ABSTRACT
Healthcare provider recommendation is a key predictor of HPV vaccination among adolescents, yet little is known about how parents’ receipt of a provider recommendation differs across Hispanic/Latinx subgroups in the United States. We analyzed data from the 2012–2016 National Immunization Survey – Teen on Hispanic/Latinx adolescent ages 13–17 (n = 16,335). Analyses used weighted logistic regression models. Overall, 62.6% of parents of Hispanic/Latinx females and 46.4% of parents of Hispanic/Latinx males reported that they had received a provider recommendation for HPV vaccination. Among parents of females, receipt of a provider recommendation ranged from 55.0% among Central Americans to 73.3% among parents of Puerto Ricans. Among parents of males, the range was from 44.5% among Mexicans and multi-subgroup males to 53.4% among Cubans. There were no differences across Hispanic/Latinx subgroups in adjusted models among either males or females (all p > .05). Among parents of females, provider recommendation was less common among those whose preferred language was Spanish for Central Americans and South Americans (both p < .05). Efforts are needed to improve provider communication about and recommendations for HPV vaccination among the Hispanic/Latinx population and to ensure the availability of language assistance services for individuals with limited English proficiency.
Almost half of adolescents in the United States (US) are not up-to-date with the human papillomavirus (HPV) vaccine series, and over 30% have not even initiated the series.Citation1 Past research has consistently shown that one of the strongest predictors of HPV vaccination among adolescents is whether their parents have received a recommendation from a healthcare provider to vaccinate.Citation2,Citation3 In fact, recent national data show that nearly 75% of adolescents whose parents had received a provider recommendation had initiated the HPV vaccine series, compared to only 47% of adolescents whose parents had not received a recommendation.Citation1
Hispanic/Latinx individuals experience several HPV-related disparities.Citation4–7 and can therefore benefit greatly from HPV vaccination, yet many Hispanic/Latinx parents are not receiving a provider recommendation to vaccinate their child.Citation8,Citation9 This could be attributable to several reasons including a lack of effective communication about HPV vaccine from providers, which may be especially true for parents with limited English proficiency.Citation10 A limitation of past research in this area is that Hispanic/Latinx individuals have been examined as a single, homogeneous group. The Hispanic/Latinx population in the US is quite heterogeneous and includes persons of Mexican, Cuban, Puerto Rican, Central American, South American, and other Spanish descent.Citation11 There are important differences between these subgroups, including characteristics that may affect provider recommendation for HPV vaccination (i.e., English proficiency).Citation11–14 However, little is currently known about how receipt of a provider recommendation for HPV vaccination differs across Hispanic/Latinx subgroups. We conducted a secondary data analysis to assess HPV vaccination outcomes across Hispanic/Latinx subgroups in the US. A secondary aim of this project was to examine receipt of a provider recommendation for HPV vaccination across these subgroups.
We describe the methods for this project in detail elsewhereCitation15 and briefly here. We analyzed data from the National Immunization Survey – Teen (NIS-Teen), an annual survey that monitors adolescent vaccination among 13–17 year-olds in the US.Citation16 The NIS-Teen collects data via a random-digit-dialed telephone survey with parents of adolescent ages 13–17 and a mailed survey to adolescents’ healthcare providers. Our analyses included NIS-Teen data from 2012 to 2016 (i.e., the five most recent data years available at the start of our study) on 16,335 Hispanic/Latinx adolescents. All of these adolescents had information available on Hispanic/Latinx subgroup from the parent survey and had healthcare providers who completed the mailed survey. The NIS-Teen obtained informed consent from all participants.
The Institutional Review Board at the Ohio State University determined our secondary data analysis was exempt from review. The NIS-Teen is sponsored and conducted by the National Center for Immunization and Respiratory Diseases (NCIRD) of the Centers for Disease Control and Prevention (CDC) and authorized by the Public Health Service Act [Sections 306]. Data collection for NIS-Teen survey was approved by the National Center for Health Statistics (NCHS) Research Ethics Review Board (ERB). Analysis of de-identified data from the survey is exempt from the federal regulations for the protection of human research participants. Analysis of restricted data (as described below) through the NCHS Research Data Center is also approved by the NCHS ERB.
The dependent variable was parents’ receipt of a recommendation from a healthcare provider to vaccinate their child against HPV. The NIS-Teen assessed receipt of a provider recommendation (yes or no) by asking parents, “Has a doctor or other healthcare professional ever recommended that [TEEN] receive HPV shots?” The main independent variable was Hispanic/Latinx subgroup. The parent survey collected information on Hispanic/Latinx subgroup among parents who indicated their adolescent was Hispanic/Latinx. Based on this information, adolescents were categorized as Mexican, Cuban, Puerto Rican, Central American, South American, other Spanish descent, or multi-subgroup (i.e., parents indicated at least two of the other subgroups). Data on Hispanic/Latinx subgroups were not available in the public use NIS-Teen datasets, so we accessed these restricted data through the NCHS Research Data Center. We also examined preferred language in a potential interaction with Hispanic/Latinx subgroup, as described further below. Preferred language was based on whether parents completed the NIS-Teen telephone survey in English or Spanish. Additional covariates included demographic and health-related characteristics collected by the NIS-Teen. Sample characteristics are shown in .
Table 1. Characteristics of Hispanic/Latinx adolescents and their parents (n = 16,335)
We stratified analyses by sex since routine HPV vaccination was first recommended for adolescent females in 2006, and this type of recommendation was not issued for adolescent males until 2011.Citation17,Citation18 For each sex, we used logistic regression to compare Hispanic/Latinx subgroups on parents’ receipt of a provider recommendation for HPV vaccination. We constructed an unadjusted model that included only Hispanic/Latinx subgroup and then an adjusted model that controlled for year of data collection and the demographic and health-related characteristics in . These models produced odds ratios (ORs) and 95% confidence intervals (CIs).
To determine the effect of preferred language across Hispanic/Latinx subgroups, we examined if an interaction existed between these two variables. In examining this interaction, we constructed an adjusted logistic regression model that controlled for year of data collection and demographic and health-related characteristics. We used Holm’s stepdown approach to adjust p-values from interaction models to account for multiple comparisons.Citation19 All analyses applied sampling weights and accounted for the complex design of the NIS-Teen,Citation20 though frequencies are not weighted. Statistical tests were two-tailed with a critical alpha of 0.05. We performed analyses using SAS Version 9.4 (SAS Inc., Cary, NC).
Overall, 62.6% of parents of Hispanic/Latinx females reported that they had received a provider recommendation for HPV vaccination. Across subgroups, receipt of a provider recommendation ranged from 55.0% among parents of Central American females up to 73.3% among parents of Puerto Rican females (). In the unadjusted model, receipt of a provider recommendation was more common among parents of Puerto Rican females compared to parents of Mexican females (OR = 1.73, 95% CI: 1.26–2.38). However, there were no differences across subgroups in the adjusted model for females (all p > .05).
Table 2. Parents’ receipt of provider recommendation to vaccinate their child against HPV across Hispanic/Latinx subgroups
There was an interaction between Hispanic/Latinx subgroup and preferred language among females (interaction p < .001). In this model, receipt of a provider recommendation was less common among parents who completed the NIS-Teen telephone survey in Spanish compared to those who completed the survey in English for the following subgroups (, panel A): Central American (39.7% vs. 73.1%; OR = 0.31, 95% CI: 0.14–0.69; p = .03) and South American (42.4% vs. 79.7%; OR = 0.16, 95% CI: 0.07–0.38; p < .001). A qualitatively similar pattern was found for the other subgroups among females, though these differences did not reach statistical significance (all p > .05).
Figure 1. Receipt of provider recommendation for HPV vaccination by parents’ preferred language across Hispanic/Latinx subgroups for adolescent females (panel A) and adolescent males (panel B). Bars indicate the standard errors. ‘*’ indicates subgroups with comparisons with p < .05 after adjustment via Holm’s stepdown approach
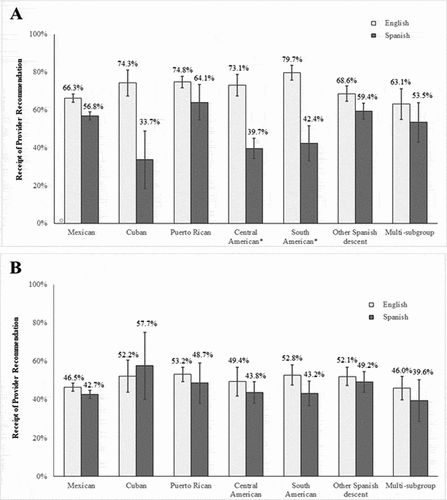
Just under half (46.4%) of parents of Hispanic/Latinx males reported that they had received a provider recommendation for HPV vaccination. Receipt of a provider recommendation ranged from 44.5% among parents of Mexican and multi-subgroup males up to 53.4% among parents of Cuban males (). In the unadjusted model, receipt of a provider recommendation was more common among parents of Puerto Rican males compared to parents of Mexican males (OR = 1.38, 95% CI: 1.03–1.87). Similar to females, there were no differences across subgroups in the adjusted model for males (all p > .05). There was also no interaction between Hispanic/Latinx subgroup and preferred language among males (interaction p = .99). Although the percentage of parents who had received a provider recommendation was lower among those who completed the NIS-Teen telephone survey in Spanish compared to those who completed the survey in English for all but one subgroup (, panel B), these differences did not reach statistical significance (all p > .05).
Many parents of Hispanic/Latinx adolescents in our study had not received a healthcare provider recommendation to vaccinate their child against HPV. Receipt of a provider recommendation was more common among parents of female adolescents than parents of male adolescents. This is not surprising since routine HPV vaccination was first recommended for females in 2006 but not for males until 2011.Citation17,Citation18 Receipt of a provider recommendation did not differ greatly across Hispanic/Latinx subgroups among females or males. The lack of differences across subgroups is actually encouraging and may be one of the reasons why HPV vaccine coverage is similar across Hispanic/Latinx subgroups in the US.Citation15
Our findings do highlight that, within some of the Hispanic/Latinx subgroups, receipt of a provider recommendation for HPV vaccination was less common among parents whose preferred language was Spanish compared to those whose preferred language was English. This is consistent with past research on provider communication about and recommendation for HPV vaccination,Citation21,Citation22 but it is interesting that statistically significant differences were found only among parents of females who were Central American or South American. In terms of why differences were found only among parents of females, it may be due in part to HPV vaccine protecting against a sexually transmitted infection (STI). Healthcare providers are less likely to provide high-quality HPV vaccine recommendations if they believe that having to talk about an STI makes conversations about HPV vaccine uncomfortable,Citation23 and it is possible that this effect is accentuated in situations with parents who have adolescent daughters and limited English proficiency.
In terms of the Hispanic/Latinx subgroups where differences by preferred language were found, Central Americans and South Americans tend to have lower English proficiency in general compared to other subgroups (e.g., Puerto Ricans and Mexicans).Citation14 It is therefore possible that parents in these subgroups whose preferred language was Spanish had especially low English proficiency, which in turn may make provider recommendations for HPV vaccination more challenging. Lack of health insurance is also more common among Central Americans and South Americans compared to other subgroupsCitation14 and among Hispanic/Latinx individuals with limited English proficiency in general.Citation24 This may lead to lower utilization of preventive care services and opportunities to receive a provider recommendation for HPV vaccination among parents in these subgroups with limited English proficiency.
We think there are a few key strategies for increasing provider recommendation for HPV vaccination among the Hispanic/Latinx population, including individuals with limited English proficiency. First, ongoing efforts are needed to improve healthcare providers’ communication about and recommendations for HPV vaccination. For example, training providers to use presumptive announcements in their communications with parents (i.e., statements that presume parents are ready to vaccinate) is more effective at improving HPV vaccine uptake than training providers to use participatory conversations.Citation25 In these communications with parents about HPV vaccine, it is also important that providers emphasize messages about cancer prevention.Citation26 Second, it is critical to ensure the availability of language assistance services for individuals with limited English proficiency. The Hispanic/Latinx population is the largest racial/ethnic minority population in the US,Citation27 and nearly 30% of this population reports speaking English less than “very well.”Citation28 The ability to align Hispanic/Latinx individuals with linguistically and culturally concordant medical care improves patient satisfaction and understanding.Citation29,Citation30 Federal law requires health programs and clinicians receiving federal financial assistance to take reasonable steps to provide meaningful access to free language assistance services for individuals with limited English proficiency,Citation31,Citation32 yet such services are still not universally available.Citation33 Lastly, and related, we think it is also important that physicians are enabled to become more fluent in Spanish. About a third of medical schools in the US do not offer a medical Spanish curriculum, and among those that do offer this type curriculum, few use a validated approach to measure language proficiency after curriculum completion.Citation34
Study strengths include a large sample of Hispanic adolescents from throughout the US and the ability to examine Hispanic/Latinx subgroup and preferred language. Study limitations include modest household response rates for the NIS-Teen, though the weights generated for NIS-Teen data are adjusted for nonresponse. In assessing provider recommendation for HPV vaccination, data were only available from the parents’ perspectives, with no data available from the providers’ perspectives. We were not able to examine the other Spanish descent subgroup and multi-subgroup at a more granular level due to the large number of response categories included in each of these subgroups and the small sample size of each individual category. We also could not include additional factors (e.g., religiosity) in our statistical models due to lack of data on these factors in the NIS-Teen datasets.
In summary, healthcare provider recommendation is strongly associated with HPV vaccination among adolescents, yet many parents of Hispanic/Latinx adolescents in the US have not yet received a recommendation. Receipt of a provider recommendation did not differ greatly across Hispanic/Latinx subgroups, but recommendations were less common among parents whose preferred language was Spanish within multiple subgroups. Efforts are needed to improve provider communication about and recommendations for HPV vaccination among the Hispanic/Latinx population and to ensure the availability of language assistance services for individuals with limited English proficiency. The findings and conclusions in this paper are those of the authors and do not necessarily represent the views of the Research Data Center, the National Center for Health Statistics, or the Centers for Disease Control and Prevention.
Disclosure of potential conflicts of interest
None of the authors have disclosures to report.
Additional information
Funding
References
- Walker TY, Elam-Evans LD, Yankey D, Markowitz LE, Williams CL, Fredua B, Singleton JA, Stokley S. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years - United States, 2018. MMWR Morb Mortal Wkly Rep. 2019;68(33):718–23. doi:10.15585/mmwr.mm6833a2.
- Rodriguez SA, Mullen PD, Lopez DM, Savas LS, Fernandez ME. Factors associated with adolescent HPV vaccination in the U.S.: A systematic review of reviews and multilevel framework to inform intervention development. Prev Med. 2020;131:105968. doi:10.1016/j.ypmed.2019.105968.
- Ylitalo KR, Lee H, Mehta NK. Health care provider recommendation, human papillomavirus vaccination, and race/ethnicity in the US national immunization survey. Am J Public Health. 2013;103(1):164–69. doi:10.2105/AJPH.2011.300600.
- Shi R, Devarakonda S, Liu L, Taylor H, Mills G. Factors associated with genital human papillomavirus infection among adult females in the United States, NHANES 2007-2010. BMC Res Notes. 2014;7(1):544–544. doi:10.1186/1756-0500-7-544.
- Hariri S, Unger ER, Sternberg M, Dunne EF, Swan D, Patel S, Markowitz LE. Prevalence of genital human papillomavirus among females in the United States, the national health and nutrition examination survey, 2003-2006. J Infect Dis. 2011;204(4):566–73. doi:10.1093/infdis/jir341.
- Han JJ, Beltran TH, Song JW, Klaric J, Choi YS. Prevalence of genital human papillomavirus infection and human papillomavirus vaccination rates among US adult men: national health and nutrition examination survey (NHANES) 2013-2014. JAMA Oncol. 2017;3:810–16.
- American Cancer Society. Cancer facts & figures for Hispanics/Latinos 2018-2020. Atlanta (GA): American Cancer Society; 2018.
- Reiter PL, Gupta K, Brewer NT, Gilkey MB, Katz ML, Paskett ED, Smith JS. Provider-verified HPV vaccine coverage among a national sample of Hispanic adolescent females. Cancer Epidemiol Biomarkers Prev. 2014;23(5):742–54. doi:10.1158/1055-9965.EPI-13-0979.
- Reiter PL, Brewer NT, Gilkey MB, Katz ML, Paskett ED, Smith JS. Early adoption of the human papillomavirus vaccine among Hispanic adolescent males in the United States. Cancer. 2014;120:3200–32017.
- Albright K, Barnard J, O’Leary ST, Lockhart S, Jimenez-Zambrano A, Stokley S, Dempsey A, Kempe A. Noninitiation and noncompletion of HPV vaccine among english- and spanish-speaking parents of adolescent girls: A qualitative study. Acad Pediatr. 2017;17(7):778–84. doi:10.1016/j.acap.2017.03.013.
- Siegel RL, Fedewa SA, Miller KD, Goding-Sauer A, Pinheiro PS, Martinez-Tyson D, Jemal A. Cancer statistics for Hispanics/ Latinos, 2015. CA Cancer J Clin. 2015;65:457–80.
- Cokkinides VE, Bandi P, Siegel RL, Jemal A. Cancer-related risk factors and preventive measures in US Hispanics/Latinos. CA Cancer J Clin. 2012;62(6):353–63. doi:10.3322/caac.21155.
- Dominguez K, Penman-Aguilar A, Chang MH, Moonesinghe R, Castellanos T, Rodriguez-Lainz A, Schieber R. Centers for Disease control and prevention, (CDC). Vital signs: leading causes of death, prevalence of diseases and risk factors, and use of health services among Hispanics in the United States - 2009-2013. MMWR Morb Mortal Wkly Rep. 2015;64:469–78.
- Motel S, Patten E. The 10 largest Hispanic origin groups: characteristics, rankings, top counties. 2012. [Accessed 2020 Aug 26]. http://www.pewhispanic.org/2012/06/27/the-10-largest-hispanic-origin-groups-characteristics-rankings-top-counties/.
- Reiter PL, Pennell ML, Martinez GA, Perkins RB, Katz ML. HPV vaccine coverage across Hispanic/Latinx subgroups in the United States. Cancer Causes Control. Cancer Causes and Control. 2020;31(10):905–14.
- Jain N, Singleton JA, Montgomery M, Skalland B. Determining accurate vaccination coverage rates for adolescents: the National Immunization Survey-Teen 2006. Public Health Rep. 2009;124(5):642–51. doi:10.1177/003335490912400506.
- Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER. Centers for Disease control and prevention, (CDC), Advisory committee on immunization practices, (ACIP). Quadrivalent human papillomavirus vaccine: recommendations of the Advisory committee on immunization practices (ACIP). MMWR Recomm Rep. 2007;56:1–24.
- Centers for Disease Control and Prevention, (CDC). Recommendations on the use of quadrivalent human papillomavirus vaccine in males–Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep 2011;60:1705–08.
- Holm S. A simple sequentially rejective multiple test procedure. Scandinavian J Statis. 1979;6:65–70.
- Centers for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases. National immunization survey-teen. A user’s guide for the 2016 public-use data file. 2017. [Accessed 2020 Aug 24]. https://www.cdc.gov/vaccines/imz-managers/nis/downloads/NIS-TEEN-PUF16-DUG.pdf.
- Moss JL, Gilkey MB, Rimer BK, Brewer NT. Disparities in collaborative patient-provider communication about human papillomavirus (HPV) vaccination. Hum Vaccin Immunother. 2016;12(6):1476–83. doi:10.1080/21645515.2015.1128601.
- Gerend MA, Zapata C, Reyes E. Predictors of human papillomavirus vaccination among daughters of low-income Latina mothers: the role of acculturation. J Adolesc Health. 2013;53(5):623–29. doi:10.1016/j.jadohealth.2013.06.006.
- Gilkey MB, Malo TL, Shah PD, Hall ME, Brewer NT. Quality of physician communication about human papillomavirus vaccine: findings from a national survey. Cancer Epidemiol Biomarkers Prev. 2015;24(11):1673–79. doi:10.1158/1055-9965.EPI-15-0326.
- DuBard CA, Gizlice Z. Language spoken and differences in health status, access to care, and receipt of preventive services among US Hispanics. Am J Public Health. 2008;98(11):2021–28. doi:10.2105/AJPH.2007.119008.
- Brewer NT, Hall ME, Malo TL, Gilkey MB, Quinn B, Lathren C. Announcements versus conversations to improve HPV vaccination coverage: A randomized trial. Pediatrics. 2017;139(1):e20161764. doi:10.1542/peds.2016-1764.
- Gilkey MB, Zhou M, McRee AL, Kornides ML, Bridges JFP. Parents’ views on the best and worst reasons for guideline-consistent HPV vaccination. Cancer Epidemiol Biomarkers Prev. 2018;27(7):762–67. doi:10.1158/1055-9965.EPI-17-1067.
- Colby SL, Ortman JM. Projections of the size and composition of the U.S. population: 2014 to 2060. In: Current population reports. Washington (DC): U.S. Census Bureau; 2014. p. 25.
- United States Census Bureau. American community survey (ACS). 2020. [accessed 2020 Aug 17]. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf.
- Cowden JD, Thompson DA, Artman M. Getting past getting by: training culturally and linguistically competent bilingual physicians. J Pediatr. 2012;160(6):891–892.e1. doi:10.1016/j.jpeds.2012.02.032.
- Dunlap JL, Jaramillo JD, Koppolu R, Wright R, Mendoza F, Bruzoni M. The effects of language concordant care on patient satisfaction and clinical understanding for Hispanic pediatric surgery patients. J Pediatr Surg. 2015;50(9):1586–89. doi:10.1016/j.jpedsurg.2014.12.020.
- US Department of Justice. Executive order 13166: improving access to services for persons with limited english proficiency. 2000. [accessed 2020 Aug 20]. https://www.justice.gov/sites/default/files/crt/legacy/2010/12/14/eolep.pdf.
- US Department of Health and Human Services. Nondiscrimination in health programs and activities. 2016. [accessed 2020 Aug 20]. https://www.govinfo.gov/content/pkg/FR-2016-05-18/pdf/2016-11458.pdf.
- Schiaffino MK, Nara A, Mao L. Language services in hospitals vary by ownership and location. Health Aff. 2016;35(8):1399–403. doi:10.1377/hlthaff.2015.0955.
- Morales R, Rodriguez L, Singh A, Stratta E, Mendoza L, Valerio MA, Vela M. National survey of medical Spanish curriculum in U.S. medical schools. J Gen Intern Med. 2015;30(10):1434–39. doi:10.1007/s11606-015-3309-3.
