ABSTRACT
Studies have shown similarities in the structure of influenza and coronaviruses, in their binding receptors and in patterns of immune responses; and that influenza vaccine can induce cross-immunity. We examined the association of previous influenza vaccination and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, resulting in coronavirus disease-2019 (COVID-19), among 715,164 members of a health maintenance organization. In a multivariate regression model, the odds ratios for SARS-CoV-2 infection among individuals vaccinated for influenza in 2018–2019, 2019–2020, and in both seasons, compared to non-vaccinated individuals, were 0.82 (95% CI 0.68–0.99, p = .048), 0.79 (95% CI 0.67–0.98, p = .005), and 0.76 (95% CI 0.61–0.97, p = .004), respectively. Based on our findings, administration of influenza vaccine before the influenza season is highly recommended to reduce the burden of influenza, which is critical in scenarios of outbreaks of both influenza and SARS-CoV-2 infections, and also regarding its association with reduced rate of COVID-19.
Introduction
The new severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has crossed the species barrier, causing the current coronavirus disease 2019 (COVID-19) pandemic, with serious morbidity and mortality.Citation1,Citation2 The severity of the infection is variable, ranging from asymptomatic infection to a complicated prolonged course. Case series have identified risk factors for poor outcome, including older age, male gender, and certain underlying diseases.Citation1,Citation3 A community-based study of 3,802 individuals tested for SARS-CoV-2 identified that variables as male gender, age 40–64 years, living in underserved areas, obesity, and chronic kidney disease were associated with a positive SARS-CoV-2 result.Citation4
The host immune response to SARS-CoV-2 is of prime importance regarding the severity of the infection and its associated mortality and morbidity.Citation5 In a subgroup of patients, dysregulation of the innate immune response occurs, which is manifested by macrophage activation and overproduction of interleukin-6, leading to sudden clinical deterioration with severe respiratory distress 7–10 days after symptom onset.Citation1,Citation6 Likewise, although children usually have a very mild course of COVID-19, a small proportion develops a life-threatening complication, designated as hyperinflammation or cytokine storm syndrome, with multi-organ involvement.Citation7
Although SARS-CoV-2 does not mutate as frequently as influenza virus, the structures of these respiratory viruses share similarities, and they both contain sialic acid residues linked to glycoproteins or gangliosides as receptors for their binding proteins.Citation8,Citation9 A report of a comprehensive longitudinal analysis of the humoral and cellular immune responses to SARS-CoV-2 showed similar findings to those reported by the same group in patients with influenza.Citation10,Citation11 Epidemiologic studies revealed complex relations between influenza vaccination and the rates of other viral respiratory pathogens.Citation12–14
The relations between influenza vaccination and SARS-CoV-2 infection is unknown, although both infections might co-exist in the coming winter. Therefore, the objective of the present study was to elucidate the association of previous influenza vaccination and COVID-2019 by conducting a comprehensive population-based analysis.
Methods
Study design
We conducted a population-based study utilizing data from a large health maintenance organization (HMO) – Leumit Health Services, which provides services to around 715,000 members dispersed in the country. The HMO has a comprehensive computerized database, continuously updated since 2000, regarding demographics, medical diagnoses, medical visits, hospitalizations, and laboratory examinations. All laboratory results and vaccinations are incorporated automatically into the patients’ medical files. All HMO members have similar access to all health services. During each physician visit, a diagnosis is entered or updated according to the International Classification of Diseases 9th revision (ICD-9). The validity of diagnoses in the registry was confirmed as high.Citation15 The current study was approved by the HMO Institutional Ethics Committee (number 1292–LEU).
Study period and population
The study period was from February 1st, 2020 to April 30th, 2020 (the first COVID-19 patient was diagnosed in the country in February 2020). The 715,164 individuals who were registered in the HMO throughout this period constituted the study population. According to the country national policy since 2012,Citation16 influenza vaccination is offered free of charge to all citizens older than 6 months, including HMO members; persons aged ≥65 years and individuals with underlying chronic medical conditions receive reminders before the influenza season. The influenza vaccine is usually given during the fall, starting in October, mainly in November, and occasionally continued in January and even February of the next year. In 2019–2020, the four-valent split influenza vaccines (Vaxigrip-Tetra, Sanofi-Pasteur, France or Fluarix-Tetra, GSK, United Kingdom) were used; in 2018–2019, a three-valent vaccine (Influvac, Abbot, USA) was also used in <10% of the vaccinees. The Israeli Ministry of Health recommends vaccination for the entire population over the age of 6 months. The vaccine is strongly recommended for people who may suffer from influenza complications: patients over 65 years and individuals with defined underlying illnesses.Citation16
Study design A – a cross-sectional study based on the results of SARS-CoV-2 PCR test
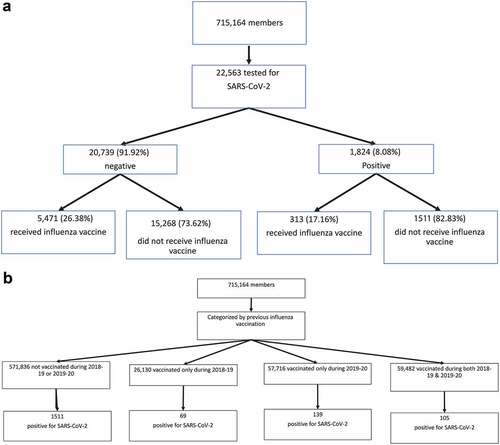
22,563 (3.15%) members of HMO underwent examinations for SARS-CoV-2 during the study period and were included in this analysis . Testing was performed only by physician referral. The HMO and the national criteria for SARS-CoV-2 examination included: direct exposure to a confirmed COVID-19 patient or presenting symptoms suggesting COVID-19 (essentially cough, shortness of breath or any other respiratory symptoms, with fever). Nasopharyngeal swabs were taken and examined for SARS-CoV-2 by a real-time RT-PCR assay with internal positive and negative controls according to the guidelines of the World Health Organization.Citation16 AllplexTM 2019-nCoV Assay (Seegene Inc., Seoul, Republic of Korea) was used until March 10th, 2020, and since then – the COBAS SARS-Cov-2 6800/8800 (Roche Pharmaceuticals, Switzerland). Individuals with positive and negative results of the SARS-CoV-2 examination were compared according to their receiving influenza vaccines for the winters of 2018–2019 and 2019–2020. Multiple additional data that could affect the rate of COVID-19 were collected for each individual from the HMO computerized database. This included age, gender, residential socioeconomic status (SES), weight, height, body mass index, smoking status, selected somatic or psychiatric comorbidities, and hospitalizations.
Study design B – population-based analysis
As the criteria for performing the SARS-CoV-2 examination were rather strict, a significant selection bias in performing the test is unlikely. However, to control for a bias caused by different rates of testing by various populations (individuals vaccinated against influenza vs. those not vaccinated, individuals with certain underlying diseases vs. those with no underlying diseases, etc.) – an additional population-based analysis was performed . All 715,164 members of the HMO throughout the study period were included in this analysis. Rates of positive SARS-CoV-2 results among all the members of the HMO were compared by previous influenza vaccination.
Definitions
SES was defined according to a person’s home address. The Central Bureau of Statistics classifies all cities and settlements into 20 levels of SES. As usually accepted and reported before, three categories of SES were defined for the present study: levels one to seven were categorized as low SES, eight to 13 as middle SES, and 14 to 20 as high SES.Citation17 Ethnicity was also defined according to the home address of the HMO member, and categorized into three groups: secular Jewish population, orthodox Jews, and Arabs.
Obesity was defined as BMI>30 Kg/m2. All the somatic and psychiatric diagnoses were based on ICD-9 codes. We concentrated on medical conditions that might affect the rates or severity of COVID-19, including mainly chronic lung disorders (asthma, chronic obstructive pulmonary disease), diabetes mellitus, hypertension, ischemic heart disease, heart failure, depressive and anxiety disorders, schizophrenia, dementia, smoking, and obesity.Citation1,Citation3,Citation4,Citation18
Statistical analysis
Statistical analysis was conducted using STATA 12 software (StataCorp LP, College Station, TX, USA). Assumptions were two-sided with α of 0.05. Initial analysis compared demographic characteristics between the study groups (positive vs. negative SARS-CoV-2 results and rates of SARS-CoV-2 positive results), using Student’s t-test and Fischer’s exact χ2 test for continuous and categorical variables, respectively, based on normal distribution and variable characteristics. These characteristics included age, gender, ethnicity, underlying medical conditions, obesity, and smoking status. Categorical data were shown in counts and percentages. Data on continuous variables with normal distribution were presented as means and standard deviation or 95% confidence intervals (CIs).
Risk estimates were first evaluated by stratified analyses. Subsequently, multivariable regression models were used to estimate crude and adjusted odds ratios (ORs) and CIs for associations between receipt of influenza vaccine and a positive PCR test for SARS-CoV-2, while controlling for potential confounders. The problem of multicollinearity among the variables in the models was tested by calculating the variance inflation factor (VIF).
Results
Demographic characteristics and medical conditions associated with SARS-CoV-2 positivity
The age range of the 22,563 individuals who were examined for SARS-CoV-2 was 6 months to 106 years; the mean age was 39.2 years. Of these, 1,824 (8.1%) had at least one positive result, representing 0.26% of the HMO population, and 20,739 (91.9%) had only negative results . In a primary univariate analysis, individuals who tested positive were younger, and more likely to be male and to reside in a city or town with a lower SES than those who tested negative . Orthodox Jews was the ethnic group with the highest proportion testing positive for SARS-CoV-2 (20.5%); Arabs were the groups with the lowest proportion testing positive (2.3%).
Table 1. Demographic variables and their association with SARS-CoV-2 results
Most of the underlying medical conditions examined were associated with SARS-CoV-2 positivity . In particular, smoking, chronic lung diseases, diabetes mellitus, hypertension, heart diseases, and mental illnesses, were significantly associated with SARS-CoV-2 positivity (details in ).
Table 2. Underlying medical conditions and their association with SARS-CoV-2 results
SARS-CoV-2 positivity by previous influenza vaccination
The study population was classified into four strata according to previous influenza vaccination: not vaccinated for the 2018–2019 or 2019–2020 seasons, vaccinated only for 2018–2019, vaccinated only for 2019–2020, or vaccinated in both 2018–2019 and 2019–2020. Compared to non-vaccinated persons (the reference group), receiving at least one influenza vaccination was associated with a significantly reduced rate of SARS-CoV-2 positivity. Influenza vaccination only during 2018–2019, only during 2019–2020, and during both seasons was gradually and significantly associated with lower SARS-CoV-2 positivity; the crude ORs were 0.69, 0.65, and 0.47, respectively (details in ).
Table 3. Crude odds ratio of previous influenza vaccination status and SARS-CoV-2 results (sensitivity analysis)
Multivariate analysis
Significant associates (possible confounding factors for the association with influenza vaccination) were entered to the regression model. Only a few variables remained significantly associated with SARS-CoV-2 positivity, including low residential SES, smoking, chronic lung diseases, and heart diseases (details in ). After controlling for the demographic variables and underlying conditions, previous influenza vaccination was still significantly associated with reduced rates of SARS-CoV-2 positive result. Compared to individuals who were not vaccinated for influenza, those who were vaccinated only in 2018–2019 had an adjusted OR of 0.82 (95% CI 0.68–0.99, p = .048), those vaccinated only in 2019–2020 had an OR of 0.79 (95% CI 0.67–0.98, p = .005), and those vaccinated both in 2018–2019 and in 2019–2020 had an OR of 0.76 (95% CI 0.61–0.97, p = .004). The VIFs were all less than 5, indicating that there was a very mild multicollinearity between the vaccination and other conditions in our data, which was not significant enough to warrant further corrective measures.
Table 4. Multivariate analysis of the variables associated with SARS-CoV-2 positivity
Population-based analysis
The previous influenza vaccination statuses of the 715,164 individuals who were registered in HMO throughout the study period were as follows: 571,836 (80%) were not vaccinated for the 2018–2019 or 2019–2020 influenza seasons, 26,130 (3.6%) were vaccinated only for the 2018–2019 season, 57,716 (8.1%) were vaccinated only for the 2019–2020 season, and 59,482 (8.3%) were vaccinated for both influenza seasons . Vaccination rates of individuals aged ≥65 years were higher, with 54% receiving an influenza vaccine in at least one of these two seasons compared to 13% in those younger than 65 years. The cumulative prevalence of infection with SARS-CoV-2 during the three-month study period in the whole population was related to previous influenza vaccination: vaccination in 2019–2020 showed a relative risk of 0.91 (p = .023) and vaccination in both influenza seasons – a relative risk of 0.67 (p < .001), as detailed in .
Table 5. Prevalence of population-based SARS-CoV-2 positivity by previous influenza vaccination status
Discussion
The present study documents an association between previous influenza vaccination and reduced SARS-CoV-2 positivity, namely reduced COVID-19 infection. This novel association is strengthened by examining a number of levels of previous influenza vaccination and by two methodological approaches. First, using a cross-sectional approach, we compared all the members of the HMO who tested positive for SARS-CoV-2 by RT-PCR, with those who tested negative. All the variables that were significantly associated with SARS-CoV-2 positivity were further examined in a multivariate regression model, which documented the significant association of previous influenza vaccination. We found a hierarchy in the impact of influenza vaccination on COVID-19 infection. In the multivariate model, compared to individuals who were not vaccinated, for those who were vaccinated only for the 2018–2019 influenza season, about 14 months before the COVID-19 epidemic, the OR was 0.82 (95% CI 0.68–0.99, p = .048). For those vaccinated only for the 2019–2020 season, a few weeks before the epidemic, the OR was 0.79 (95% CI 0.67–0.98, p = .005), and for those vaccinated for both seasons, the OR was 0.76 (95% CI 0.61–0.97, p = .004).
The univariate and multivariate analyses of this community-based cross-sectional study showed similar findings as reported by previous community- and hospital-based studies. Specifically, smoking, low SES, and certain underlying medical conditions were significantly associated with increased infection in the multivariate analysis.Citation1,Citation3,Citation4 However, we added the variable of previous influenza vaccination and showed its significant association with SARS-CoV-2 positivity. We studied the rates of COVID-19 infection, not its severity; for example, although young age has high infectious rates, older people present a more severe course with higher morbidity and mortality.Citation1,Citation3
To confirm our findings in the cross-sectional approach, we examined the prevalence of SARS-CoV-2 positivity among the entire population of 715,164 members of HMO, according to their influenza vaccination during the current and previous seasons. The significant impact of receiving an influenza vaccine was documented again, with the hierarchy documented as well. Compared to influenza non-vaccinated individuals, vaccination for only the 2018–2019 influenza season, over 1 year before the COVID-19 epidemic, had no significant association. Vaccination for the 2019–2020 season showed a relative risk of 0.91 (95% CI 0.85–0.98, p = .023). Finally, vaccination for both the 2018–2019 and 2019–2020 influenza seasons showed a relative risk of 0.67 (95% CI 0.56–0.79, p < .001). Thus, the OR for COVID-19 positivity was lower when the influenza vaccine was given more recently and during two consecutive influenza seasons.
The documented association between previous influenza vaccination and COVID-19 infection does not prove causality. However, it is the first crucial step in elucidating the relation between influenza vaccination and SARS-CoV-2. In an ecological Italian study investigating the role of Influenza vaccine in reducing COVID-19 prevalence, influenza vaccination coverage rates correlated negatively with all COVID-19 outcomes, the coverage rate of the influenza vaccination in people aged 65 and over was associated with a reduced spread and a less severe clinical expression of COVID-19.Citation19 Another research from U.S explored the influence of influenza vaccine on covid-19 infection in the country level. They found that a 10% increase in vaccination coverage was associated with a statistically significant 28% decrease in the COVID-19 death rate.Citation20
Previous studies have shown some similarities in the structure of influenza and coronaviruses,Citation8,Citation21 in their binding receptors,Citation8 and in the patterns of the induced immune responses.Citation10,Citation11 Therefore, the mechanisms of the association between influenza vaccination and COVID-19 should be further investigated.
In our multivariate regression model, we attempted to control for variables – demographic, ethnic, and comorbidities – that might affect SARS-CoV-2 positivity, and confirmed the independent association of a previous influenza vaccination and COVID-19 infection. Other confounders may play a role. We cannot rule out, for example, that individuals who received the influenza vaccine just a few weeks before the COVID-19 pandemic care more about their health, have higher awareness to prevention of infection, have distinct health-seeking patterns, or more strictly followed recommendations of health authorities regarding hygiene measures and social distancing, which are crucial to preventing SARS-CoV-2 infection or have other unforeseen residual confounders. The bottom line, however, is that individuals who have been vaccinated against influenza had lower rates of COVID-19 after controlling for other related variables.
Our research has several limitations. First, we performed a case-control analysis to evaluate the association between influenza vaccination and COVID-19, not a randomized-controlled study that is always preferred. However, since an influenza vaccine is recommended in many locations, universally or to high-risk individuals, who are usually at high risk for COVID-19 – such a study is not feasible and probably not ethical. The possibility that an influenza vaccine was administered outside the HMO or not recorded is very unlikely. It is questionable whether the HMO members represent other populations. While they probably represent populations in developed countries, we recommend that the study be repeated in other locations. Associations of influenza vaccination with the severity of COVID-19 infection were beyond the scope of the present study, and should be investigated in future research.
A major strength of our study is the relatively large cohort size, which includes all individuals registered at the HMO, with a computerized database that is updated regularly and that includes demographic details, medical diagnoses, and vaccine recording. In addition, we approached the research question by several methodologies, with four strata of influenza vaccine administration. These revealed similar conclusions on the association between influenza vaccination and COVID-19.
A co-infection of the viruses influenza A and SARS-CoV-2 in a patient with pneumonia was documented.Citation22 As seasonal influenza outbreaks are expected, with their significant burden on morbidity and mortality, a double-hit with both influenza and SARS-CoV-2 infections can occur and challenge the capabilities of the health systems to respond appropriately. Infection control measures are similar for both infections, as they actually share transmission routes.Citation23 As a vaccine against SARS-CoV-2 is not yet available, vaccination against seasonal influenza, which is recommended in many locations is crucial in preparing for the near future,Citation16,Citation24,Citation25 although vaccine hesitancy is a complex problem.Citation26,Citation27
In conclusion, based on our findings, it is recommended to administer the influenza vaccine before the influenza season. This will reduce the burden of influenza, the widespread circulation of influenza strains, and is critical in scenarios of outbreaks or even increased incidence of both influenza and SARS-CoV-2 infections. Importantly, reduced rates of COVID-19 may also be expected. Special attention and priority should be given to individuals with high-risk conditions,Citation16,Citation24 which are very similar for influenza and COVID-19. Healthcare services should prepare accordingly.
Disclosure of potential conflicts of interest
SA has received institutional research grant support from: MeMed and Ablynx, and is a medical adviser to the start-up company BiondVax Pharmaceuticals.
Additional information
Funding
Notes on contributors
Ilan Green
Dr. Ilan Green, MD, MHA Board Certified Specialist in Family Medicine. Head of Leumit Research Institute, Medical Division, Leumit Health Services. Head of the Department and Director of Family Physician Residency program, Leumit health services. Clinical instructor of the department of family medicine, sackler school of medicine, Tel-Aviv University.
Shai Ashkenazi
Prof Shai Ashkenazi, MD Shai Ashkenazi completed his medical education and residency in pediatrics in Israel and a fellowship in pediatric infectious diseases in Houston, Texas, USA (1987-90). His research expertise includes the molecular and cellular pathogenicity of infectious diseases with development of appropriate experimental animal models, novel antimicrobial agents and basic and clinical studies (phases 1-3) of novel human vaccines. He is currently Professor of Medicine and Dean at the Adelson School of Medicine, Ariel University; Chairman of the Israel Pediatric Association, member of the National Council for Child Health and member of the Education Committee of the World Society for Pediatric Infectious Diseases. Dr. Ashkenazi is a member of the Editorial Boards of several national and international medical journals, a co-author of over 280 medical publications, over 30 chapters in books and Editor of the Hebrew Textbook of Pediatrics (9 editions). He has organized local, national and international medical meetings, of which the majority were on the topic of pediatric infectious diseases, and has been invited as a speaker to international medical symposia and meetings. Dr. Ashkenazi has received dozens of research grants, including from the NIH (USA), European Union (FP-7), Chief Scientist, the USA-Israel Bi-national Science Foundation (BSF), pharmaceutical companies, and in 2017 – the Education Award of the European Society for Paediatric Infectious Diseases.
Eugene Merzon
Dr. Eugene Merzon, MD Board Certified Specialist in Family Medicine, Israeli Ministry of Health Certified ADHD Diagnostician, Head of the Department of Managed Care, Medical Division, Leumit Health Services, Clinical instructor of the Department of Family Medicine, Sackler School of Medicine, Tel Aviv University, Executive Board Member of Israeli National Diabetes Council. Executive Board Member of Israeli Society of ADHD (ISA). Executive Board Member of Israeli National Program for the Dementia prevention.
Shlomo Vinker
Prof. Shlomo Vinker, MD, MHA Board Certified Specialist in Family Medicine. Head of the Medical Division, Leumit Health Services. Head of the Department of family medicine, Sackler Faculty of Medicine, Tel Aviv University. Full Professor in Family Medicine, Department of Family Medicine, Sackler School of Medicine, Tel Aviv University. Vice-Dean for Community teaching, Sackler School of Medicine, Tel Aviv University. Executive board member of European General Partitions Research Network. President Elect of WONCA Europe Past Chairman, Israeli Association of Family Physician, Treasurer of Israeli Association of Family Physician Founder and chief scientific editor of a “Wikipedia” website for peer reviewed medical knowledge in Hebrew– about 3,500 topics and millions of entries per year - https://www.wikirefua.org.il.
Avivit Golan-Cohen
Dr. Avivit Golan-Cohen, MD, MHA Board Certified Specialist in Family Medicine, Head of the Department of Health Care Quality, Medical Division, Leumit Health Services. Until recently – Chief Physician of the Central District of LHS. An active family physician, working in an urban clinic in the city of Tel Aviv, Israel. Lecturer of the Department of Family Medicine, Sackler School of Medicine, Tel Aviv University.
References
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–62. PMID: 32171076. doi:10.1016/S0140-6736(20)30566-3.
- Itelman E, Wasserrstrum Y, Segev A, Avaky C, Negru L, Cohen D, Turpashvili N, Anani S, Zilber E, Lasman N, et al. Clinical characterization of 162 COVID-19 patients in Israel: preliminary report from a large tertiary center. Isr Med Assoc J. 2020;22:271–74. PMID: 32378815.
- Adams ML, Katz DL, Grandpre J. Population-based estimates of chronic conditions affecting risk for complications from coronavirus disease, United States. Emerg Infect Dis. 2020;26. PMID: 32324118. doi:10.3201/eid2608.200679.
- de Lusignan S, Dorward J, Correa A, Jones N, Akinyemi O, Amirthalingam G, Andrews N, Byford R, Dabrera G, Elliot A, et al. Risk factors for SARS-CoV-2 among patients in the Oxford royal college of general practitioners research and surveillance centre primary care network: a cross-sectional study. Lancet Infect Dis. 2020;15:1034–42. PMID: 32422204. doi:10.1016/S1473-3099(20)30371-6.
- Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pacific J Allergy Immunol. 2020;38(1):1–9. PMID: 32105090. doi:10.12932/AP-200220-0772.
- Giamarellos-Bourboulis EJ, Netea MG, Rovina N, Akinosoglou K, Antoniadou A, Antonakos N, Damoraki G, Gkavogianni T, Adami ME, Katsaounou P, et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27(6):992–1000. PMID: 32320677. doi:10.1016/j.chom.2020.04.009.
- Pain CE, Felsenstein S, Cleary G, Mayell S, Conrad K, Harave S, Conrad K, Harave S, Duong P, Sinha I, et al. Novel paediatric presentation of COVID-19 With ARDS and cytokine storm syndrome without respiratory symptoms. Lancet Rheumatol. 2010;2(7):e376–e379. PMID: 32427161. doi:10.1016/S2665-9913(20)30137-5.
- Zeng Q, Langereis MA, van Vliet ALW, Huizinga EG, de Groot RJ. Structure of coronavirus hemagglutinin-esterase offers insight into corona and influenza virus evolution. Proc Natl Acad Sci. 2008;105(26):9065–69. PMID: 18550812. doi:10.1073/pnas.0800502105.
- Matrosovich M, Herrler G, Klenk HD. Sialic acid receptors of viruses. Top Curr Cem. 2015;367:1–28. PMID: 23873408. doi:10.1007/128_2013_466.
- Thevarajan I, Nguyen THO, Koutsakos M, Druce J, Caly L, van de Sandt CE, Jia X, Nicholson S, Catton M, Cowie B, et al. Breadth of concomitant immune responses prior to patient recovery: a case report of non-severe COVID-19. Nat Med. 2020;26(4):453–55. PMID: 32284614. doi:10.1038/s41591-020-0819-2.
- Koutsakos M, Wheatley AK, Loh L, Clemens EB, Sant S, Nüssing S, Fox A, Chung AW, Laurie KL, Hurt AC, et al. Circulating T FH cells, serological memory, and tissue compartmentalization shape human influenza-specific B cell immunity. Sci Transl Med. 2018;10(428):eaan8405. PMID: 29444980. doi:10.1126/scitranslmed.aan8405.
- Wolff GG. Influenza vaccination and respiratory virus interference among department of defence personnel during the 2017–2018 influenza season. Vaccine. 2020;38(2):350–54. PMID: 31607599. doi:10.1016/j.vaccine.2019.10.005.
- Horns F, Dekker CL, Quake SR. Memory B cell activation, broad anti-influenza antibodies, and bystander activation revealed by single-cell transcriptomics. Cell Rep. 2020;30:905–13. PMID: 31968262. doi:10.1016/j.celrep.2019.12.063.
- Skowronski DM, Zou M, Clarke Q, Chambers C, Dickinson JA, Sabaiduc S, Olsha R, Gubbay JB, Drews SJ, Charest H, et al. Influenza vaccine does not increase the risk of coronavirus or other non-influenza respiratory viruses: retrospective analysis from Canada 2010–11 to 2016–17. Clin Infect Dis. 2020;ciaa626. PMID: 32442261. doi:10.1093/cid/ciaa626.
- Sagie S, Na’amnih W, Frej J, Cohen D, Alpert G, Muhsen K, Buttigieg SC. Correlates of hospitalizations in internal medicine divisions among Israeli adults of different ethnic groups with hypertension, diabetes and cardiovascular diseases. PLoS One. 2019 Apr;14(4):e0215639. PMID: 31017972. doi:10.1371/journal.pone.0215639.
- n.d. https://www.who.int/publications/i/item/diagnostic-testing-for-sars-cov-2
- Merzon E, Manor I, Rotem A, Schneider T, Vinker S, Golan Cohen A, Lauden A, Weizman A, Green I. ADHD as a risk factor for infection with covid-19. J Atten Disord. 2020 Jul 22. 1087054720943271. PMID: 32697120. doi:10.1177/1087054720943271.
- Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, Ji R, Wang H, Wang Y, Zhou Y. Prevalence of comorbidities and its effects in patients infected with SARS-C0V-2: a systematic review and meat-analysis. Int J Infect Dis. 2020;94:91–95. PMID: 32173574. doi:10.1016/j.ijid.2020.03.017.
- Amato M, Werba JP, Frigerio B, Coggi D, Sansaro D, Ravani A, Ferrante P, Veglia F, Tremoli E, Baldassarre D. Relationship between influenza vaccination coverage rate and COVID-19 outbreak: an italian ecological study. Vaccines (Basel). 2020 Sep 16;8(3):E535. PMID: 3294798. doi:10.3390/vaccines8030535.
- Zanettini C, Omar M, Dinalankara W, Imada EL, Colantuoni E, Parmigiani G, Marchionni L. Influenza vaccination and COVID19 mortality in the USA. medRxiv [Preprint]. 2020 Jun 26;20129817. 2020.06.24. PMID: 32607525. doi:10.1101/2020.06.24.20129817
- Abdella R, Aggarwal M, Okura T, Lamb RA, He Y. Structure of a paramyxovirus polymerase complex reveals a unique methyltransferase-CTD conformation. Proc Natl Acad Sci. 2020;117(9):4931–41. PMID: 32075920. doi:10.1073/pnas.1919837117.
- Wu X, Cai Y, Huang X, Yu X, Zhao L, Wang F, Li Q, Gu S, Xu T, Li Y, et al. Co-infection with SARS-CoV-2 and influenza A virus in patient with pneumonia, China. Emerg Infect Dis. 2020;26(6):1324–26. PMID: 32160148. doi:10.3201/eid2606.200299.
- Soo RJJ, Chiew CJ, Ma S, Pung R, Lee V. Decreased influenza incidence under COVID-19 control measures, Singapore. Emerg Infect Dis. 2020;26(8):1933–35. PMID: 32339092. doi:10.3201/eid2608.201229.
- Grohskopf LA, Alyanak E, Broder KR, Walter EB, Fry AM, Jernigan Grohskopf LA, Alyanak E, Broder KR, Blanton LH, Fry AM, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices - United States, 2020–21 influenza season. MMWR Recomm Rep. 2020 Aug 21;69(8):1–24. PMID: 32820746. doi:10.15585/mmwr.rr6908a1.
- Mendelson M. Could enhanced influenza and pneumococcal vaccination programs help limit the potential damage from SARS-CoV-2 to fragile health systems of southern hemisphere countries this winter? Int J Infect Dis. 2020;94:32‐3. PMID: 32194236. doi:10.1016/j.ijid.2020.03.030.
- Livni G, Chodik G, Yaari A, Tirosh N, Ashkenazi S. Attitudes, knowledge and factors related to acceptance of influenza vaccine by pediatric healthcare workers. J Pediatr Infect Dis. 2008;3:111–17. doi:10.1055/s-0035-1556979.
- Grossman Z, Ashkenazi S, Rubin L. How are we responding to vaccine-hesitant parents? Lancet Child Adolescent Med. 2017;1:9–11. PMID: 30169232. doi:10.1016/S2352-4642(17)30040-8.