ABSTRACT
Meningococcal meningitis caused by Neisseria meningitidis is a reportable infectious disease in China, due to the high incidence of meningitis in the era before the availability of vaccines. The disease incidence was markedly reduced after meningococcal vaccination was introduced in the 1980s. Currently, there are polysaccharide, conjugate, and combined vaccine formulations against meningococcal meningitis in the Chinese market, almost all of which are produced by domestic manufacturers. It is necessary to further enhance national meningococcal surveillance to improve the level of prevention and control of meningococcus. However, the immune efficacy and persistence of immunity of vaccines should be monitored. More importantly, additional investments should be made to develop serogroup B meningococcal vaccines.
1. Introduction
Neisseria meningitidis is a leading cause of invasive meningococcal disease (MD).Citation1 According to the different structures of its extracellular polysaccharide capsule, 12 distinct capsular groups of meningococcus have been classified; MD is mainly caused by 6 meningococcal capsular groups: A, B, C, W, Y, and X.Citation2 MD is characterized by a broad clinical spectrum that can involve meningitis, meningococcemia, or both, as well as other clinical presentations, with a 6–10% case-fatality rate.Citation3 Furthermore, permanent sequelae associated with MD, such as brain damage and amputations of limbs or digits, can occur.Citation3 To date, the only recognized reservoirs of N. meningitidis are humans. It is reported that asymptomatic nasopharyngeal carriage is frequent and involves ~10% of the general population.Citation4 Transmission of the bacterium from an infected individual to another person occurs via direct contact with droplet respiratory secretions. An estimated 1.2 million cases of MD are reported each year worldwide, including 135,000 deaths, and most cases occur in developing and underdeveloped countries. The incidence of MD is highest for children <1 year, but in epidemic outbreaks, older children and adolescents can have high incidence of this disease.Citation5,Citation6
In China, meningococcal meningitis is a reportable infectious disease due to the high incidence of MD before the introduction of meningococcal vaccination.Citation7–9 In this review, we briefly describe the epidemiological characteristics of MD and summarize the development of various meningococcal vaccines in China. Quality control assessments of meningococcal vaccines currently used in the country are also reviewed.
2. Meningococcal meningitis in China
In the era when vaccines were unavailable, five pandemics of MD were recorded in China, and nationwide pandemics appeared every 8–10 years, each of which lasted approximately 3–4 years.Citation8,Citation9 The highest incidence of MD occurred in the spring of 1967. More than 3.04 million cases were reported, with greater than 160,000 deaths and a 5.5% fatality rate. The next largest pandemic happened in 1977, and had a morbidity of 59.7 cases per 100,000 and a 4.0% fatality rate. Meningococcal polysaccharide vaccines (MenPV) have been used since the 1980s; the incidence of the disease has been sharply reduced and continues to be reduced.Citation7,Citation8,Citation10 According to the data from the Chinese infectious disease reporting system, the incidence of MD is less than 1 per 100,000 since the 1990s and only 104 and 111 MD cases were reported in 2018 and 2019, respectively (). The incidence is higher in children <5 years, and the younger the child, the higher the incidence.Citation11 Previous studies have investigated N. meningitidis carriage rates among the healthy population from different provinces in China, and recent meta-analysis estimates that the overall N. meningitidis carriage rate was 2.7% (95% CI 2.0–3.5%) in 2005–2015.Citation12
Figure 1. Reported meningococcal meningitis and death cases in China, 2002–2019
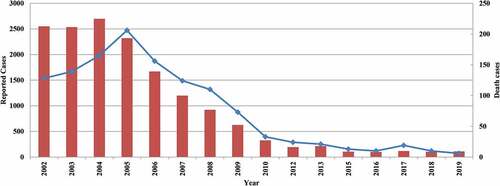
In mainland China, routine epidemiological surveillance, including acute MD case reports, outbreak surveys, and collections of cerebrospinal fluid and blood of suspected cases, have been conducted, and the National Centers for Disease Control and Prevention and its provincial branches are legally required to formally report MD cases to the Ministry of Health monthly. However, considering MD is clinically diagnosed by physicians, and not all hospitals diagnose MD using the current laboratory methods, this suggests a significant underestimation of burden of N. meningitidis infection in China.Citation10
N. meningitidis serogroup A is the most common serogroup involved in MD pandemics; the earliest serogroup A strain was isolated in the 1950s in China. Several historic large outbreaks were caused by serogroup A before the 1980s.Citation13 Serogroup distribution of 922 N. meningitidis strains isolated from 22 provinces in China from 1956–2002 included 638 serogroup A (69.2%), 254 serogroup B (27.6%), 9 serogroup C (1.0%), and another group of 21 strains (2.3%),Citation14 suggesting serogroup C seldom caused MD before 2000 in China. However, serogroup C ST4821 has emerged and caused a large MD outbreak in Anhui province, China during 2003–2004.Citation15 The new strain appears to be more invasive, causing serious complications and a higher case-fatality rate than serogroup A. It is interesting that the ST4821 lineage became the predominant clone and rapidly spread nationwide, causing several local outbreaks.Citation16 Zhou et al reported that 47.1% serogroup C (n = 164), 35.3% serogroup A (n = 123), and 12.9% serogroup B (n = 45) strains were found in 348 laboratory-confirmed patients with MD from 2005–2012, and almost all serogroup C (92.4%, 123/131) and 34.5% (10/29) of serogroup B strains belonged to the ST4821 clonal complex.Citation17 Genomic analysis has demonstrated that the serogroup C ST4821 clonal complex is likely the origin of serogroup B ST4821.Citation18 The emergence and circulation of serogroup B ST4821 may increase the incidence of MD, which causes epidemics and outbreaks in China.Citation18,Citation19 Recently, phylogenetic analysis has suggested that successive recombination events within genes encoding surface antigens and acquisition of quinolone resistance mutations may have contributed to the emergence of the ST4821 clone in China.Citation20 Furthermore, a few cases of serogroup W ST4821 MD were reported in southeast China in 2013,Citation21 although all 14 serogroup W strains isolated from 2005–2012 were ST11 complex.Citation17 Other serogroup N. meningitidis strains, including serogroup Y and X have occasionally been reported in China.Citation17,Citation22
3. Meningococcal vaccines in the Chinese market
Licensed vaccines against MD have been available for more than 50 years. It has been demonstrated that vaccination of at-risk populations is the most viable strategy to control MD.Citation23 In China, the history of meningococcal vaccine-related studies can be traced back to 1939, but failed to be used. In 1963, an inactivated whole cell vaccine was developed, but this type of vaccine was not widely used due to adverse reactions.
There are polysaccharide, conjugate, and combined vaccine formulations available in the Chinese market. Almost all meningococcal vaccines are produced by domestic manufacturers. One imported bivalent (A and C) MenPV, has been licensed since the 2000s, but no products were sent to the National Institutes for Food and Drug Control (NIFDC) for lot release after 2013. Untill now, no meningococcal protein-polysaccharide conjugate vaccines (MenCV) from other countries have been licensed in China. This may be due to the fact that there are various domestic meningococcal vaccines available in the Chinese market. Currently, the routine vaccination schedule for MenPV in China includes a total of four doses, two of which include doses of serogroup A univalent MenPV for children ages 6–18 months and another two doses of serogroups A and C bivalent MenPV (one dose for children at 3 years and one dose for children at 6 years).Citation24 Vaccination via conjugate and combined vaccines for children are paid by the parent. It has been shown that the reported routine vaccination coverage of meningococcal vaccines was more than 99% in China (not including Hong Kong, Macao, and Taiwan) in 2015.Citation25
Many studies have demonstrated that serum bactericidal antibody (SBA) activity is highly correlated with immunity to meningococcal disease.Citation26–29 To date, it is generally accepted that SBA is the best surrogate of protection for all serogroups. An SBA titer of either ≥ 4 or ≥ 8 using human or rabbit complement, respectively, or a ≥ 4-fold rise in SBA pre- to post-vaccination are considered as the cutoff value for protection after vaccination with meningococcal polysaccharide or conjugate vaccines.Citation24 Therefore, the immunogenicity of meningococcal polysaccharide or conjugate vaccines is commonly evaluated using seroconversion to SBA-positivity.Citation30–32
3.1 Polysaccharide vaccines
Since the 1960s, serogroup A MenPV have been successfully developed.Citation27 Randomized, controlled trials have been conducted in many countries and regions,Citation28,Citation33,Citation34 demonstrating the safety and effectiveness of serogroup A MenPVs in children and young adults. To date, they are available in bivalent (A and C), trivalent (A, C, and W135), and quadrivalent (A, C, W135, and Y) polysaccharide vaccines licensed by different countries.Citation23
In China, MenPV-related studies were conducted in the 1970s and a MenPV was successfully developed in 1980.Citation35 Different from the serogroup A MenPVs of other countries,Citation23 a single-dose serogroup A MenPV containing 30 μg of polysaccharides is used in China.Citation7 It is reported that more than 10 million subjects were immunized with serogroup A MenPV from 1977–1980, and the efficacy of the vaccine was 86.5–92.3%. Between 3.8–12.8%, and 0.8–1.3% of 3,874 subjects developed local reactions at 6 and 24 h after vaccination, respectively.Citation7 Many studies have demonstrated that extensive use of serogroup A MenPV to immunize children greatly contributed to reduce the burden of MD in China.Citation36 To date, serogroup A MenPVs are still included in the national vaccination program. In 2019, more than 20 million human doses of serogroup A MenPVs were released by the NIFDC onto the market ().
Figure 2. Number of lot release of different types of meningococcal vaccines in China, 2010–2019
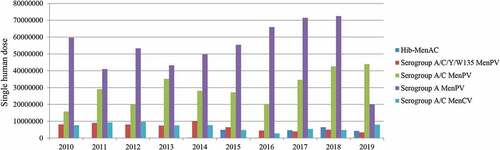
Due to the national rapid spread of N. meningitidis serogroup C, a national vaccination program using a serogroup A and C bivalent MenPV was initiated to replace the serogroup A polysaccharide-based vaccines in 2005.Citation10 The immunogenicity and clinical efficacy of serogroup A and C MenPVs are well established in China.Citation37,Citation38 In the past five years, more than 20 million human doses of bivalent (A and C) MenPV were released onto the Chinese market each year (). Furthermore, trivalent or pentavalent MenPVs, which should protect against more serogroups, have also been shown to be as well-tolerated and immunogenic in other countries.Citation39,Citation40 A phase III clinical trial has also been performed to evaluate the efficacy and safety of quadrivalent (A, C, W135, and Y) vaccines produced by Chinese manufacturers in children aged ≥ 2 years and in adults in China.Citation32 Adverse reactions to quadrivalent (A, C, W135, and Y) MenPVs were mild and no significant difference was found when compared to bivalent (A and C) MenPVs. Consistent with other studies, more than 90% of subjects showed a ≥ 4-fold increase of SBAs against the four serogroups after vaccination with a single dose of test vaccine, confirming the efficacy of quadrivalent vaccines.Citation32 Since 2006, serogroup A, C, W135, and Y MenPVs have been licensed for use in China and the vaccination cost is paid by the individual. Six local manufacturers produce the quadrivalent (A, C, W135, and Y) vaccine and approximately 3.3 million human doses of quadrivalent vaccines were released in 2019 ().
3.2 Protein-polysaccharide conjugate vaccines
It is reported that MenPVs are T cell-independent antigens which induce humoral responses but not T-cell responses or immunological memory, which means that immune protection will be relatively short-lived.Citation41 To address the limitations posed by MenPVs, meningococcal capsular polysaccharides were chemically conjugated to protein carriers, such as tetanus toxoid (TT), diphtheria toxoid, and a nontoxic mutant of diphtheria toxin, the Corynebacterium diphtheriae cross-reactive material 197 (CRM-197), which was shown to stimulate a T-cell response, since the 1990s.Citation23,Citation30,Citation31 Many studies have demonstrated that meningococcal conjugate vaccines are safe and immunogenic, and result in immunologic memory when administered as vaccines to infants.Citation29–31,Citation42,Citation43 Licensed MenCV are currently monovalent (A or C), bivalent (A and C) or quadrivalent (A, C, W135, and Y) worldwide.Citation23
To offer better protection against MD, conjugated formulations were also developed during the last decades in China. Different from other countries, only bivalent (A and C) MenCV are currently licensed.Citation44 The conjugate vaccines contain 10 μg each of the individual polysaccharides conjugated to the TT carrier (except for a MenB outer membrane protein used as the carrier), and do not contain preservatives or adjuvants. The vaccine has been demonstrated to be safe and immunogenic in subjects aged 6–23 months, 2–15 years, and 16–30 years after 1–3 doses, inducing higher SBA titers against serogroup A and C, and no severe systemic adverse reaction has been observed.Citation45 No significant difference for local reaction rates were found when compared to the previously licensed monovalent A or bivalent A and C MenPVs. A post-marketing study on the safety of bivalent A and C MenCV has shown that the overall incidence rate of systemic and local adverse effects is 1.6% (565/34 411; 95% CI: 1.5%–1.8%) and 1.6% (554/34 411; 95% CI: 1.5%–1.7%), respectively.Citation46 Similar results have also been found in other safety studies of serogroup A and C MenCV from Chinese manufacturers.Citation47 A lower rate of adverse reactions to the examined vaccine in comparison to a previous post-marketing examination of a MenCV in another country may be associated with differences in national definitions of adverse reactions that resulted in the underestimation of certain mild adverse reactions.Citation47 Consistent with the results from other countries, immune memory after immunization of different ages of infants and children persisted to at least 3 years of age following 2–3 doses of serogroup A and C MenCV,Citation48 further indicating long-term protection induced by conjugate vaccines in children. Bivalent A and C MenCV made by three local manufacturers are available on the Chinese market; approximately 7.9 million human doses of conjugate vaccines were released in 2019 ().
Furthermore, quadrivalent A, C, W, and Y MenCV providing broader protection have been developed by Chinese local manufacturers. According to the data shown, phase II and III clinical trials of quadrivalent A, C, W, and Y MenCV from two local manufacturers have recently been finished (http://www.chinadrugtrials.org.cn).
3.3 Combination vaccines
It is generally accepted that the development of combined vaccines would be advantageous to increase the coverage of a vaccine. Considering both N. meningitidis A and C capsular groups, together with Haemophilus influenza type b (Hib), are responsible for most of the cases of MD in China, a combination vaccine based on Hib and meningococcal A and C polysaccharide conjugated with TT (Hib-MenAC) has been developed. Clinical trials demonstrated that the seroconversion rate after administration with Hib-MenAC was 99.0%, 96.1%, and 97.7% for rSBA-MenA, rSBA-MenC, and anti-polyribosyl-ribitol phosphate antibodies, respectively, in the 3–5-month-old group, 95.3%, 97.1%, and 96.9% in 6–23-month-old group, and 96.0%, 94.6%, and 94.6% in the 5-year-old group, respectively.Citation49,Citation50 Most adverse reactions were mild or moderate; no serious adverse event were judged to be related to the vaccination, suggesting the vaccine is well-tolerated.Citation51
In 2014, a Hib-MenAC was approved in China, which contains 10 μg each of the individual polysaccharides conjugated to the TT carrier absorbed with the adjuvant Al(OH)3. To obtain sustained protection, a primary series of three doses for the 2–5-month age group or two doses for the 6–11-month age group with a 1-month interval, or one dose administered at 12–71 months of age is recommended by the manufacturer.Citation44
3.4 MenB vaccines
N. meningitidis serogroup B (MenB) is one of the world’s major epidemic serogroups.Citation52 Although various meningococcal polysaccharide and conjugate vaccines with four (A, C, W, and Y) serogroups are currently available, it is a great challenge to develop a broadly protective polysaccharide-based MenB vaccine. This is because its chemical composition is identical to polysaccharides decorating the human fetal neural cell-adhesion, which is crucial for central and peripheral nervous systems. This similarity between host and organism cell surface molecules results in poor immunogenicity and the theoretical risk of developing autoantibodies.Citation53
Although outer membrane vesicles (OMV) purified from MenB have also been developed in several countries, OMV vaccines induce immunity mostly against the highly variable membrane protein, which have shown serosubtype-specific protection.Citation54 With the development of reverse vaccinology approaches, 4CMenB containing three main Neisseria protein antigens and OMV from the MenB outbreak strain have been successfully developed.Citation55,Citation56 In September 2015, the UK was the first country to introduce the 4CMenB vaccine into their national immunization program. One year after the introduction of 4CMenB, the number of MenB cases in vaccine-eligible infants decreased 50% compared with the prevaccine period, indicating two-dose 4CMenB vaccination was highly effective in preventing Men B disease in infants.Citation57 To date, 4CMenB has been licensed in many countries.Citation56Furthermore, a second MenB vaccine, rLP2086, containing two fHbp variants, was licensed in the USA for active immunization in 10–25-year-olds in 2014 and in the EU for individuals aged ≥ 10 years in 2017.Citation58,Citation59 To date, these two broadly protective MenB vaccines have not been licensed in China. Previous studies demonstrated that current circulating serogroup B N. meningitidis in China is dominated by the ST4821 clone group, which is different from the existing serogroup B protein vaccines in the world. The effectiveness and impact of these MenB vaccines in China is unknown. Several MenB-related studies have been reported, but all are in preclinical studies in China.
4. Quality control of meningococcal vaccines in China
4.1 Lot release system in China
Since vaccines are used in healthy populations, it is essential to ensure consistent quality in each vaccine lot released to the market. A system was piloted for five EPI vaccines on December 31, 2001, in China. Monovalent A and bivalent (A and C) MenPV were included in October 1, 2005. Accordingly, the Chinese government has required since January 1, 2006, that all marketed preventative vaccines be released, lot by lot, by the national regulatory authority,Citation8,Citation60 following review and independent testing. The review evaluates whether critical raw materials and manufacturing processes are consistent with approved parameters, and whether the vaccine bulk and final product meet the current national pharmaceutical criteria.Citation8,Citation61 Independent tests include tests of identity and sterility. In addition, the key test (such as a polysaccharide content test) for vaccine quality control is required to be conducted in 10–30% of vaccine products.
After reviewing the relevant protocols, the vaccine test data from the manufacturer’s and NIFDC are collected, and used to perform trend analysis. The polysaccharide contents of the products are the most important parameters in the quality control of meningococcal vaccines. It is suggested that through trend analysis any significant deviation or shift from the trends could be detected. Usually, the following parameters are used in the analysis: mean, standard deviation (SD), ± 2 SD, and ± 3 SD. Results outside the ranges of the ± 2 SD and ± 3 SD lines are taken as the warning and actions limits, respectively, and trigger actions by the regulator and manufacturer. The trend analysis has shown that almost all results of the content of four polysaccharides have been within the alert limit (mean ± 2 SD) for quadrivalent A, C, W, and Y MenPVs in 2012–2013, and no significant difference has been found in the four polysaccharide contents of 27 different lots by NIFDC compared to that of the manufacturer, suggesting that the quality of domestic MenPV products is stable.Citation62
4.2 Quality control tests for meningococcal vaccines
Vaccine bulk is the most important intermediate product in the vaccine production process, and the meningitis vaccine bulk is a uniform substance used for the final product. Both the bivalent and tetravalent polysaccharide meningitis vaccines are prepared from a monovalent serogroup bulk. The quality control of vaccine products should be based on the characteristics of vaccine strains, the composition of the culture medium, the metabolites in the cultivation process, the chemical agents used in the purification process, the protective agents for vaccines, and the raw and auxiliary materials used.
In addition to the identification test, the quality control for MenPV bulks is mostly chemical testing, such as protein, nucleic acid content, O-acetyl content, phosphorus content (for serogroup A), sialic acid content (for serogroup C, Y, and W135), molecular size determination, an endotoxin/pyrogen test, and a sterility test ().Citation62 It was also noted that several test items for the serogroup A polysaccharide bulk, such as the amount of protein content, and nucleic acid and molecular size determination was slightly different from the group A + C polysaccharide vaccine (). Different from the bulk, the quality control for the final products involved the test of the final product reconstituted with diluents, as well as the diluents themselves.
Table 1. Quality control tests for the bulk of different meningococcal vaccines used in China
Different methods are used to determine the content of the vaccine main component polysaccharide. Monovalent A or bivalent A + C vaccines are often determined by chemical methods. The content of serogroup A polysaccharides can be determined by the phosphorus content; each gram of polysaccharide contains 75 mg of phosphorus. The group C polysaccharides can be determined by the content of sialic acid, and each gram of polysaccharide contains 750 mg N-acetylneuraminic acid (sialic acid).Citation62 The polysaccharide content of serogroup A/C/Y/W135 MenPVs cannot be detected by chemical methods, and can only be detected by immunological methods, such as rocket electrophoresis assays. It is reported that a high-performance liquid chromatography with pulsed amperometric detector method has been used for serogroup quantitation of multivalent polysaccharide and polysaccharide-conjugate meningococcal vaccines.Citation63 Meanwhile, an immune rate turbidimetric method for determination of polysaccharide content in Hib-MenAC combined vaccines has been developed. The specific antibody against various polysaccharides shows a reaction only to the corresponding polysaccharide antigens, whereas no interference was observed between various polysaccharide antigens.Citation64
Due to the different production processes, the quality control of the MenCV is not only involved with the polysaccharide bulk, but also the conjugated bulks. On the basis of the polysaccharide bulk, the detection of the polysaccharide derivatization rate has been added (). For the quality control of conjugated bulks, the content of bound polysaccharide, free polysaccharide, protein, polysaccharide/protein ratio, molecular size distribution, and sterility tests are required. In addition to the appearance and pH value, the identification tests, sterility inspection, the content of polysaccharide, free polysaccharide, free protein content, and pyrogen inspection are required for the final conjugated vaccine product. To further strength the quality control according to requirements in China and to evaluate the antibody response induced by MenCV, a potency assay based on immunogenicity in a mouse model is used. The anti-A and C polysaccharide IgG titers in the serum of immunized mice (two-dose regimen) are determined by ELISA; the seroconversion rates should be at least 80%.Citation50 Due to the addition of Al(OH)3, the adjuvant content should also be tested in the Hib-MenAC.
5. Conclusion
This remarkable progress toward MD control is followed by new challenges. The rapidly changing epidemiology of N. meningitidis strains, the waning of vaccine-induced immune responses over time, the variable impact of vaccination in carriage and herd immunity, and the increased reactogenicity observed after coadministration of meningococcal and routine vaccination must be properly addressed to constantly maintain an acceptable level of protection. Continuous surveillance and coverage studies are hence required so that national vaccination programs can be promptly adapted and optimized.
To further improve the level of prevention and control of meningococcus, it is necessary to further enhance meningococcal surveillance in China. The current content of meningococcal surveillance includes cases, vaccination rates, antibiotic resistance, population antibody levels, and carrier infection rates in healthy populations. It is necessary to carry out surveillance of encephalitis/meningitis cases so that early detection, early diagnosis, and early treatment of all suspected cases are possible. According to spatiotemporal sequence analysis, actively detecting cases of meningococcal clusters is required to prevent meningococcal outbreaks or epidemics. According to the actual situation in various places, the serogroups, types and subtypes of meningococcal cases need to be actively monitored.Citation44
Additionally, the immune efficacy and persistence of the vaccine should be monitored. Meta–analysis has shown that the prevalence of N. meningitidis serogroup A and C antibodies is 70.9 and 23.6% in the healthy 0–4 year population, respectively.Citation12 The lower seroprevalence of serogroup C within the population may be associated with the more than 90% use of bivalent A and C MenPVs before 2015 in China. It is generally accepted that immunogenicity and persistence of conjugated vaccines are better than MenPVs, especially serogroup C, in children aged 0–2 years.Citation65 Based on epidemiology, vaccinology and health economics, replacing MenPVs with conjugated vaccines should be considered in the future in China. It is also noted that a difference exists in the immunization program recommended by the current bivalent A and C conjugate vaccines manufacturers, which has caused problems for vaccinating personnel. It is recommended that relevant departments strengthen joint research and unify the vaccination procedures recommended by vaccine instructions. Furthermore, to simplify the vaccination procedure, reduce injection times, reduce the risk of adverse reactions, and save cost, combination vaccines need to be investigated.Citation65
Although widespread vaccination with meningococcal vaccine has caused the incidence of meningoencephalitis nationwide to be lower than that of some developed countries, there has been an obvious upward trend in MenB cases in China. Various types of MenB vaccines have been used in other countries.Citation52,Citation56,Citation59 but they are unavailable in the Chinese market. Therefore, we should strengthen guidance and focus on supporting the development of MenB vaccines in China.
Acknowledgments
This work was supported by the National Key R&D Program of China (No. 2018YFC1603900) and Medical Microbiology Resources Sub-platform Operation and Service Funds, China (NIMR-2019).
Disclosure of potential conflicts of interest
The authors declare that they have no competing interests.
Additional information
Funding
References
- Dwilow R, Fanella S. Invasive meningococcal disease in the 21st century-an update for the clinician. Curr Neurol Neurosci Rep. 2015;15(3):2. doi:10.1007/s11910-015-0524-6.
- Harrison OB, Claus H, Jiang Y, Bennett JS, Bratcher HB, Jolley KA, Corton C, Care R, Poolman JT, Zollinger WD. Description and nomenclature of Neisseria meningitidis capsule locus. Emerg Infect Dis. 2013;19(4):566–73. doi:10.3201/eid1904.111799.
- Brooks R, Woods CW, Benjamin DK Jr., Rosenstein NE. Increased case-fatality rate associated with outbreaks of Neisseria meningitidis infection, compared with sporadic meningococcal disease, in the United States, 1994-2002. Clin Infect Dis. 2006;43(1):49–54. doi:10.1086/504804.
- Laver JR, Hughes SE, Read RC. Neisserial molecular adaptations to the nasopharyngeal niche. Adv Microb Physiol. 2015;66:323–55.
- Rouphael NG, Stephens DS. Neisseria meningitidis: biology, microbiology, and epidemiology. Methods Mol Biol. 2012;799:1–20.
- Jafri RZ, Ali A, Messonnier NE, Tevi-Benissan C, Durrheim D, Eskola J, Fermon F, Klugman KP, Ramsay M, Sow S. Global epidemiology of invasive meningococcal disease. Popul Health Metr. 2013;11(1):17. doi:10.1186/1478-7954-11-17.
- Ding SQ. Group A meningococcal polysaccharide vaccine widely used in China. Bull Med Res. 1981;6:19.
- Xu M, Liang Z, Xu Y, Wang J. Chinese vaccine products go global: vaccine development and quality control. Expert Rev Vaccines. 2015;14(5):763–73. doi:10.1586/14760584.2015.1012503.
- Li JH, Wu D, Yin ZD, Li YX. Analysis of epidemic characteristics for meningococcal meningitis in China during 2015-2017. Zhonghua yu fang yi xue za zhi [Chin J Prev Med]. 2019;53:159–63.
- Li J, Shao Z, Liu G, Bai X, Borrow R, Chen M, Guo Q, Han Y, Li Y, Taha M-K. Meningococcal disease and control in China: findings and updates from the Global Meningococcal Initiative (GMI). J Infect. 2018;76(5):429–37. doi:10.1016/j.jinf.2018.01.007.
- Li J, Li Y, Shao Z, Li L, Yin Z, Xu L, Luo H. Prevalence of meningococcal meningitis in China from 2005 to 2010. Vaccine. 2015;33:1092–97.
- Zhang Y, Wei D, Guo X, Han M, Yuan L, Kyaw MH. Burden of Neisseria meningitidis infections in China: a systematic review and meta-analysis. J Glob Health. 2016;6(2):20409. doi:10.7189/jogh.06.020409.
- Ling J, Li Y, Luo H. Prevention and control of epidemic meningococcal meningitis. Disease surveillance. 2009;24:155–59.
- Shao Z, Xu L, Gao Y, Li M, Li Y, Yin Z, Liang X. Epidemiologic trend of serogroup switching of Neisseria meningitidis strains in China. Chin J Vaccin Immun. 2007;13:541–44.
- Shao Z, Li W, Ren J, Liang X, Xu L, Diao B, Li M, Lu M, Ren H, Cui Z. Identification of a new Neisseria meningitidis serogroup C clone from Anhui province, China. Lancet. 2006;367(9508):419–23. doi:10.1016/S0140-6736(06)68141-5.
- Zhang X, Shao Z, Yang E, Xu L, Xu X, Li M, Ren J, Zhu Y, Yang F, Liang X. Molecular characterization of serogroup C Neisseria meningitidis isolated in China. J Med Microbiol. 2007;56(9):1224–29. doi:10.1099/jmm.0.47263-0.
- Zhou H, Shan X, Sun X, Xu L, Gao Y, Li M, Shao Z. Clonal characteristics of invasive Neisseria meningitidis following initiation of an A + C vaccination program in China, 2005-2012. J Infect. 2015;70:37–43.
- Zhu B, Xu Z, Du P, Xu L, Sun X, Gao Y, Shao Z. Sequence type 4821 clonal complex serogroup B Neisseria meningitidis in China, 1978-2013. Emerg Infect Dis. 2015;21(6):925–32. doi:10.3201/eid2106.140687.
- Zhu BQ, Gao WY, Xu L, Gao Y, Shao ZJ. Molecular characteristics of serogroup B neisseria meningitidis, China. Zhonghua yu fang yi xue za zhi [Chin J Prev Med]. 2019;53:153–58.
- Guo Q, Mustapha MM, Chen M, Qu D, Zhang X, Chen M, Chen M, Wang M, Harrison LH. Evolution of sequence type 4821 clonal complex meningococcal strains in China from prequinolone to quinolone era, 1972-2013. Emerg Infect Dis. 2018;24(4):683–90. doi:10.3201/eid2404.171744.
- He B, Jia Z, Zhou H, Wang Y, Jiang X, Ma H, Qian Z, Liu X, Shao Z, Chen S. CC4821 serogroup W meningococcal disease in China. Int J Infect Dis. 2014;29:113–14. doi:10.1016/j.ijid.2014.08.022.
- Guo LC, Liu XC, Xu QY, Yiu YS Cai Y Jiang GQ Shao Z. Epidemiological analysis on serogroup Y neisseria meningitidis firstly isolated from patient in Tianjin. Zhonghua yu fang yi xue za zhi [Chin J Prev Med]. 2016;50:825–27.
- World Health Organization. Meningococcal vaccines: WHO position paper, November 2011. Releve epidemiologique hebdomadaire. 2011; 86:521–39.
- Borrow R, Balmer P, Miller E. Meningococcal surrogates of protection–serum bactericidal antibody activity. Vaccine. 2005;23(17–18):2222–27. doi:10.1016/j.vaccine.2005.01.051.
- Cui J, Cao L, Zheng J, Cao L, Duan M, Xiao Q. Reported coverage of vaccine in the national Immunizaiton program of China, 2015. Chin J Vaccin Immun. 2017;23:601–07.
- Goldschneider I, Gotschlich EC, Artenstein MS. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969;129(6):1307–26. doi:10.1084/jem.129.6.1307.
- Gotschlich EC, Liu TY, Artenstein MS. Human immunity to the meningococcus. 3. Preparation and immunochemical properties of the group A, group B, and group C meningococcal polysaccharides. J Exp Med. 1969;129(6):1349–65. doi:10.1084/jem.129.6.1349.
- Gold R, Lepow ML, Goldschneider I, Draper TL, Gotschlich EC. Clinical evaluation of group A and group C meningococcal polysaccharide vaccines in infants. J Clin Invest. 1975;56(6):1536–47. doi:10.1172/JCI108235.
- Leach A, Twumasi PA, Kumah S, Banya WS, Jaffar S, Forrest BD, Granoff DM, LiButti DE, Carlone GM, Pais LB. Induction of immunologic memory in Gambian children by vaccination in infancy with a group A plus group C meningococcal polysaccharide-protein conjugate vaccine. J Infect Dis. 1997;175(1):200–04. doi:10.1093/infdis/175.1.200.
- Campagne G, Garba A, Fabre P, Schuchat A, Ryall R, Boulanger D, BYBEL M, CARLONE G, BRIANTAIS P, ANOFF B. Safety and immunogenicity of three doses of a Neisseria meningitidis A + C diphtheria conjugate vaccine in infants from Niger. Pediatr Infect Dis J. 2000;19(2):144–50. doi:10.1097/00006454-200002000-00013.
- MacLennan JM, Shackley F, Heath PT, Deeks JJ, Flamank C, Herbert M, Griffiths H, Hatzmann E, Goilav C, Moxon ER. Safety, immunogenicity, and induction of immunologic memory by a serogroup C meningococcal conjugate vaccine in infants: A randomized controlled trial. Jama. 2000;283(21):2795–801. doi:10.1001/jama.283.21.2795.
- Wu C, Wang Y, Hang J, Xu J, Yuan W, Ma F, Tao H, Gong P. The efficacy and safety of A,C,W135,Y Meningococcal polysaccharide vaccines. Pract Prev Med. 2007;14:1768–70.
- Wahdan MH, Rizk F, el-Akkad AM, el-Ghoroury AA, Hablas R, Girgis NI, Amer A, Boctar W, Sippel JE, Gotschlich EC, et al. A controlled field trial of a serogroup A meningococcal polysaccharide vaccine. Bull World Health Organ. 1973;48:667–73.
- Peltola H, Makela H, Kayhty H, Jousimies H, Herva E, Hallstrom K, Sivonen A, Renkonen O-V, Pettay O, Karanko V. Clinical efficacy of meningococcus group A capsular polysaccharide vaccine in children three months to five years of age. N Engl J Med. 1977;297(13):686–91. doi:10.1056/NEJM197709292971302.
- Wei RT. Immunizing reactivity following vaccination with a purified fraction of Neisseria meningitidis and observations of effectiveness throughout 3 years. Zhonghua liu xing bing xue za zhi = Zhonghua liuxingbingxue zazhi. 1982;3:4–7.
- Ma Z. Observation on the epidemiologic efficacy for 9 years on epidemic meningococcal polysaccharide vaccine of group A by district immunization method. Zhonghua liu xing bing xue za zhi = Zhonghua liuxingbingxue zazhi. 1991;12:69–71.
- Yang J, Gong J, Liao Z. Observation on the injection reaction and immunogenicity of group A+C meningococcal polysaccharide vaccine. Applied Prev Med. 2006;12:338–42.
- Huang Z, QIan W, Yuan L, Chen Y, Cheng N, Zhou H, Li W, Yang M, Liu H. Preparation of group A+ C meningococcal polysaccharide conjugate vaccine. Chin J Biol. 2005;18:483–86.
- Peltola H, Safary A, Kayhty H, Karanko V, Andre FE. Evaluation of two tetravalent (ACYW135) meningococcal vaccines in infants and small children: a clinical study comparing immunogenicity of O-acetyl-negative and O-acetyl-positive group C polysaccharides. Pediatrics. 1985;76:91–96.
- Vodopija I, Baklaic Z, Hauser P, Roelants P, Andre FE, Safary A. Reactivity and immunogenicity of bivalent (AC) and tetravalent (ACW135Y) meningococcal vaccines containing O-acetyl-negative or O-acetyl-positive group C polysaccharide. Infect Immun. 1983;42(2):599–604. doi:10.1128/IAI.42.2.599-604.1983.
- Weintraub A. Immunology of bacterial polysaccharide antigens. Carbohydr Res. 2003;338(23):2539–47. doi:10.1016/j.carres.2003.07.008.
- Lieberman JM, Chiu SS, Wong VK, Partidge S, Chang SJ, Chiu CY, Gheesling LL, Carlone GM, Ward JI. Safety and immunogenicity of a serogroups A/C Neisseria meningitidis oligosaccharide-protein conjugate vaccine in young children. A randomized controlled trial. Jama. 1996;275:1499–503.
- Sow SO, Okoko BJ, Diallo A, Viviani S, Borrow R, Carlone G, Tapia M, Akinsola AK, Arduin P, Findlow H. Immunogenicity and safety of a meningococcal A conjugate vaccine in Africans. N Engl J Med. 2011;364(24):2293–304. doi:10.1056/NEJMoa1003812.
- Chai Z, Li J, Shao Z, Li F, Diao L, Li L, Yin Z. Minutes of the meningococcal meningitis surveillance and immunization prevention seminar. Chin Prev Med. 2015;16:901–03.
- Tao H, Li Y, Wu C, Ye Q, Hong J, Xu J, Yuan W, Ma F. Study on safety and immunogenicity of group A/C meningococcal polysaccharide conjugate vaccine. Chin J Vaccin Immun. 2009;15:531–35.
- Zhou H, Wang JY, Tan Y, Lu HY, Wang M, Cai QC, Zhang HZ. Evaluation of safety of meningococcal group AC bivalent polysaccharide conjugate vaccine in children aged 5-24 months old. Zhonghua yu fang yi xue za zhi [Chin J Prev Med]. 2013;47:920–23.
- Fu C, Huang G, Cui M, Wang M. Post-marketing study on the safety of a meningococcal group A, C bivalent polysaccharide conjugate vaccine. Hum Vaccin Immunother. 2014;10(1):138–39. doi:10.4161/hv.26347.
- Zheng J, Zhu X, Liu G, Du L. Immunogenicity and immune persistence of groups A&C meningococcal polysaccharide conjugate vaccine. Chin J Biol July. 2015;28:707–10.
- Wang YX, Tao H, Hu JL, Li JX, Dai WM, Sun JF, Liu P, Tang J, Liu W-Y, Zhu F-C. The immunogenicity and safety of a Hib-MenAC vaccine: a non-inferiority randomized, observer-blind trial in infants aged 3-5 months. Expert Rev Vaccines. 2017;16(5):515–24. doi:10.1080/14760584.2017.1303380.
- Hu JL, Tao H, Li JX, Dai WM, Song B, Sun JF, Liu P, Tang J, Liu W-Y, Wang S-Y, et al. Safety and immunogenocity of a novel combined Haemophilus influenzae type b-Neisseria meningitidis serogroups A and C-tetanus-toxoid conjugate vaccine in healthy Chinese children aged 6 months to 5 years old. Hum Vaccin Immunother. 2015;11(5):1120–28. doi:10.1080/21645515.2015.1033592.
- Li J, Yang J, Liu X, Shi K, Yang H, Zheng W, Li S, Liu A, Zhang Z. Safety of post-marketing group A and C meningococcal conjugate and haemophilus type b conjugate combined vaccine among children aged 2-71 months. Chin J Vaccin Immun. 2016;22:578–81.
- Villena R, Safadi MAP, Valenzuela MT, Torres JP, Finn A, O’Ryan M. Global epidemiology of serogroup B meningococcal disease and opportunities for prevention with novel recombinant protein vaccines. Hum Vaccin Immunother. 2018;14(5):1042–57. doi:10.1080/21645515.2018.1458175.
- Finne J, Leinonen M, Makela PH. Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development and pathogenesis. Lancet. 1983;2(8346):355–57. doi:10.1016/S0140-6736(83)90340-9.
- Holst J, Oster P, Arnold R, Tatley MV, Naess LM, Aaberge IS, Galloway Y, McNicholas A, O’Hallahan J, Rosenqvist E. Vaccines against meningococcal serogroup B disease containing outer membrane vesicles (OMV): lessons from past programs and implications for the future. Hum Vaccin Immunother. 2013;9(6):1241–53. doi:10.4161/hv.24129.
- Rappuoli R, Pizza M, Masignani V, Vadivelu K. Meningococcal B vaccine (4CMenB): the journey from research to real world experience. Expert Rev Vaccines. 2018;17(12):1111–21. doi:10.1080/14760584.2018.1547637.
- Flacco ME, Manzoli L, Rosso A, Marzuillo C, Bergamini M, Stefanati A, Cultrera R, Villari P, Ricciardi W, Ioannidis JPA. Immunogenicity and safety of the multicomponent meningococcal B vaccine (4CMenB) in children and adolescents: a systematic review and meta-analysis. Lancet Infect Dis. 2018;18(4):461–72. doi:10.1016/S1473-3099(18)30048-3.
- Parikh SR, Andrews NJ, Beebeejaun K, Campbell H, Ribeiro S, Ward C, White JM, Borrow R, Ramsay ME, Ladhani SN. Effectiveness and impact of a reduced infant schedule of 4CMenB vaccine against group B meningococcal disease in England: a national observational cohort study. Lancet. 2016;388(10061):2775–82. doi:10.1016/S0140-6736(16)31921-3.
- Perez JL, Absalon J, Beeslaar J, Balmer P, Jansen KU, Jones TR, Harris S, York LJ, Jiang Q, Radley D. From research to licensure and beyond: clinical development of MenB-FHbp, a broadly protective meningococcal B vaccine. Expert Rev Vaccines. 2018;17(6):461–77. doi:10.1080/14760584.2018.1483726.
- Marshall HS, Richmond PC, Beeslaar J, Jiang Q, Jansen KU, Garces-Sanchez M, Martinón-Torres F, Szenborn L, Wysocki J, Eiden J. Meningococcal serogroup B-specific responses after vaccination with bivalent rLP2086: 4 year follow-up of a randomised, single-blind, placebo-controlled, phase 2 trial. Lancet Infect Dis. 2017;17(1):58–67. doi:10.1016/S1473-3099(16)30314-0.
- Jia L, Yang R, Dong G. Study on improvement of national lot release regulations on biological products. Chin Pharm Affairs. 2010;24:523–30.
- National pharmacopoeia committee.The Chinese pharmacopoeia (2020 version) 3rd part. Beijing (China): Chinese Medical Science and Technology Press; 2020.
- Li Y, Zhao D, Li M, Li H, Liang L, Ye Q, Chen C. Trend analysis and quality evaluation for Group ACYW meningococcal polysaccharide vaccine in China. Chin J Biol. 2005;28:657–61.
- Cook MC, Bliu A, Kunkel JP. Quantitation of serogroups in multivalent polysaccharide-based meningococcal vaccines: optimisation of hydrolysis conditions and chromatographic methods. Vaccine. 2013;31(36):3702–11. doi:10.1016/j.vaccine.2013.05.098.
- Li Y, Zhu X, Ma Q, Cheng S, Zhao D, Wang S. Development and verification of immune rate turbidimetric method for quantitative determination of polysaccharide content in groups A and C meningococcus and Haemophilus influenzae type b combined vaccine. Chin J Biol. 2017;30:645–48.
- Wu J, Li J, Li Y, Shao Z, Li J, Zhu B, Wu D, Yao K, Liu G, Ye Q, et al. Chinese preventive medicine A. [Experts’ consensus on immunization with meningococcal vaccines in China]. Zhonghua yu fang yi xue za zhi [Chin J Prev Med]. 2019; 53:141–45.
