ABSTRACT
Introduction: Exercise holds the potential to be beneficial if used during vaccination processes by 1)exercise-induced analgesia to reduce pain associated with vaccination, 2)immune-enhancing effects, improving antibody responses to the vaccine, and 3)reducing local and systemic adverse reactions to the vaccine. This study examines whether analgesic responses could be enhanced locally in the exercising limb to further benefit the use of exercise during influenza vaccination processes to minimize vaccine-related pain and improve antibody response to inactivated influenza vaccines.
Methods: 57 participants (22.6 ± 3.2 years, 33 females) randomized into a control (n = 19) or one of two exercise groups: pre-vaccine arm (n = 19) or pre-vaccine leg (n = 19). Intervention groups performed exercise (15 minutes), prior to administration of the vaccine. Vaccine-related pain and pressure pain threshold (PPT) were measured at baseline and post-vaccination for all groups. Blood samples were taken on the day of vaccination and one month later to measure serum antibody titers to influenza.
Results: No significant difference in vaccine-related pain or change in PPT was found with exercise, however, there was a trend in higher reports of vaccine-related pain in females compared to males(p = .06). Significantly higher fold increase (p = .02) of the B/Brisbane/60/2008 strain was found in the exercise group compared to the control group.
Conclusion: The current study failed to observe an analgesic effect of exercise to improve vaccine-related pain in young adults. However, immune-enhancing effects in one of four strains suggest potential adjuvant effects of exercise. Importantly, the sex difference in pain sensitivity suggests the need for separate analysis, especially when examining pain perception.
Australian New Zealand Clinical Trial Registry (ACTRN:12617000374369)
Introduction
Vaccines are estimated to prevent 2–3 million deaths every year and are further proposed to prevent an additional 1.5 million deaths if global coverage rates improve.Citation1 Some individuals are unable to be vaccinated due to medical (immunocompromised or non-responders) reasons, hence it is vital for healthy individuals to be vaccinated to achieve the optimum coverage rate to reach effective herd immunity (80% in healthy persons and 90% in high-risk persons for influenza virusesCitation2). Recent outbreaks of vaccine-preventable diseases around the globe show evidence of barriers against achieving appropriate coverage rates. While economic barriers are predominant in developing nations, globally identified barriers, including pain, fear and anxiety associated with vaccination, contribute to the hesitancy to vaccinate.Citation3–5 However, relatively little research has attempted to address vaccine-related pain and side effects, or to identify effective interventions to improve the vaccination experience. The World Health Organization (WHO) recently recognized the need for work in this area, calling for interventions to improve the vaccination process.Citation6 While some studies have examined different interventions to improve vaccination-related processes, most work has been conducted on infants, with mixed results.Citation7
Exercise holds the potential to be beneficial if used during vaccination processes. Firstly, exercise is known to increase pain tolerance and threshold, exhibiting analgesic properties across a broad range of painful stimuli.Citation8 The effect size of aerobic exercise is reported to be moderate, and larger effect sizes have been reported for isometric and resistance exercise.Citation9 Surprisingly this analgesic effect has received little attention in the setting of vaccination pain, although we recently found beneficial effects of upper body resistance-band exercise on reported vaccine-related pain and anxiety in female adolescents receiving human papillomavirus (HPV) vaccine.Citation10 Secondly, a review of exercise-induced analgesia has found a larger effect size (>1.5) in studies using lower leg exercises compared to smaller muscle groups such as fingers or hands.Citation9 This suggests that exercising the larger muscle groups of the legs may allow a greater analgesic effect compared to exercising the smaller arm muscle groups. Interestingly, an increasing number of studies are showing analgesic effects to be enhanced locally. Two of the three published studies using isometric exercise found greater analgesic effects in the exercising limb compared to the non-exercised limb, Citation11,Citation12 while the other showed similar effects on both sides.Citation13 These findings suggest that exercising the limbs that will receive the painful stimuli may be most effective in producing an analgesic effect.
As well as improving analgesia, we recently found exercise to significantly reduce local and systemic adverse reactions to a vaccine, with more pronounced effects seen in adolescent females compared to adolescent males.Citation14 Exercise also holds the potential to improve immune response to vaccination as several studies have shown adjuvant effects, especially on less immunogenic antigens.Citation15,Citation16 Although multiples studies have reported enhanced antibody levels with exercise compared to rested control groups, no significant effects from variations in exercise tasks have been found. For example, the timing of exercise (immediately, 6 or 48 hours before vaccine administration) showed no difference in antibody response, Citation17 and the only study comparing exercise intensities found no differences in effect, although an overall effect was found for exercise compared to control.Citation18
We propose that enhanced local analgesic effects in the exercising limb may further benefit the use of exercise during vaccination processes to improve vaccine-related pain as well as improve antibody response to vaccinations. To assess this, we examined whether the analgesic effect of exercise was more pronounced when the arms, a smaller muscle group but where the vaccine is injected, is exercised compared to the legs, where a larger muscle group is exercised but not localized to the site of injection. Potential immune-enhancing effect of exercise was also examined along with any sex differences in these outcomes.
Methods
Participants
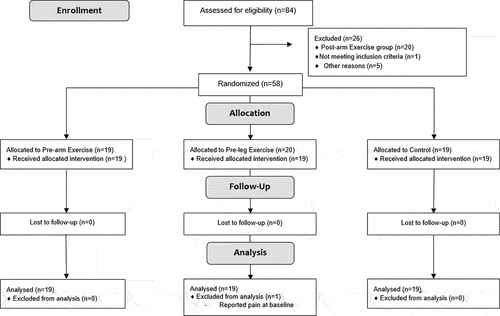
Participants were recruited from The University of Sydney, Cumberland campus during April-May 2018. A total of 84 participants were screened for their eligibility () and 78 young adults (age range: 18–30 year old; median age: 22 year old) participated in the study. Participants with any documented history of anaphylaxis to previous vaccine dose, unable to participate in exercise (according to screening using the Physical Activity Readiness Questionnaire), or who had received a dose of the influenza vaccine in the current year were excluded from the study. Written consent was given through a secure online consent form. The study was approved by the University of Sydney Human Research Ethics Committee (Protocol number: 2015/945). All participants were given an AUD$10 voucher for their participation.
Procedure
After consent, participants were randomly allocated to control (n = 19) or one of three intervention groups: pre-vaccine arm exercise (Pre-arm, n = 19), pre-vaccine leg exercise (Pre-leg, n = 20), and post-vaccine arm exercise (Post-arm, n = 20). The Post-arm group was excluded from the current analysis but was included in a previous study that examined the effects of exercise timing on other longer-term adverse events after vaccination.Citation14 The computer-generated randomization sequence and allocation were concealed from all research staff and participants until the participant attended the vaccination clinic.
Before the day of testing, participants were asked to complete a Life Events Scale for Students (LESS-v)Citation19 questionnaire as a marker of stress levels, which are associated with differences in vaccination responses, and a baseline characteristic questionnaire (age, height and weight) online. On the testing day, group allocation was revealed upon arrival, and a baseline measure of the pain of the arm was taken. Control group participants proceeded through the standard vaccination process while Pre-arm and Pre-leg groups completed the 15-minute exercise task prior to vaccine administration. All participants received the 2017 Australia Fluquadri adult vaccine (Sanofi-Aventis Australia, 0.25 mL) intramuscularly in the non-dominant deltoid muscle. A single nurse administered all vaccinations to try and minimize the variation in administration techniques which may influence vaccine-related pain.Citation20 Post-vaccination pain measures were taken immediately after the vaccine (within 1 minute following administration) was received and followed by a blood sample taken by venipuncture from the contralateral arm. The participants rested for 15 minutes prior to leaving and were asked to complete an adverse events diary for 7-days.Citation14 Another blood sample was collected one month following vaccination. Blood samples were stored at room temperature for at least 30 minutes and stored on ice until centrifugation (3000 rpm, 10 min, 7°C), and aliquoted serum was stored at −80°C for later assessment of antibody titers.
Exercise tasks
The arm exercise task used elastic resistance bands and consisted of three upper body exercises (bicep curls, lateral raises and upright rows). The leg exercise task included body-weighted lower body exercises (squats, lunges and calf raises). For both tasks, each of the three movements was performed for 30 seconds followed by 30 seconds of rest, with each movement repeated five times to a total of 15 minutes. Participants were encouraged to do as many repetitions as they could for each of the 30 second exercise periods. Participants were asked to report their Rating of Perceived Exertion (RPE) immediately after completion of the task.
Antibody titers
Plasma samples were sent the World Health Organization (WHO) Collaborating Center for Reference and Research on Influenza, Victorian Infectious Diseases Reference Laboratory where influenza-specific hemagglutinin inhibition antibody (HI) titers were performed against each vaccine strain by standardized assay. Sera were titrated (in two-fold dilutions) against the same egg-grown viruses as were contained in the vaccine (A(H1N1pdm09: IVR-180 A/Singapore/GP1908/2015 (An A/Michigan/45/2015-like virus), A(H3N2): X-263B A/Hong Kong/4801/2014, B/Victoria-lineage: B/Brisbane/60/2008, B/Yamagata-lineage: BPhuket/3073/2013) using turkey red blood cells (RBC) for the A(H1N1)pdm09 and B viruses and guinea pig RBC for the A(H3N2) virus.
Pain measures
A Visual Analogue Scale (VAS, 1–10 scale) was used to measure pain at the deltoid in the non-dominant arm (site of the vaccine injection), participants were asked to ‘rate the pain that you feel on your arm now’. Pressure pain threshold was measured afterward (within 1 minute after injection), using an FPX 50 Algometer (Wagner Instruments, Greenwich, USA). The participants were asked to indicate when they felt the pressure was painful, and the pressure noted. The PPT measures were taken three times and results averaged.
Statistical analysis
Initial analysis to examine the effect of exercising different limbs was conducted using the relevant exercise groups. Further analysis to examine the overall effect of exercise on reported pain and the antibody titer was conducted by combining the exercise groups (Pre-arm and Pre-leg). The main effect of group and sex, as well as the interaction effect of group by sex effects were examined using Generalized Linear Models using the difference between baseline and follow-up measures. Antibody titers were log-transformed due to skewed distribution. The differences between the two time points, fold differences and seroconversion (dichotomized by any fold of 4 or more) were used to test for statistical differences. Independent sample t-test was used to examine the difference in the fold increases, while seroconversion differences between groups were examined using the Chi-squared test. One participant that reported pain at baseline was excluded from the analysis of analgesia. The significance level was set at p < .05 for all analyses, and all analyses were performed using IBM SPSS 25.0 (SPSS Inc., Chicago, IL). Baseline data are reported by the mean and standard deviation, while the dependent variables are reported by the mean and 95% confidence interval.
The sample size was calculated using the effect size of a study that examined the adjuvant effect of exercise in young adults using similarly reduced dose vaccinations.Citation18 Allowing for a drop-out rate of 20%, a sample size of n = 20 per group (n = 80 total) was proposed.
Results
Participant description
Baseline characteristics of the participants, including stress levels, were not significantly different across groups, as outlined in . However, there were significant differences in height (1.8 m, 95%CI 1.8–1.8; 1.6 m, 95%CI 1.6–1.7; p < .001) and weight (76.0 kg, 95%CI 73.0–78.9; 58.2 kg, 95%CI 54.7–61.6; p < .001) between males and females, respectively. RPE between the two exercise groups was significantly different (p < .001), with higher RPE reported in the Pre-arm exercise group compared to the Pre-leg exercise group.
Table 1. Baseline characteristics of the participants (mean±SD). * = significant difference between exercise groups (p < .05)
Vaccination pain
When examining the effect of exercise (combined exercise groups vs control, ) on post-vaccination pain, there were no significant group (p = .4) or group by sex interactions (p = .6). However, there was a trend for a larger difference in pain scores from baseline to post-vaccination in females (11.2, 95%CI 9.0–13.4) than males (5.0, 95%CI 2.6–7.4, p = .06). Similarly, there were no significant group (p = .1) or group by sex (p = .10) interaction effect when examining the effect of different exercise mode (Control vs Pre-arm vs Pre-Leg).
Table 2. Vaccine-related pain (A) and pressure pain threshold (B) at baseline and post-vaccination separated by group
Pressure pain threshold (PPT)
There were no significant group (p = .4), sex (p = .9) or group by sex (p = .6) interaction effects in the change in PPT with exercise (combined exercise groups vs control, ). Similarly, no significant group (p = .6) or group by sex (p = .6) effects were found when examining the effect of exercise mode (Control vs Pre-arm vs Pre-Leg).
Antibody response to vaccination
Significantly higher fold increases in the B/Brisbane/60/2008 (B/Bris/08) strain was found in the exercise group (7.6 ± 12.9) compared to the control group (2.0 ± 11.1, p = .02, ). However, exercise did not affect the geometric mean antibody titers, fold increases or seroconversion rate against A/Singapore/GP1908/2015 (A/Sing/15), B/Phuket/3073/2013 (B/Phuket/13) or A/Hong Kong/4801/2014 (A/Hong Kong/14) viruses (). Similarly, neither of the exercise modes (Pre-arm or Pre-leg) induced any significant effect on antibody titers, fold increases or seroconversion rate of A/Sing/15, B/Phuket/13, or A/Hong Kong/14 strains. No sex effects were observed in any of the four strains (B/Bris/08, B/Phuket/13, A/Sing/15 or A/Hong Kong/14).
Table 3. Fold increases in antibody titers separated by group. * = significant difference between exercise and control group (p < .05)
Table 4. Geometric mean antibody titers (GMT) at baseline and at 1 month follow-up separated by group
Discussion
This study aimed to examine the presence of exercise analgesia, as well as the potential for pronounced local analgesic effects on the arms after vaccination. We also explored changes to the antibody titers with vaccination in response to exercise. While the analgesic effect was not found in participants that exercised, nor enhanced locally in the exercised limbs, a higher antibody response to one of the four vaccine strains was observed with exercise. A trend of females reporting higher levels of pain after vaccination compared to males was also observed.
In contrast to our hypothesis, we did not find enhanced local analgesic effects in the exercised arm. Together with the null findings of the overall effects of exercise on vaccine-related pain or pain threshold, this suggests the exercise failed to elicit an analgesic effect. While exercise is found to exhibit analgesic properties across a broad range of painful stimuli, Citation8 exercise is also suggested to cause muscle pain. For example, repeated brief contractions of the quadriceps muscle until exhaustion found pain ratings in the quadriceps to increase.Citation21 Similarly, the current study protocol asked individuals to perform as many repetitions as possible within a 30-second exercising period. Therefore, this repeated bout of exercise may have caused localized exercise-induced muscle pain independent of vaccination pain. Secondly, exercising to fatigue is also associated with muscle pain due to exercise-induced mechanical changes in the muscle (e.g. heat, noxious pressure and endogenous pain-producing substances).Citation22 In the current study, earlier onset of fatigue would have been reached by those doing the arm exercises in comparison to the leg exercises due, in part, to the relatively small muscle mass used. The observed higher RPE among participants performing the arm, compared to the leg, exercise is evidence of earlier fatigue. Future studies may examine levels of biochemical markers in the muscle associated with fatigue to identify potential correlation effects of fatigue. Furthermore, how and where the pain stimuli are applied when measuring pain should be carefully considered.
Contrary to the current findings, we have previously found arm exercise to reduce vaccine-related pain and anxiety in female adolescents administered with the HPV vaccine.Citation10 Several study factors may account for the disagreement. Firstly, influenza and HPV vaccines are known to evoke different levels of reported pain. Influenza vaccinations typically include lower rates of reported pain (10–64%)Citation23 when compared to HPV vaccination (49–83%).Citation24 Therefore, the analgesic effects of exercise may have been present with the greater rates of pain experienced with the HPV vaccine, but not the influenza vaccine. However, factors such as techniques used by the administrator of the vaccine, and handling and storing of vaccines are also known to influence pain levels and need to be interpreted with caution.Citation20,Citation25 Secondly, isometric exercises are suggested to have a stronger analgesic effect when compared to dynamic resistance exercises.Citation9 While isometric exercise is used often in studies examining analgesic effects, the use of dynamic resistance exercise is less prevalent. In contrast to the exercise used in the current study, the two studies that found analgesic effects with dynamic resistance exercise were of higher intensity (3 sets of 10 reps at 75% 1RM) and duration (45 minutes).Citation26,Citation27 The lack of research conducted using dynamic exercises limit our ability to determine the potential implications. However, the aforementioned studies suggest higher intensity and duration of exercise or isometric exercises may be needed to elicit a significant analgesic effect. Lastly, vaccine-related pain can vary dependent on various factors such as administration techniques relating to needle angle, length, site and depth of injection.Citation28 The current study tried to minimize the potential effect this may have by having one nurse administer the vaccine. However, the variability that may have been caused by this also needs to be acknowledged.
While females have lower pain tolerance and threshold compared to males, Citation29 evidence for a sex difference on the analgesic effect of exercise is divided. Three studies using different modes of exercise (track running or isometric exercises) found analgesic effects in both males and females, Citation30–32 while two studies (treadmill running and isometric exercise) found this effect was only present in females.Citation32,Citation33 This difference is suggested to be associated with biological contributions (e.g., gonadal hormones and endogenous pain modulations) as well as psychosocial influences such as gender roles.Citation34 Measuring gonadal hormones in participants or controlling for menstrual cycles and/or contraceptive use in female participants would have allowed for further investigation into the potential role of gonadal hormones but was not possible here due to financial and time constraints.
Antibody responses to vaccination is reported to be influenced by psychological status, Citation35 genetics, Citation36 sex, Citation37 age, Citation38 as well as activity levels.Citation39 While psychological stress levels are negatively associated with peak antibody titers in the young and the elderly, Citation35 the stress level of participants in the current study at baseline did not differ. Enhanced antibody response to vaccination in fit or active individuals participating in regular exercise is evident, Citation39,Citation40 however, the effect of an acute exercise bout is less clear. Currently, the literature suggests exercise intensity and sex influences immune-enhancing effects of exercise.Citation15,Citation16 While we found weak evidence for an improved antibody response following exercise in one of the four antibody strains, we did not observe any sex differences. In contrast, other studies have shown greater antibody responses with exercise in females but not in malesCitation16 and in males but not females and only for the weakest strain.Citation15 Influence of thymus-dependent and independent antigens eliciting a different response in males and females has been proposed as a possible cause.Citation15,Citation16,Citation41 Exercise intensity is also suggested to play a role as the immune-enhancing effects were not seen in ‘very light’ intensity exercise, Citation15 but benefits were observed with ‘moderate’ intensity exercise.Citation26,Citation27 Indeed, the authors of the study using ‘very light’ intensity exercise acknowledged that the intensity of exercise might not have been sufficient to induce a response. Limited studies on the acute effects of exercise restrict our ability to draw conclusions based on these findings and warrant further research on why sex and strain effects are observed inconsistently between studies and provide a better understanding of the benefits of exercise to antibody and pain responses.
Limitations
Pain is influenced by physiological factors as well as psychological factorsCitation42 and is difficult to measure. Although the use of VAS and PPT to measure pain and pain thresholds are common, there are still limitations to each measure. Firstly, it is difficult to differentiate pain from other factors that may influence the outcome, such as anxiety and vigilance, when using psychophysical methods such as VAS.Citation43 Secondly, although pressure algometry is the most frequently applied technique for quantification of pain, the results are influenced by several factors. These include the need to accurately locate the anatomical structure that is being tested, correct application of pressure (perpendicular), uniform size of instrument surface in contact with tissue, consistent rate of pressure increase, patterned instructions to examination subjects, and a uniform endpoint.Citation44 However, as these two instruments are the most commonly used methods to measure pain, the current study utilized these methods. Thirdly, because gonadal hormones may play a role in sex differences in pain responses, controlling for menstrual cycle and contraceptive use in female participants or analyzing salivary levels are suggested for future studies. Lastly, due to the novelty of the study, the sample size calculation was based on the effect size of a study examining the effects of exercise with reduced vaccination dosage in young adults.Citation18 The small sample size of the current study needs to be acknowledged, giving rise to the potential for type II error.
Conclusion
The current study failed to show the analgesic effects of exercise to improve vaccine-related pain in young adults receiving an inactivated influenza vaccine. However, immune-enhancing effects in one of four antibody strains suggested potential adjuvant effects of exercise. Future study designs examining exercise analgesia should ensure exercise protocols are designed to consider the potential effect of muscle size and fatigue. Importantly, the sex difference in pain sensitivity suggests the need for separate analysis, especially when examining pain sensations.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors would like to thank Malet Aban for technical assistance in performing the HI assays. The Melbourne WHO Collaborating Centre for Reference and Research on Influenza is supported by the Australian Government Department of Health.
Additional information
Funding
References
- Immunization coverage: fact sheet. World Health Organization (WHO); 2017 [accessed 2017 Jul]. http://www.who.int/news-room/fact-sheets/detail/immunization-coverage.
- Plans-Rubió P. The vaccination coverage required to establish herd immunity against influenza viruses. Prev Med. 2012;55:72–77.
- Kimmel SR, Burns IT, Wolfe RM, Zimmerman RK. Addressing immunization barriers, benefits, and risks. J Fam Pract. 2007;56(2 Suppl Vaccines):S61–9.
- Jani JV, De Schacht C, Jani IV, Bjune G. Risk factors for incomplete vaccination and missed opportunity for immunization in rural Mozambique. BMC Public Health. 2008;8(1):161. doi:10.1186/1471-2458-8-161.
- Favin M, Steinglass R, Fields R, Banerjee K, Sawhney M. Why children are not vaccinated: a review of the grey literature. Int Health. 2012;4(4):229–38. doi:10.1016/j.inhe.2012.07.004.
- World Health Organization. Reducing pain at the time of vaccination: WHO position paper – September 2015. Weekly Epidemiological Rec. Relevé épidémiologique hebdomadaire. 2015;90(39):505–10.
- Lee VY, Caillaud C, Fong J, Edwards KM. Improving vaccine-related pain, distress or fear in healthy children and adolescents–a systematic search of patient-focused interventions. Hum Vaccin Immunother. 2018;14(11):2737–47.
- Koltyn KF. Analgesia following exercise. Sports Med. 2000;29(2):85–98. doi:10.2165/00007256-200029020-00002.
- Naugle KM, Fillingim RB, Riley JL. A meta-analytic review of the hypoalgesic effects of exercise. J Pain. 2012;13(12):1139–50. doi:10.1016/j.jpain.2012.09.006.
- Lee VY, Booy R, Skinner R, Edwards KM. The effect of exercise on vaccine-related pain, anxiety and fear during HPV vaccinations in adolescents. Vaccine. 2018;36(23):3254–59. doi:10.1016/j.vaccine.2018.04.069.
- Persson AL, Hansson G-Å, Kalliomäki J, Moritz U, Sjölund BH. Pressure pain thresholds and electromyographically defined muscular fatigue induced by a muscular endurance test in normal women. Clin J Pain. 2000;16(2):155–63. doi:10.1097/00002508-200006000-00009.
- Kosek E, Lundberg L. Segmental and plurisegmental modulation of pressure pain thresholds during static muscle contractions in healthy individuals. Eur J Pain. 2003;7(3):251–58. doi:10.1016/S1090-3801(02)00124-6.
- Lemley KJ, Hunter SK, Bement MKH. Conditioned pain modulation predicts exercise-induced hypoalgesia in healthy adults. Med Sci Sports Exerc. 2015. doi:10.1249/MSS.0000000000000381.
- Lee V, Booy R, Skinner SR, Fong J, Edwards KM. The effect of exercise on local and systemic adverse reactions after vaccinations–outcomes of two randomized controlled trials. Vaccine. 2018;36(46):6995–7002. doi:10.1016/j.vaccine.2018.09.067.
- Edwards KM, Burns VE, Adkins AE, Carroll D, Drayson M, Ring C. Meningococcal A vaccination response is enhanced by acute stress in men. Psychosom Med. 2008;70(2):147–51. doi:10.1097/PSY.0b013e318164232e.
- Edwards KM, Burns VE, Reynolds T, Carroll D, Drayson M, Ring C. Acute stress exposure prior to influenza vaccination enhances antibody response in women. Brain Behav Immun. 2006;20(2):159–68. doi:10.1016/j.bbi.2005.07.001.
- Campbell JP, Edwards KM, Ring C, Drayson MT, Bosch JA, Inskip A, Long JE, Pulsford D, Burns VE. The effects of vaccine timing on the efficacy of an acute eccentric exercise intervention on the immune response to an influenza vaccine in young adults. Brain Behav Immun. 2010;24(2):236–42. doi:10.1016/j.bbi.2009.10.001.
- Edwards KM, Campbell JP, Ring C, Drayson MT, Bosch JA, Downes C, Long JE, Lumb JA, Merry A, Paine NJ, et al. Exercise intensity does not influence the efficacy of eccentric exercise as a behavioural adjuvant to vaccination. Brain Behav Immun. 2010;24(4):623–30. doi:10.1016/j.bbi.2010.01.009.
- Clements K, Turpin G. The life events scale for students: validation for use with British samples. Pers Individ Dif. 1996;20(6):747–51. doi:10.1016/0191-8869(96)00005-0.
- Taddio A, Ilersich AL, Ipp M, Kikuta A, Shah V. Physical interventions and injection techniques for reducing injection pain during routine childhood immunizations: systematic review of randomized controlled trials and quasi-randomized controlled trials. Clin Ther. 2009;31:S48–S76. doi:10.1016/j.clinthera.2009.07.024.
- Kadetoff D, Kosek E. The effects of static muscular contraction on blood pressure, heart rate, pain ratings and pressure pain thresholds in healthy individuals and patients with fibromyalgia. Eur J Pain. 2007;11(1):39–39. doi:10.1016/j.ejpain.2005.12.013.
- O’Connor PJ, Cook DB. 5 exercise and pain: the neurobiology, measurement, and laboratory study of pain in relation to exercise in humans. Exerc Sport Sci Rev. 1999;27:119–66.
- Information sheet - observed rate of vaccine reactions: influenza vaccine. The World Health Organization (WHO). 2012 [accessed 2017 Jul]. http://www.who.int/vaccine_safety/initiative/tools/Influenza_Vaccine_rates_information_sheet.pdf?ua=1.
- Macartney KK, Chiu C, Georgousakis M, Brotherton JML. Safety of human papillomavirus vaccines: a review. Drug Saf. 2013;36(6):393–412. doi:10.1007/s40264-013-0039-5.
- Taddio A, Appleton M, Bortolussi R, Chambers C, Dubey V, Halperin S, Hanrahan A, Ipp M, Lockett D, MacDonald N, et al. Reducing the pain of childhood vaccination: an evidence-based clinical practice guideline. Cmaj. 2010;182(18) E843-E855. doi: 10.1503/cmaj.092048.
- Focht BC, Koltyn KF. Alterations in pain perception after resistance exercise performed in the morning and evening. J Strength Conditioning Res. 2009;23(3):891–97. doi:10.1519/JSC.0b013e3181a05564.
- Koltyn KF, Arbogast RW. Perception of pain after resistance exercise. Br J Sports Med. 1998;32(1):20–24. doi:10.1136/bjsm.32.1.20.
- Petousis-Harris H. Vaccine injection technique and reactogenicity—evidence for practice. Vaccine. 2008;26(50):6299–304. doi:10.1016/j.vaccine.2008.08.052.
- Riley Iii JL, Robinson ME, Wise EA, Myers CD, Fillingim RB. Sex differences in the perception of noxious experimental stimuli: a meta-analysis. Pain. 1998;74(2):181–87. doi:10.1016/S0304-3959(97)00199-1.
- Hoeger MKB, DICAPO J, RASIARMOS R, HUNTER SK. Dose response of isometric contractions on pain perception in healthy adults. Med Sci Sports Exerc. 2008;40(11):1880–89. doi:10.1249/MSS.0b013e31817eeecc.
- Umeda M, Newcomb LW, Ellingson LD, Koltyn KF. Examination of the dose-response relationship between pain perception and blood pressure elevations induced by isometric exercise in men and women. Biol Psychol. 2010;85(1):90–96. doi:10.1016/j.biopsycho.2010.05.008.
- Sternberg WF, Boka C, Kas L, Alboyadjia A, Gracely RH. Sex-dependent components of the analgesia produced by athletic competition. J Pain. 2001;2(1):65–74. doi:10.1054/jpai.2001.18236.
- Koltyn KF, TRINE MR, STEGNER AJ, TOBAR DA. Effect of isometric exercise on pain perception and blood pressure in men and women. Med Sci Sports Exerc. 2001;33(2):282–90. doi:10.1097/00005768-200102000-00018.
- Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL. Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain. 2009;10(5):447–85. doi:10.1016/j.jpain.2008.12.001.
- Pedersen AF, Zachariae R, Bovbjerg DH. Psychological stress and antibody response to influenza vaccination: a meta-analysis. Brain Behav Immun. 2009;23(4):427–33. doi:10.1016/j.bbi.2009.01.004.
- Kimman T, Vandebriel R, Hoebee B. Genetic variation in the response to vaccination. Public Health Genomics. 2007;10(4):201–17. doi:10.1159/000106559.
- Flanagan KL, Fink AL, Plebanski M, Klein SL. Sex and gender differences in the outcomes of vaccination over the life course. Annu Rev Cell Dev Biol. 2017;33(1):577–99. doi:10.1146/annurev-cellbio-100616-060718.
- Seidman JC, Richard SA, Viboud C, Miller MA. Quantitative review of antibody response to inactivated seasonal influenza vaccines. Influenza Other Respi Viruses. 2012;6(1):52–62. doi:10.1111/j.1750-2659.2011.00268.x.
- Stewart A, Vanderkooi OG, Reimer RA, Doyle-Baker PK. Immune response in highly active young men to the 2014/2015 seasonal influenza vaccine. Appl Physiol Nutr Metab. 2018;43(8):769–74. doi:10.1139/apnm-2017-0683.
- Keylock KT, Lowder T, Leifheit KA, Cook M, Mariani RA, Ross K, Kim K, Chapman-Novakofski K, McAuley E, Woods JA, et al. Higher antibody, but not cell-mediated, responses to vaccination in high physically fit elderly. J Appl Physiol. 2007;102(3):1090–98. doi:10.1152/japplphysiol.00790.2006.
- Edwards KM, Burns VE, Allen LM, McPhee JS, Bosch JA, Carroll D, Drayson M, Ring C. Eccentric exercise as an adjuvant to influenza vaccination in humans. Brain Behav Immun. 2007;21(2):209–17. doi:10.1016/j.bbi.2006.04.158.
- Calvino B, Grilo RM. Central pain control. Joint Bone Spine. 2006;73:10–16.
- Staahl C, Drewes AM. Experimental human pain models: a review of standardised methods for preclinical testing of analgesics. Basic Clin Pharmacol Toxicol. 2004;95(3):97–111. doi:10.1111/j.1742-7843.2004.950301.x.
- Fischer AA. Introduction: Pressure algometry in quantification of diagnosis and treatment outcome. Journal of Musculoskeletal Pain 1998;6(1):1–3.