was P[8] (85.1%). Only two P[6] and one P[4] cases were found.
Distribution of HBGA phenotypes in Chinese children
Among 915 children who provided saliva specimens, A, B, and O blood types were responsible for 36.3%, 38.3%, and 34.6%, respectively, AB type (9.2%) was less common, but H1 antigens were detected from 439 samples (48.0%). As for Lewis HBGA classification, the highest occurrences were Leb (89.9%) and Ley (85.9%), followed by Lea (75.2%), Lex (34.6%). Based on the pre-set definitions, 80.3% (735) and 16.5% (151) of the recruited children were secretors and weak secretors, respectively, only 3.2%Citation29 were non-secretors.
To further validate the EIA results, 126 (13.8%) saliva samples with A/B/H1/Leb/Ley antigen signals near the cutoff (0.2), as well as 29 samples that were defined as non-secretors were measured for the three most common mutations (A385T, G428A, and C571T) of FUT2 gene, which found in 125 (80.6%), 34 (21.9%), and 2 (1.3%) children, respectively, including heterozygous and homozygous mutations. At the A385 position, almost all tested children were wild type (94.1%) or with heterozygous mutation (A385T, 83.7%), leading to secretor status, while homozygous mutation at this location led to weak secretor (43.1%) or non-secretor (33.3%), rather than secretor (23.6%) ().
Table 2. Consistence between FUT2 A385T mutation and secretor status
Associations between HBGA phenotype and RV infection
Looking into individual ABO and Lewis types, significantly a high proportion of H1 (59.1%), B (46.4%), Leb (94.5%), and Ley (94.5%) types were found in children with RV diarrhea during the surveillance (P <.05). Moreover, 216 (91.9%) out of 235 children with RV diarrhea were found to be secretors, only 17 (7.2%) and 2 (0.9%, who were infected by G3P[8] and G9P[8] RV strain) were weak secretors or non-secretors, respectively. Conversely, 76.3% (519) children without diarrhea were secretors, while the weak and non-secretors made up 19.7% (134) and 4.0%Citation27 respectively (Fisher’s exact test, P < .0001), resulted in an OR of 3.5 (95% CI: 2.1–5.8; P < .0001) for those children in secretor status (). It was noted that 164 children, including 44 in RV case group and 120 in non-diarrhea control group, received an LLR vaccine before 12 months of age. When these children were removed from the analysis, similar profiles (for secretor, OR = 3.3, 95% CI: 1.9–5.7, P < .0001; for weak secretor, OR = 0.3, 95%CI: 0.2–0.6, P < .0001, and for non-secretor, OR = 0.3, 95%CI: 0.1–1.2, P = .08) to secretor status were obtained. After adjusting age and gender using a logistic model, a significant association was detected between secretor status and RV diarrhea (OR = 3.0, 95%CI: 1.9–4.7, P < .0001), conversely, significant association between secretor status and vaccination status was not observed (OR = 0.9, 95%CI: 0.6–1.3, P = .55).
Table 3. Association between HBGA phenotype and RV infection
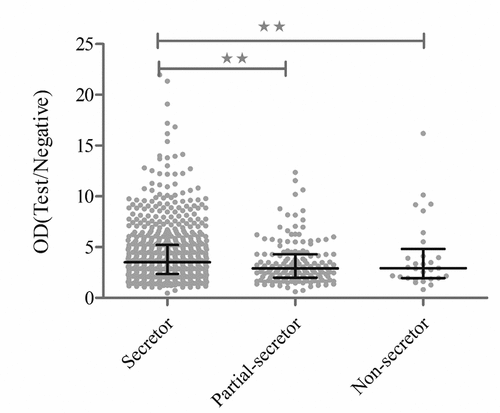
Four years after the completion of the surveillance, RV-specific IgG antibody was measured in 754 (82.4%) out of 915 children. Nearly all children (212/235, 90.2%) who were confirmed as RV diarrhea during the surveillance were RV IgG positive. By contrast, more RV IgG negative samples (138/680, 20.3%) were noted in non-diarrhea control group (P < .0001). Out of 754 RV-infected children, 618 (82.0%) were categorized as secretors, while 114 (15.1%) and 22 (2.9%) were weak secretors or non-secretors, respectively. Notably, less secretors (117/161, 72.7%) were found in non-infected children (P = .02), resulted in an OR of 1.7 (95% CI: 1.2–2.5; P < .01) for those children with secretor status (). Again, after adjusting age and gender, risk of RV infection was found to associate with secretor status (OR = 1.5, 95%CI: 1.1–2.0, P < .05), rather than with vaccination (OR = 1.2, 95%CI: 0.7–1.9, P = .44). Moreover, a significant association between RV IgG antibody titers in OD values and secretor status was observed (P < .05) (). In addition, when 915 children were further grouped into both RV IgG/RV diarrhea positive, and both RV IgG/RV diarrhea negative, a remarkable increase of risk to RV attack (OR = 4.0, 95%CI: 2.2–6.9, P < .0001) was found in children with secretor status ().
The proposed association between severity of RV diarrhea and secretor status was not observed, the distribution of secretors (90.8% vs 93.9%), weak secretors (8.5% vs 4.9%), and non-secretors (0.7% vs 1.2%) in mild and moderate/severe RV diarrhea patients was almost equal (P = .54). After adjusting age and gender, a similar risk to the severity of RV diarrhea was found in children with secretor status (OR = 1.3, 95%CI: 0.5–3.3) and vaccination (OR = 1.3, 95%CI: 0.6–2.8) both did not reach statistical significant levels.
Discussion
Literature indicates that HBGAs are host attachment factors or glycan receptors for noroviruses.Citation34–36 Similar scenario has been proposed to RV, which affects RV infection and host susceptibility.Citation11,Citation15,Citation17,Citation37,Citation38 In this large population-based case–control study, a moderate association (OR = 1.7, 95%CI: 1.2–2.5) between HBGA secretor in Chinese children and RV infection based on serological definition was determined. To our knowledge, this is the first study on the role of HBGAs in RV host susceptibility through an integration of serologic and clinical evidence of RV infection, as well as FUT2 gene mutations. Our data demonstrated that secretor status is also the crucial genetic risk factor to RV infection for Chinese children. Interestingly, the strength of the association increased with the increasing stringency of the definition, from serological definitions, clinical virology definitions, to serological and clinical virology definitions. Compared to the association derived from serological definition, a threefold higher risk was observed in children with the demanding definition – both RV IgG and RV antigen positive. The most likely explanation might be that some RV strains are not related to the secretor status.Citation39–43 Some studies would support this conclusion. Generally, the intestinal tract of a neonate may synthesize only the HBGA precursor glycans at birth, but then starts to generate matured fucosylated antigens at a later stage, including ABH and Lewis antigens, along with changes in diet and bacterial colonization.Citation44–46 On the other hand, VP8* of an RV strain like a P[11] virus could bind to type I and type II precursor glycans, rather than matured HBGAs whose biosynthesis depends on a α1,2 fucosyltransferase encoded by the FUT2 gene.Citation11,Citation47 In addition, findings from epidemiological studies suggest that RV infections in infants were distinct from infections in a toddler.Citation3,Citation48 Most infections in the first half-year of life were asymptomatic, and the symptomatic infection occurred more frequently in the second and third half-year of life. By the age of 36 months, almost 100% of children had been infected with RVs.Citation7,Citation8
Literatures showed that almost all human RV strains could bind to either the H type 1, Lewis b, or A antigens whose biosynthesis depends on a α1,2 fucosyltransferase encoded by the FUT2 gene.Citation17 Mutations of FUT2 lead to either a complete lack or a severely decreased α1,2 fucosyltransferase activity and therefore appear as non-secretor or weak secretor.Citation38,Citation49 Several common mutations are known in the FUT2 gene, and some are highly ethnic specificity. The G428A nonsense mutation is typically found in the Caucasian population, Citation50 and the nonsense C571T mutation is found mainly in Pacific Islanders.Citation51 In addition, missense mutation at position A385T, which correlates commonly with weak secretor, even non-secretor phenotype, Citation52 is detected frequently in Asians.Citation50,Citation53,Citation54 In this study, G428A and C571T mutations were less commonly detected from the samples in comparison with A385T mutation. Furthermore, the profile of A385T mutation was perfectly consistent with the HBGA secretor status. Almost all wild type (94.1%) corresponded to secretor, while missense mutation was mainly responsible for weak secretor (43.1%) and non-secretor (33.3%). This finding is considered as mechanistic support for the association between host HBGA phenotypes and risk of RV infection.
The discovery that RVs recognize HBGAs as potential host receptors via their VP8*s has significantly highlighted the role of the VP8* protein in RV infection. Hence, distributions of HBGAs and secretor statuses affect RV host ranges in different populations. These new findings help our understanding on different performances of the current RV vaccines (Rotarix and RotaTeq) in different populations. In vitro glycan-binding studies indicated that P[8] RVs prefer to bind to H-type I and Leb antigens, while P[6] appear to interact with H-type I antigen and type I precursor. Because Lewis-negative secretor is relatively common in African children, they may be less susceptible to the Rotarix and RotaTeq vaccine strains, due to possible resistance to infection and replication of the vaccine RV (P[8]) strains, and thus eventually reduced vaccine efficacy in these populations.Citation11,Citation38 Surprisingly, vast majority of children (80.3%) who were secretors were genetically susceptible to RV infection in this study, by contrast, the non-susceptible non-secretors account only for 3.2%, while the Lewis-negative secretor was even more rare (0.5%). The low prevalence of non-secretor and Lewis-negative individuals in Chinese children may explain the satisfactory performance of Rotarix and RotaTeq vaccines tested in Chinese children.Citation55,Citation56 This scenario may also serve as a great encouragement for the development of non-replicating vaccines in China, including inactivated and VP8* subunit vaccines that both are P[8] type-based.Citation57,Citation58
This study has several potential limitations. Firstly, the G1 to G4 RV antigens were used to coat ELISA plates, whereas G9 RV antigen was not included. This might reduce the prevalence of RV infection in the study population, and eventually resulted in an overestimate of association between HBGAs and RV infection. However, out of 235 RV cases who were able to be followed, G9 genotype accounted for 7.7% (18/235). Moreover, of the 18 G9 RV cases, IgG was detected from 16 cases. Secondly, the participants in this study were derived from a 6-month surveillance. Though serum samples were collected and serological status of infection was determined at the 4th year after the completion of surveillance, the clinical status before and after the surveillance was absent. This absence might ultimately lead to an underestimate for the association, since when the participants used for analysis were limited to the demanding definition – both RV IgG and RV antigen positive, a higher OR value was calculated. Finally, since by the end of 6-month surveillance study, some of the participants have not reached 3 y. In order to ensure that children have enough exposure time, a serological test was performed 4 y after the surveillance study. Even though, a notable percentage (20%) of non-diarrhea children with negative anti-RV antibodies was observed. The most likely explanation could be either the waning of antibody or lack of RV receptors, and the former was the inherent weakness of study design. It would lead to a weakened association. In fact, a stronger association was found when the RV diarrhea, rather than RV infection, was applied for grouping variables. The abovementioned issues could be clarified by future studies using birth cohort design.
In summary, a significant association between HBGA secretor status and risk of RV infection was determined in Chinese children. The observed prevalence of Lewis-positive secretor in this population strongly suggested that vast majority of Chinese children are genetically susceptible to current circulating RV strains. Due to the lack of a specific antiviral treatment of RV disease, a general intervention approach to block transmission is indicated. It is strongly recommended to incorporate RV vaccines into national immunization programs and launch universal immunization in China as soon as possible.
Authors’ contributions
Song-Mei Wang and Xuan-Yi Wang contributed to the study’s conception and design. Yan-Hong Zhang, Zhi-Yong Hao, Xin-Jiang Zhang, Jing-Chen Ma, and Yu-Liang Zhao conducted the fieldwork. Jin-Xia Wang, Can-Jing Zhang, Hong-Lu Zhou, Chao Qiu, and Song-Mei Wang carried out the laboratory testing. Jin-Xia Wang and Xuan-Yi Wang analyzed the data. Xuan-Yi Wang wrote the paper. Ming Tan and Xi Jiang provided important contributions in laboratory testing and the writing of the paper. All authors read and approved the final manuscript.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Competing interests
The authors declare that they have no competing interests.
Acknowledgments
We would like to thank the people of Zhengding County who participated in the study and the dedicated staff of Zhengding Center for Disease Control and Prevention who made this study possible. In particularly, we are grateful to Dr. Xu Zhou (Shanghai Institute of Biological Products, Co., Ltd.), and Drs. Hong Bao, Chao Ma, Yun-Jin Wang (Lanzhou Institute of Biological Products, Co., Ltd.) for providing RV IgG assay, and helping on the performance.
Additional information
Funding
References
- Troeger C, Khalil IA, Rao PC, Cao S, Blacker BF, Ahmed T, Armah G, Bines JE, Brewer TG, Colombara DV, et al. Rotavirus vaccination and the global burden of rotavirus diarrhea among children younger than 5 years. JAMA Pediatr. 2018;172(10):958–65.
- Khalil IA-M. The global burden of rotavirus diarrheal diseases: results from the global burden of diseases study 2016. Open Forum Infect Dis. 2017;4(Suppl 1):S363–S. doi:10.1093/ofid/ofx163.885.
- Lewnard JA, Lopman BA, Parashar UD, Bar-Zeev N, Samuel P, Guerrero ML, Ruiz-Palacios GM, Kang G, Pitzer VE. Naturally acquired immunity against rotavirus infection and gastroenteritis in children: paired reanalyses of birth cohort studies. J Infect Dis. 2017;216(3):317–26. doi:10.1093/infdis/jix310.
- Velázquez FR, Matson DO, Calva JJ, Guerrero L, Morrow AL, Carter-Campbell S, Glass RI, Estes MK, Pickering LK, Ruiz-Palacios GM. Rotavirus infection in infants as protection against subsequent infections. N Engl J Med. 1996;335(14):1022–28. doi:10.1056/NEJM199610033351404.
- Parashar UD, Burton A, Lanata C, Boschi-Pinto C, Shibuya K, Steele D, Birmingham M, Glass R. Global mortality associated with rotavirus disease among children in 2004. J Infect Dis. 2009;200(Suppl 1):S9–s15. doi:10.1086/605025.
- Tate JE, Burton AH, Boschi-Pinto C, Parashar UD. Global, regional, and national estimates of rotavirus mortality in children <5 years of age, 2000-2013. Clin Infect Dis. 2016;62:S96–s105.
- Wang XY, Riewpaiboon A, von Seidlein L, Chen XB, Kilgore PE, Ma JC, Qi S-X, Zhang Z-Y, Hao Z-Y, Chen J-C. Potential cost-effectiveness of a rotavirus immunization program in rural China. Clinical Infect Dis Off Publ Infect Dis Soc Ama. 2009;49(8):1202–10. doi:10.1086/605632.
- Wang JX, Zhou HL, Mo ZJ, Wang SM, Hao ZY, Li Y, Zhen -S-S, Zhang C-J, Zhang X-J, Ma J-C, et al. Burden of viral gastroenteritis in children living in rural China: population-based surveillance. Int J Infect Dis. 2020;90:151–60. doi:10.1016/j.ijid.2019.10.029.
- Tan M, Jiang X. Norovirus-host interaction: multi-selections by human histo-blood group antigens. Trends Microbiol. 2011;19(8):382–88. doi:10.1016/j.tim.2011.05.007.
- Nordgren J, Svensson L. Genetic susceptibility to human norovirus infection: an update. Viruses. 2019;11(3):3. doi:10.3390/v11030226.
- Jiang X, Liu Y, Tan M. Histo-blood group antigens as receptors for rotavirus, new understanding on rotavirus epidemiology and vaccine strategy. Emerging Microbes Infect. 2017;6(1):e22. doi:10.1038/emi.2017.30.
- Sharma S, Hagbom M, Svensson L, Nordgren J. The impact of human genetic polymorphisms on rotavirus susceptibility, epidemiology, and vaccine take. Viruses. 2020;12(3):3. doi:10.3390/v12030324.
- Bucardo F, Nordgren J, Reyes Y, Gonzalez F, Sharma S, Svensson L. The Lewis A phenotype is a restriction factor for Rotateq and Rotarix vaccine-take in Nicaraguan children. Sci Rep. 2018;8(1):1502. doi:10.1038/s41598-018-19718-y.
- Barbé L, Le Moullac-Vaidye B, Echasserieau K, Bernardeau K, Carton T, Bovin N, Nordgren J, Svensson L, Ruvoën-Clouet N, Le Pendu J, et al. Histo-blood group antigen-binding specificities of human rotaviruses are associated with gastroenteritis but not with in vitro infection. Sci Rep. 2018;8(1):12961. doi:10.1038/s41598-018-31005-4.
- Gozalbo-Rovira R, Ciges-Tomas JR, Vila-Vicent S, Buesa J, Santiso-Bellon C, Monedero V, Yebra MJ, Marina A, Rodríguez-Díaz J. Unraveling the role of the secretor antigen in human rotavirus attachment to histo-blood group antigens. PLoS Pathog. 2019;15(6):e1007865. doi:10.1371/journal.ppat.1007865.
- Sun X, Li D, Qi J, Chai W, Wang L, Wang L, Peng R, Wang H, Zhang Q, Pang L, et al. Glycan binding specificity and mechanism of human and porcine P[6]/P[19] rotavirus VP8*s. J Virol. 2018;92(14):14. doi:10.1128/JVI.00538-18.
- Hu L, Sankaran B, Laucirica DR, Patil K, Salmen W, Ferreon ACM, Tsoi PS, Lasanajak Y, Smith DF, Ramani S, et al. Glycan recognition in globally dominant human rotaviruses. Nat Commun. 2018;9(1):2631.
- Sun X, Li D, Peng R, Guo N, Jin M, Zhou Y, Xie G, Pang L, Zhang Q, Qi J, et al. Functional and structural characterization of P[19] rotavirus VP8* interaction with histo-blood group antigens. J Virol. 2016;90(21):9758–65. doi:10.1128/JVI.01566-16.
- Hu L, Crawford SE, Czako R, Cortes-Penfield NW, Smith DF, Le Pendu J, Estes MK, Prasad BVV. Cell attachment protein VP8* of a human rotavirus specifically interacts with A-type histo-blood group antigen. Nature. 2012;485(7397):256–59. doi:10.1038/nature10996.
- Hu L, Ramani S, Czako R, Sankaran B, Yu Y, Smith DF, Cummings RD, Estes MK, Venkataram Prasad BV. Structural basis of glycan specificity in neonate-specific bovine-human reassortant rotavirus. Nat Commun. 2015;6(1):8346. doi:10.1038/ncomms9346.
- Sun X, Dang L, Li D, Qi J, Wang M, Chai W, Zhang Q, Wang H, Bai R, Tan M. Structural basis of glycan recognition in globally predominant human P[8] rotavirus. Virol Sin. 2020;35(2):156–70. doi:10.1007/s12250-019-00164-7.
- Zhen SS, Li Y, Wang SM, Zhang XJ, Hao ZY, Chen Y, Wang D, Zhang Y-H, Zhang Z-Y, Ma J-C, et al. Effectiveness of the live attenuated rotavirus vaccine produced by a domestic manufacturer in China studied using a population-based case-control design. Emerging Microbes Infect. 2015;4(10):e64. doi:10.1038/emi.2015.64.
- VESIKARI TRaT. Rotavirus disease in finnish children use of numerical scores for clinical severity of diarrhoea1 episodes. Scand J Infect Dis. 1990;22(3):259–67. doi:10.3109/00365549009027046.
- Department of Immunization Vaccines and Biologicals World Health Organization. Manual of rotavirus detection and characterization methods. Switzerland: the WHO document production services; 2009. http://www.who.int/immunization/documents/WHO_IVB_08.17/en/.
- Huang P, Farkas T, Marionneau S, Zhong W, Ruvoën-Clouet N, Morrow AL, Altaye M, Pickering L, Newburg D, LePendu J, et al. Noroviruses bind to human ABO, Lewis, and secretor histo-blood group antigens: identification of 4 distinct strain-specific patterns. J Infect Dis. 2003;188(1):19–31. doi:10.1086/375742.
- Sun X, Guo N, Li J, Yan X, He Z, Li D, Jin M, Xie G, Pang L, Zhang Q, et al. Rotavirus infection and histo-blood group antigens in the children hospitalized with diarrhoea in China. Clin Microbiol Infect Off Publ Euro Soc Clin Microbiol Infect Dis. 2016;22(8):740.e1-3. doi:10.1016/j.cmi.2016.06.007.
- Tan M, Jin M, Xie H, Duan Z, Jiang X, Fang Z. Outbreak studies of a GII-3 and a GII-4 norovirus revealed an association between HBGA phenotypes and viral infection. J Med Virol. 2008;80:1296–301.
- Ferrer-Admetlla A, Sikora M, Laayouni H, Esteve A, Roubinet F, Blancher A, Calafell F, Bertranpetit J, Casals F. A natural history of FUT2 polymorphism in humans. Mol Biol Evol. 2009;26(9):1993–2003. doi:10.1093/molbev/msp108.
- Soejima M, Pang H, Koda Y. Genetic variation of FUT2 in a Ghanaian population: identification of four novel mutations and inference of balancing selection. Ann Hematol. 2007;86(3):199–204. doi:10.1007/s00277-006-0203-4.
- Zhang D, Tan M, Zhong W, Xia M, Huang P, Jiang X. Human intestinal organoids express histo-blood group antigens, bind norovirus VLPs, and support limited norovirus replication. Sci Rep. 2017;7(1):12621. doi:10.1038/s41598-017-12736-2.
- Moon SS, Groome MJ, Velasquez DE, Parashar UD, Jones S, Koen A, van Niekerk N, Jiang B, Madhi SA. Prevaccination rotavirus serum IgG and IgA are associated with lower immunogenicity of live, oral human rotavirus vaccine in South African infants. Clinical Infect Dis Off Publ Infect Dis Soc Ama. 2016;62(2):157–65. doi:10.1093/cid/civ828.
- Dupont WD. Power calculations for matched case-control studies. Biometrics. 1988;44(4):1157–68. doi:10.2307/2531743.
- Fleiss JL, Levin B, Paik MC, Fleiss J. Statistical methods for rates and proportions. 3rd ed. New York: John Wiley & Sons; 2003.
- Tan M, Jiang X. Norovirus and its histo-blood group antigen receptors: an answer to a historical puzzle. Trends Microbiol. 2005;13(6):285–93. doi:10.1016/j.tim.2005.04.004.
- Fiege B, Leuthold M, Parra F, Dalton KP, Meloncelli PJ, Lowary TL, Peters T. Epitope mapping of histo blood group antigens bound to norovirus VLPs using STD NMR experiments reveals fine details of molecular recognition. Glycoconj J. 2017;34(5):679–89. doi:10.1007/s10719-017-9792-5.
- Atmar RL, Ramani S, Estes MK. Human noroviruses: recent advances in a 50-year history. Curr Opin Infect Dis. 2018;31:422–32.
- Imbert-Marcille BM, Barbe L, Dupe M, Le Moullac-Vaidye B, Besse B, Peltier C, Ruvoën-Clouet N, Le Pendu J. A FUT2 gene common polymorphism determines resistance to rotavirus A of the P[8] genotype. J Infect Dis. 2014;209(8):1227–30.
- Ramani S, Hu L, Venkataram Prasad BV, Estes MK. Diversity in rotavirus-host glycan interactions: a “sweet” spectrum. Cell Mol Gastroenterol Hepatol. 2016;2(3):263–73. doi:10.1016/j.jcmgh.2016.03.002.
- Rodriguez-Diaz J, Garcia-Mantrana I, Vila-Vicent S, Gozalbo-Rovira R, Buesa J, Monedero V, Collado MC. Relevance of secretor status genotype and microbiota composition in susceptibility to rotavirus and norovirus infections in humans. Sci Rep. 2017;7(1):45559. doi:10.1038/srep45559.
- Harris VC, Armah G, Fuentes S, Korpela KE, Parashar U, Victor JC, Tate J, de Weerth C, Giaquinto C, Wiersinga WJ, et al. Significant correlation between the infant gut microbiome and rotavirus vaccine response in rural Ghana. J Infect Dis. 2017;215(1):34–41.
- Arrieta MC, Stiemsma LT, Amenyogbe N, Brown EM, Finlay B. The intestinal microbiome in early life: health and disease. Front Immunol. 2014;5:427.
- Chan J, Nirwati H, Triasih R, Bogdanovic-Sakran N, Soenarto Y, Hakimi M. Maternal antibodies to rotavirus: could they interfere with live rotavirus vaccines in developing countries? Vaccine. 2011;29(6):1242–47. doi:10.1016/j.vaccine.2010.11.087.
- Chilengi R, Simuyandi M, Beach L, Mwila K, Becker-Dreps S, Emperador DM, Velasquez DE, Bosomprah S, Jiang B. Association of maternal immunity with rotavirus vaccine immunogenicity in Zambian infants. PloS One. 2016;11(3):e0150100. doi:10.1371/journal.pone.0150100.
- Nanthakumar NN, Dai D, Newburg DS, Walker WA. The role of indigenous microflora in the development of murine intestinal fucosyl- and sialyltransferases. Faseb J. 2003;17(1):44–46. doi:10.1096/fj.02-0031fje.
- Nanthakumar NN, Meng D, Newburg DS. Glucocorticoids and microbiota regulate ontogeny of intestinal fucosyltransferase 2 requisite for gut homeostasis. Glycobiology. 2013;23:1131–41.
- Etzold S, Bode L. Glycan-dependent viral infection in infants and the role of human milk oligosaccharides. Curr Opin Virol. 2014;7:101–07. doi:10.1016/j.coviro.2014.06.005.
- Ramani S, Cortes-Penfield NW, Hu L, Crawford SE, Czako R, Smith DF, Kang G, Ramig RF, Le Pendu J, Prasad BVV, et al. The VP8* domain of neonatal rotavirus strain G10P[11] binds to type II precursor glycans. J Virol. 2013;87(13):7255–64. doi:10.1128/JVI.03518-12.
- Haffejee IE. Neonatal rotavirus infections. Rev Infect Dis. 1991;13(5):957–62. doi:10.1093/clinids/13.5.957.
- Van Trang N, Vu HT, Le NT, Huang P, Jiang X, Anh DD. Association between norovirus and rotavirus infection and histo-blood group antigen types in Vietnamese children. J Clin Microbiol. 2014;52(5):1366–74. doi:10.1128/JCM.02927-13.
- Yoshiro Koda MS, Kimura H. The polymorphisms of fucosyltransferases. Leg Med. 2001;3:2–14. doi:10.1016/S1344-6223(01)00005-0.
- Pang H, Fujitani N, Soejima M, Koda Y, Islam MN, Islam AK, Kimura H. Two distinct Alu-mediated deletions of the human ABO-secretor (FUT2) locus in Samoan and Bangladeshi populations. Hum Mutat. 2000;16(3):274. doi:10.1002/1098-1004(200009)16:3<274::AID-HUMU20>3.0.CO;2-I.
- Henry S, Mollicone R, Fernandez P, Samuelsson B, Oriol R, Larson G. Homozygous expression of a missense mutation at nucleotide 385 in the FUT2 gene associates with the Le(a+b+) partial-secretor phenotype in an Indonesian family. Biochem Biophys Res Commun. 1996;219(3):675–78. doi:10.1006/bbrc.1996.0293.
- Pang H, Koda Y, Soejima M, Fujitani N, Ogaki T, Saito A, KAWASAKI T, KIMURA H. Polymorphism of the human ABO-Secretor locus (FUT2) in four populations in Asia: indication of distinct Asian subpopulations. Ann Hum Genet. 2001;65(5):429–37. doi:10.1046/j.1469-1809.2001.6550429.x.
- Koda Y, Soejima M, Liu Y, Kimura H. Molecular basis for secretor type alpha(1,2)-fucosyltransferase gene deficiency in a Japanese population: a fusion gene generated by unequal crossover responsible for the enzyme deficiency. Am J Hum Genet. 1996;59:343–50.
- Li RC, Huang T, Li Y, Luo D, Tao J, Fu B, Si G, Nong Y, Mo Z-J, Liao X-Y. Human rotavirus vaccine (RIX4414) efficacy in the first two years of life: a randomized, placebo-controlled trial in China. Hum Vaccin Immunother. 2014;10(1):11–18. doi:10.4161/hv.26319.
- Mo Z, Mo Y, Li M, Tao J, Yang X, Kong J, Wei D, Fu B, Liao X, Chu J, et al. Efficacy and safety of a pentavalent live human-bovine reassortant rotavirus vaccine (RV5) in healthy Chinese infants: A randomized, double-blind, placebo-controlled trial. Vaccine. 2017;35(43):5897–904.
- Xue M, Yu L, Che Y, Lin H, Zeng Y, Fang M, Li T, Ge S, Xia N. Characterization and protective efficacy in an animal model of a novel truncated rotavirus VP8 subunit parenteral vaccine candidate. Vaccine. 2015;33(22):2606–13. doi:10.1016/j.vaccine.2015.03.068.
- Wu JY, Zhou Y, Zhang GM, Mu GF, Yi S, Yin N, Xie Y-P, Lin X-C, Li H-J, Sun M-S. Isolation and characterization of a new candidate human inactivated rotavirus vaccine strain from hospitalized children in Yunnan, China: 2010-2013. World J Clin Cases. 2018;6(11):426–40. doi:10.12998/wjcc.v6.i11.426.