ABSTRACT
In order to analyze the effect of EV71 vaccination on the incidence of encephalitis in patients with HFMD, 292 cases were vaccinated, and 2,486 cases were not vaccinated which were collected in 2018 and 2019. It shows that the incidence rate of encephalitis in vaccinated patients was significantly lower than that in non-vaccinated (P = .028), which suggests that EV71 vaccine has a protective effect on the occurrence of encephalitis. But some EV71 vaccinated patients still developed into encephalitis showed that they had not produced protection or protection was weak against EV71-related encephalitis; the reasons require further investigation.
Introduction
Hand, Foot and Mouth Disease (HFMD) is a common childhood illness that is mainly caused by Coxsackievirus A16 (CVA16) and Enterovirus 71 (EV71). HFMD primarily affects infants and children aged <5 years.Citation1 EV71 is a non-enveloped, positive, single-stranded RNA virus, which belongs to the Picornaviridae family, Citation2 and those under 3 years are more susceptible to severe or fatal neurological diseases caused by EV71.Citation3–5 Three inactivated monovalent EV-A71 vaccines were licensed in 2016 in China, but these must be paid for by vaccine recipients themselves, and are not covered by the national immunization program. A corresponding technical guideline on vaccine use was issued in the same year.Citation6 The EV71 vaccine has been inoculated for more than three years in China; vaccines showed high efficacy in the prevention of EV-A71-associated HFMD in randomized controlled trials.Citation7–9The inactivated enterovirus 71 vaccine used was developed by the Institute of Medical Biology, Chinese academy of Medical Sciences, and Sinovac Biotech.
However, there are relatively few reports of the effect of EV71 vaccination on the incidence of encephalitis of hospitalized patients with HFMD. We collected patients with HFMD in 2018 and 2019; 169 and 123 patients received EV71 vaccine among them in 2018 and 2019, respectively, to observe and analyze the clinical protective effect of EV71 vaccine and changes in the incidence of encephalitis.
Methods
Materials and methods
Usually, HFMD is self-limiting, but some patients may experience severe systemic and neurological complications that may be fatal. In total, 1,434 and 1,344 cases with HFMD were collected in 2018 and 2019, respectively, in Infectious Diseases Department, Shandong University Qilu Children’s Hospital, China. Among them, 169 and 123 cases were vaccinated with EV71 vaccine in 2018 and 2019, respectively. In a total of 292 vaccinated cases, 281 cases were given full vaccination, and 18 given partial vaccination. Fully vaccinated children have received two doses of EV71 vaccine before hospitalization. Likewise, patients who had received one single dose before hospitalization were classified as partly vaccinated. The inactivated enterovirus 71 vaccine was developed by Sinovac Biotech or the Institute of Medical Biology, Chinese academy of Medical Sciences. HFMD was diagnosed through clinical features of oral ulcers and skin lesion on the hands and feet. Reverse transcription-polymerase chain reaction (RT-PCR) was used to detect EV71 or/and CVA16 nucleic acid in throat swabs, stool specimens, cerebrospinal fluid (CSF), or other tissue fluids that were collected from each patient on the day of admission. Encephalitis was diagnosed by brain parenchyma lesions, which were identified by magnetic resonance imaging or computed tomography, as well as pleocytosis in CSF (CSF examination is essential in patients with HFMD who have suspected central nervous system involvement to identify the presence not only of the virus but also pleocytosis in the CSF, white blood cell count >5/mm3). Blood EV71IgM and CVA16IgM were detected. This work was a retrospective study and approved by the Institutional Ethics Board of Shandong University Qilu Children’s Hospital.
Non-normal distribution data were expressed by median (IQR), analyzed by rank-sum test: Mann-Whitney test; Categorical (nominal/ordinal) variables were described using frequencies and percentages, using the chi-square test. All analyses were performed using Statistical Package for Social Science (IBM SPSS software, USA) version 16.0, and significance was set at a P-value of <0.05.
Results
Baseline demographics and characteristics
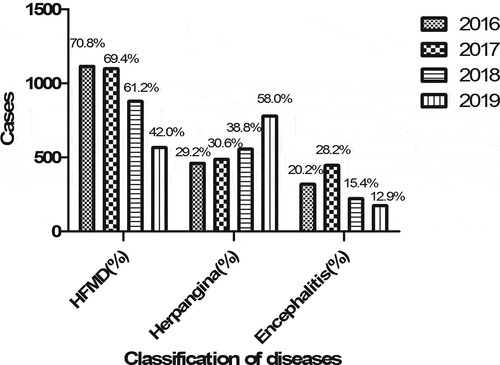
The EV71 vaccination rate was very low from 2016 to 2017 in Shandong area, China. Since 2018, the EV71 vaccination rate began to increase, and started to be recorded in Infectious Diseases Department, Shandong University Qilu Children’s Hospital. A total of 1573 cases were involved in 2016, including 1114 cases of HFMD (70.8%), and 459 cases of herpangina (29.2%), while 318 cases (20.2%) were complicated with encephalitis among them. In total, 1583 cases involved in 2017, 1098 cases of HFMD (69.4%), 485 cases of herpangina (30.6%), while 446 cases (28.2%) complicated with encephalitis among them. In total, 1434 cases involved in 2018, 878 cases of HFMD (61.2%), 556 cases of herpangina (38.8%), while 221 cases (15.4%) were complicated with encephalitis among them. In total, 1344 cases involved in 2019, 565 cases of HFMD (42.0%), 779 cases of herpangina (58.0%), while 173 cases (12.9%) were complicated with encephalitis among them ().
Among 1,434 patients in 2018, 169 (11.8%) cases were vaccinated (including 11 cases with partial-EV71 vaccination), 1,265 (88.2%) cases unvaccinated, and 221 (15.4%) cases were complicated with encephalitis. Among 1,344 patients in 2019, 123 (9.2%) cases vaccinated (including 7 cases with partial-EV71 vaccination), 1,221 (90.8%) cases unvaccinated, and 173 (12.9%) cases were complicated with encephalitis. Boys have higher rates of EV71 vaccination than girls in 2 years ().
Table 1. Basic information of patients in 2018 and 2019
Effects of vaccination on patients with encephalitis
Among patients in 2018 and 2019, 292 cases were vaccinated [176 (60.3%) cases were male], and 2,486 cases unvaccinated. Between vaccinated and unvaccinated cases, there was no significant difference in age, sex, or incidence rate of HFMD and herpangina. But the incidence rate of encephalitis in vaccinated patients was significantly lower than that in unvaccinated (P = .028) (). As shown in , a significant difference was not observed in EV71IgM, CVA16IgM EVPCR, and EV71PCR positive between cases with EV71 vaccination and non-EV71 vaccination, but except for CVA16PCR positive.
Table 2. Effects of EV71 vaccination on patients with encephalitis
Discussion
However, some study showed that the open-label and controlled phase Ⅳ trial involving a large population of 155,995 children aged 6–71 months for long-term observation of 14 months demonstrated a vaccine effectiveness of 89.7% (95% CI, 24.0 to 98.6) and good safety (the inactivated EV71 vaccine used in this study was made by Institute of Medical Biology).Citation10 The Sinovac EV71 vaccine could provide a sustained high protection against EV71-associated HFMDs for up to 2 years,Citation11 and consistently elicited immunogenicity and provided protection against mild-to-severe disease caused by EV71 for at least 1 year in infants and young children.Citation8 Interchangeability of EV71 vaccines from two manufactures (participants received Institute of Medical Biology Chinese Academy of Medical Sciences vaccine in the first dose and the Sinovac vaccine in the second dose) to complete an immunization series showed good immunogenicity and safety; the antibody response levels may vary by vaccination sequences of EV71 vaccines from the two manufacturers.Citation12 But the clinical trial observation data, effectiveness and safety evaluation of EV71 vaccine were relatively rare after the Ⅳ phase. Even with the availability of the EV71 vaccines, vaccination coverage remains low, ranging from 13.7%Citation13 to 37.6%Citation14 and insufficiently able to form a stable immune barrier. In rural areas, the coverage of EV71 vaccines was even lower.Citation15 The EV71 vaccine was launched in China in 2016, while the low rate of vaccination in Shandong area in 2016 and 2017 may be due to the beginning of inoculation less than two years, lack of publicity, awareness, and payment for vaccination. Most parents were willing to vaccinate their children against EV71-related HFMD. Parental age, location, education level, knowledge of EV71 vaccines, concern about susceptibility, and severity of HFMD were all factors that influenced willingness to vaccinate.Citation15With the knowledge of EV71 vaccines growing, the rate of inoculation increased obviously in 2018 and 2019. According to the number of patients admitted in the past 4 years in our department, the number of patients with HFMD admitted in 2018 and 2019 decreased compared with that in 2016 and 2017; the number of cases complicated with encephalitis also declined, but the incidence of herpangina increased. In particular, the number of outpatients with HFMD decreased significantly in 2019.
Among the 1,434 patients in 2018, the incidence was mainly HFMD, while the incidence was mainly herpangina among the 1,344 patients in 2019. Boys had a higher proportion of inoculation in 292 inoculated cases in the past 2 years; why boys had a higher rate than girls in EV71 vaccination in our collected patients, which may be that the protective effect of EV71 vaccine is weaker in boys, so the HFMD incidence was higher, but further research is needed. Two hundred and sixty-three (90.1%) were mild cases, which suggests that the EV71 vaccine had a protective effect; however, 29 (9.9%) patients still had encephalitis, indicating that some children may have no or weak protective effect after inoculation. In all, 292 cases were vaccinated (including 281 were given full vaccination, 11 given partial vaccination), and 2,486 cases were not vaccinated; it has shown that the incidence rate of encephalitis in vaccinated patients was significantly lower than that in non-vaccinated (P = .028), and the decreasing incidence of encephalitis may be associated with EV71 vaccination, while CVA16 infection has increased in patients with EV71 vaccine. The results were similar to Li Y, et al. who reported that the vaccine effectiveness for full vaccination was estimated to be 91.1% among non-severe cases compared with 73.3% in severe cases.Citation16 But as the proportions of other enteroviruses, such as CV-A6, have been increasing among HFMD cases in both China and worldwide,Citation17,Citation18 it is important to develop multivalent vaccines that can protect against multiple enterovirus serotypes.
Therefore, since the EV71 vaccination, this study has shown that the incidence of HFMD has declined in patients in our department, but the incidence of herpangina has increased, while the incidence of encephalitis has decreased obviously. It indicates that EV71 vaccination has a protective effect on the occurrence of severe HFMD. However, some patients do not produce a protective effect after inoculation or the protective effect was weak to develope into encephalitis, the reasons for which need further observation and study. This study has some limitations: First, only one center admitted HFMD patients. Second, only the morbidity and vaccination of the hospitalized patients were analyzed. Third, the time for collecting cases is relatively short for 2 years.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Human subjects approval statement
This study was approved by the Institutional Ethics Board of Shandong University Qilu children’s hospital.
Acknowledgments
We acknowledge all patients involved in the report.
Additional information
Funding
References
- Esposito S, Principi N. Hand, foot and mouth disease: current knowledge on clinical manifestations, epidemiology, aetiology and prevention. Eur J Clin Microbiol Infect Dis. 2018;37(3):391–98. doi:10.1007/s10096-018-3206-x.
- Yi EJ, Shin YJ, Kim JH, Kim TG, Chang SY. Enterovirus 71 infection and vaccines. Clin Experim Vaccine Res. 2017;6:4–14.
- Wang Y, Feng Z, Yang Y, Self S, Gao Y, Longini IM, Wakefield J, Zhang J, Wang L, Chen X, et al. Hand, foot, and mouth disease in China: patterns of spread and transmissibility. Epidemiology. 2011;22(6):781–92. doi:10.1097/EDE.0b013e318231d67a.
- Zhang J, Sun J, Chang Z, Zhang W, Wang Z, Feng Z. Characterization of hand, foot, and mouth disease in China between 2008 and 2009. Biomed Environ Sci. 2011;24:214–21.
- Lee TC, Guo HR, Su HJ, Yang YC, Chang HL, Chen KT. Diseases caused by enterovirus 71 infection. Pediatr Infect Dis J. 2009;28(10):904–10. doi:10.1097/INF.0b013e3181a41d63.
- Chinese Centre for Disease Control and Prevention. Technical guideline on use of inactivated enterovirus 71 vaccine; 2016. [accessed 2019 Feb 17]. http://www.chinacdc.cn/zxdt/201606/W020160608725047001222.pdf
- Li R, Liu L, Mo Z, Wang X, Xia J, Liang Z, Zhang Y, Li Y, Mao Q, Wang J, et al. An inactivated enterovirus 71 vaccine in healthy children. N Engl J Med. 2014;370(9):829–37. doi:10.1056/NEJMoa1303224.
- Zhu F, Xu W, Xia J, Liang Z, Liu Y, Zhang X, Tan X, Wang L, Mao Q, Wu J, et al. Efficacy, safety, and immunogenicity of an enterovirus 71 vaccine in China. N Engl J Med. 2014;370(9):818–28. doi:10.1056/NEJMoa1304923.
- Zhu FC, Meng FY, Li JX, Li XL, Mao QY, Tao H, Zhang Y-T, Yao X, Chu K, Chen Q-H, et al. Efficacy, safety, and immunology of an inactivated alum-adjuvant enterovirus 71 vaccine in children in China: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2013;381(9882):2024–232. doi:10.1016/S0140-6736(13)61049-1.
- Guan X, Che Y, Wei S, Li S, Zhao Z, Tong Y, Wang L, Gong W, Zhang Y, Zhao Y, et al. Effectiveness and safety of an inactivated enterovirus 71 vaccine in children aged 6-71 months in a phase IV study [published online ahead of print, 2019 Nov 18]. Clin Infect Dis. 2019;ciz1114. doi:10.1093/cid/ciz1114.
- Li JX, Song YF, Wang L, Zhang XF, Hu YS, Hu YM, Xia J-L, Li J, Zhu F-C. Two-year efficacy and immunogenicity of Sinovac Enterovirus 71 vaccine against hand, foot and mouth disease in children. Expert Rev Vaccines. 2016;15(1):129–37. doi:10.1586/14760584.2016.1096782.
- Xu Q, Cao Q, Yang W, Liu X, Liu H, Tian X, Li J, Fang X, Jia N, Zeng G, et al. Interchangeability of two Enterovirus 71 inactivated vaccines in Chinese children: A phase IV, open-label, and randomized controlled trial. Vaccine. 2020;38(12):2671–77. doi:10.1016/j.vaccine.2020.02.013.
- Guangwu W, Lijuan B. Epidemiological and etiological analysis of HFMD in border area of Chongzuo city from 2008 to 2017. Chin J Dis Control Prev. 2018;22:1028–31.
- Xiaopu Z, Jun Z. Investigation on vaccination rate of EV71 vaccine in severe hand, foot and mouth in Qujing City and analysis of population inoculation intention. Health Way. 2017;14:248–49.
- Wang Y, Meng F, Li J, Li G, Hu J, Cao J, Yu Q, Liang Q, Zhu F. Willingness of parents to vaccinate their 6-60-month-old children with EV71 vaccines: a cross-sectional study in rural areas of northern Jiangsu Province. Hum Vaccin Immunother. 2020;16(7):1579–85. doi:10.1080/21645515.2020.1737465.
- Li Y, Zhou Y, Cheng Y, Wu P, Zhou C, Cui P, Song C, Liang L, Wang F, Qiu Q, et al. Effectiveness of EV-A71 vaccination in prevention of paediatric hand, foot, and mouth disease associated with EV-A71 virus infection requiring hospitalisation in Henan, China, 2017-18: a test-negative case-control study. Lancet Child Adolesc Health. 2019;3(10):697–704. doi:10.1016/S2352-4642(19)30185-3.
- Li Y, Chang Z, Wu P, Liao Q, Liu F, Zheng Y, Luo L, Zhou Y, Chen Q, Yu S, et al. Emerging enteroviruses causing hand, foot and mouth disease, China, 2010–2016. Emerg Infect Dis. 2018;24(10):1902–06. doi:10.3201/eid2410.171953.
- Bian L, Wang Y, Yao X, Mao Q, Xu M, Liang Z. Coxsackievirus A6: a new emerging pathogen causing hand, foot and mouth disease outbreaks worldwide. Expert Rev Anti Infect Ther. 2015;13(9):1061–71. doi:10.1586/14787210.2015.1058156.