ABSTRACT
Background Rotavirus infections, prevalent in human populations, are caused mostly by group A viruses. Immunization against rotaviruses in infancy is currently the most effective and economical strategy to prevent rotavirus infection. This study evaluated the safety of a novel hexavalent rotavirus vaccine and analyzed its dose and immunogenicity.
Methods This randomized, double-blinded, placebo-controlled phase I clinical trial enrolled healthy adults, toddlers, and infants in Zhengding County, Hebei Province, northern China. 40 adults and 40 children were assigned in a 2:1:1 ratio to receive one vaccine dose, placebo 1, and placebo 2, respectively. 120 6–12 week old infants were assigned equivalently into 3 groups. The infants in each group were assigned in a 2:1:1 ratio to receive three doses of vaccine, placebo 1, and placebo 2, at a 28-day interval. Adverse events (AEs) until 28 days after each dose and serious adverse events (SAEs) until 6 months after the third dose were reported. Virus shedding until 14 days after each dose in infants was tested. Geometric mean concentrations (GMCs) and seroconversion rates were measured for anti-rotavirus IgA by using an enzyme-linked immunosorbent assay (ELISA).
Results The solicited and unsolicited AE frequencies and laboratory indexes were similar among the treatment groups. No vaccine-related SAEs were reported. The average percentage of rotavirus vaccine shedding in the infant vaccine groups was 5.00%. The post-3rd dose anti-rotavirus IgA antibody geometric mean concentrations (GMC) and seroconversion rate were higher in the vaccine groups than in the placebo groups.
Conclusions The novel oral hexavalent rotavirus vaccine was generally well-tolerated in all adults, toddlers and infants, and the vaccine was immunogenic in infants.
KEYWORDS:
1. Introduction
Rotaviruses are the most common cause of severe diarrheal disease in young children throughout the world. According to the World Health Organization (WHO), in 2013, approximately 215, 000 children aged 5 years or younger died from vaccine-preventable rotavirus infections, the vast majority of these children lived in low-income countries.Citation1,Citation2 It was estimated that 12.1 million diarrhea episodes among Chinese children under 5 years old were attributable to rotavirus infections in 2007 which resulted in 3.5 million outpatient visits and 220,000 hospital admissions.Citation3 From 2003 to 2012, the total number of deaths caused by rotavirus disease among Chinese children was 53,559.Citation4
Considering that rotavirus diarrheal disease is a significant public health problem, especially in low-income countries, the WHO recommends universal immunization with rotavirus vaccines.Citation5 Currently, there are four WHO-prequalified rotavirus vaccines. A pentavalent human-bovine (WC3) reassortant live attenuated rotavirus vaccine (RotaTeq®, Merck & Co., Inc.,Kenilworth, NJ, USA), a live-attenuated monovalent human rotavirus vaccine (Rotarix®, GlaxoSmithKline, Belgium), a live-attenuated monovalent human rotavirus 116E vaccine (Rotavac®, Bharat Biotech, India) and a live-attenuated pentavalent bovine-human rotavirus reassortant (RotasiilTM, Serum Institute of India, India).Citation6 Since 2006, more than 100 countries have introduced rotavirus vaccine into their immunization programs,Citation7 but 57% of all children worldwide still lack access to rotavirus vaccines.Citation8 In China, a domestic Lanzhou lamb rotavirus (LLR) vaccine has been available in the local market since 2000. Since RotaTeq® was licensed in 2019 in China. Nevertheless, cost-effective and safe rotavirus vaccines for the countries that need them the most remain an overwhelming need. Recently, using indigenous manufacturing technology at Wuhan Institute of Biological Products Co., Ltd. (WIBP) in China, another hexavalent rotavirus vaccine (serotypes G1, G2, G3, G4, G8, and G9) candidate was developed, which is based on the US National Institutes of Health (NIH) bovine-human reassortant rotavirus strain using human rotaviruses corresponding to serotypes G1-G4, G8, and G9 as donors of VP7 gene and the UK Compton strain of bovine rotavirus as the donor of the remaining 10 genes.Citation9 The bovine UK strain shares the VP4 genotype specificity with the bovine WC3 rotavirus strain.Citation10–12 These reassortant strains (G1-G4) have been evaluated for safety and immunogenicity in phase I and II clinical studies, and have been shown to be safe and effective.Citation13–15 The new rotavirus vaccine (serotypes G1, G2, G3, G4, G8, and G9) used in our research has been shown to be safe and highly immunogenic in animals and was approved for human trials by the China Medical Products Administration. Here, we report the findings from a phase I clinical trial of the vaccine candidate conducted in China.
2. Methods
2.1 Study design and participants
This study was a randomized, double-blinded, placebo-controlled, single-center phase I clinical trial. Healthy adults aged >18 years, healthy children aged 2–6 years and healthy infants aged 6–12 weeks living in Zhengding County, Hebei Province, China, were enrolled. Subjects were excluded if they had any history of congenital abdominal disorders, rotavirus gastroenteritis, intussusception (IS), chronic diarrhea, failure to thrive, or abdominal surgery; were immunocompromised; had received previous rotavirus vaccination; had any clinical evidence of active gastrointestinal illness; or had received systemic corticosteroid treatment, blood transfusion or blood products. Participants with fever (axillary temperature ≥37.1°C) at the time of vaccination or within the prior 72 h (axillary temperature ≥38.5°C) were deferred for vaccination.
Eligible healthy adults and children were randomized in a 2: 1:1 ratio to receive a single high-dose level (l × 106.5 FFU) of vaccine, placebo 1 (without antacid), or placebo 2 (with antacid). A total of 120 healthy infants aged 6–12 weeks received three doses of vaccine, placebo 1 or placebo 2, with the first dose administered at visit 1 and subsequent doses administered at 4-week intervals. The dose-escalation phase was designed to test three dose levels (l × 105.0 FFU, 1 × 106.0 FFU, and 1 × 106.5 FFU) of the vaccine. The researcher assessed safety findings and approved enrollment in the next stage in the absence of contraindications until vaccination of all dose groups was completed.
The study was approved by the Institutional Review Board of Hebei Center for Disease Control and Prevention and conducted in compliance with Good Clinical Practice, the Declaration of Helsinki, and local regulations in China. Parents/guardians of eligible study participants or the subjects themselves signed written informed forms consent before any study-related procedures were performed. The clinical trial is registered at National Medical Products Administration (NMPA) under number CRT20150878.
2.2 Randomization and blinding
Randomization was performed using a computer-generated randomization schedule created by a statistician who was not involved with the study. At visit 1 (day 0), a randomization number was assigned to each participant in a sequential manner as they became eligible for randomization. Each vaccine or placebo was labeled with a randomization number and unique participant identifier number. Trial participants and study site staff were not blinded to the dose level, but they were blinded to the vaccine and placebo. Laboratory analyses were performed in a blinded manner throughout the study.
2.3 Vaccines
The experimental vaccine used in the study was the live attenuated hexavalent bovine-human reasssortant rotavirus (G1, G2, G3, G4, G8, and G9) vaccine. Wuhan Institute Biological Products Co., Ltd (WIBP) obtained the right to use the second-generation hexavalent BRV(UK) vaccine originally designed by Kapikian from NIH and WIBP started to work on it in 2007. In 2009, WIBP established a 3-level virus seed. Each monovalent vaccine bulk was produced by expanding Vero cells from a frozen vial through a series of expansion steps, and the supernatant of the infected cells was harvested to yield harvested virus fluid (HVF). The HVF was clarified by microfiltration. This filtrate was concentrated by ultrafiltration and then sterile filtered through a 0.2 μm membrane. The resulting filtered virus fluids (FVF) were frozen and stored at ≤ −25°C. The FVF was thawed and redispensed into aliquots appropriately sized for use in the formulation and filling process. Three vaccine dose levels were assessed (low-dose: 1 × 105.0 FFU, intermediate-dose: 1 × 106.0 FFU, and high-dose: 1 × 106.5 FFU). Each dose of vaccine consisted of 2 ml. Two types of placebos were used in the trial: placebo 2 contained the same constituents as the vaccine without viral antigens, and the only difference between placebo 1 and placebo 2 was that placebo 1 did not contain antacids.
2.4 Assessment of safety
Subjects were observed for 30 min to monitor for any immediate adverse events (AEs) after the administration of the vaccine or placebo. Thereafter, subjects were given a thermometer and a diary card covering days 0–14 for safety follow-up. They were instructed to observe and record their axillary temperature daily and any AEs on the card for 14 days. The subjects were asked to return to the research site on day 15 after vaccination. Thereafter, contact cards were given to the subjects to collect the safety information for days 15–28. Serious adverse events (SAEs) were collected 6 months after receiving the full course of vaccination. Additionally, laboratory indexes of healthy adults and children were determined before the first dose and 4 days after vaccination by analyzing blood and urine samples. Serum glutamic-pyruvic transaminase (AST) levels, bilirubin levels, blood urea nitrogen (BUN) levels, creatinine levels, white blood cell (WBC) counts, platelet counts, and hemoglobin levels were measured. Albuminuria and red blood cell (RBC) counts in urine were assessed. All the tests were performed at local hospital.
Stool samples were collected from the infant cohort at 1–14 days after the administration of any dose to observe vaccine-strain rotavirus replication in the intestinal tract. A 3–5 g stool sample was collected. After collecting a stool sample, the investigator transported the stool sample to Zhengding Country Center for Disease Control and Prevention in a cooler box at a temperature of less than 8°C. Samples were stored at −20°C after packing until they were sent to the reference laboratory for testing. Rotavirus antigen was tested in fecal samples by enzyme-linked immunosorbent assay (ELISA) with a ProSpecT Rotavirus Kit performed by National Institute for Food and Drug Control (NIFDC). If a sample was found to be vaccine rotavirus-positive by ELISA, it will be done RNA extraction and RT-PCR by a commercial kit ((QIAamp® viral RNA mini kit). The VP7 and VP6 will be sequenced. The sequence of the VP7 determined the G serotypes of the rotavirus. The sequence of VP6 gene determined whether the rotavirus is a vaccine strain.Citation16
2.5 Assessment of immunogenicity
Serum samples were collected to determine immunological responses evaluated by anti-rotavirus IgA antibody geometric mean concentrations (GMCs) and seroconversion rates, which were determined only in the infants. Approximately 2.5–3.0 ml serum samples were collected from each infant participant at baseline and 28 days post-dose 3 (PD3) of the study vaccinations. Sera were separated at Zhengding Center for Disease Control and Prevention (CDC) laboratories and stored at −20°C. All serum samples were tested for IgA antibody in the reference laboratory at the NIFDC, Beijing, China. The rotavirus IgA antibody responses were measured with an in-house-developed enzyme-linked immunosorbent assay kit (developed by NIFDC). Mouse monoclonal antibodies (mAb) against each serotype of rotavirus vaccine strain used as coating antibodies were obtained from Murdoch Institute, Australia. IgA serum control against rotavirus was obtained from Christian Medical College, Vellore, India. The antigen used for the ELISA was rotavirus vaccine strains of the G1, G2, G3, G4, G8and G9 serotypes propagated in Vero cells. Wells of a 96-well microplate coated with the mAb were incubated with different rotavirus serotypes, followed by sequential incubations with human serum samples, biotin-conjugated goat serum against human IgA, avidin biotin peroxidase complex and TMB.
2.6 Statistical analysis
All analyses were performed using SAS version 9.2. All statistical tests were two-sided and differences resulting in p values of 0.05 or less were considered statistically significant. Student’s t-test or the Mann-Whitney U test was used to assess dimensional outcomes, and the chi-square or Fisher’s exact test was used to analyze dichotomous outcomes. Antibody seroconversion rates, GMCs, and their 95% confidence intervals were calculated. Antibody levels were log-transformed to calculate GMCs. Seroconversion rates were defined as anti-rotavirus IgA antibody concentration ≥20 U/ml in initially seronegative infants and a fourfold rise in concentration from baseline to PD3. An anti-rotavirus IgA antibody concentration<20 U/ml was calculated as 10 U/ml to count the GMCs.
3. Results
3.1 Demographic and other baseline characteristics
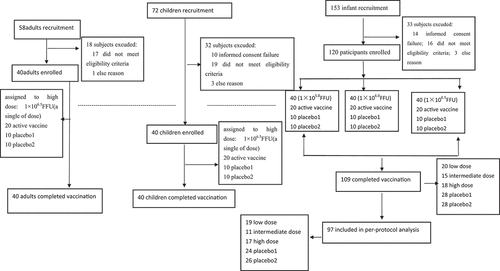
In total, 283 volunteers participated in interviews and physical examination for this trial. Of these volunteers, 83 were excluded due to failure to sign the informed consent form, meeting the exclusion criteria, and other reasons. 200 subjects (40 adults, 40 children, and 120 infants) were enrolled in the trial . show that vaccine and placebo groups were comparable regarding demography and baseline characteristics, even though a statistically significant difference (p = .02) between genders was observed in the toddler cohort. All subjects in the adult and child groups completed the follow-up, and 11 infant subjects were lost to follow-up, resulting in a final total of 90.83% (109/120) infant participants receiving three doses of vaccine or placebo. Twelve subjects who failed to follow the protocol were excluded, and 97 infants were included in the immunogenicity analysis.
Table 1. Summary of demographic characteristics-adult cohort
Table 2. Summary of demographic characteristics-toddler cohort
Table 3. Summary of demographic characteristics-infant cohort
3.2 Vaccine safety
A summary of the AEs (including solicited AEs and unsolicited AEs) reported during the 0–28 day post-vaccination period in the adult and child groups are provided in . Similar incidence rates of solicited AEs were observed in the vaccine group and the placebo group. No grade 3 solicited AEs or SAEs were reported in any of the groups. Except for a few minor changes, the hematology, biochemistry, and urine analysis results remained normal in all the groups. Among these minor changes, three adults presented abnormalities (one with an abnormal routine blood analysis, one with an abnormal blood biochemical analysis and one with an abnormal urine routine analysis), five children presented abnormalities, including four with abnormal routine blood analyses and one with an abnormal routine urine analysis.
Table 4. Summary of adverse enents (AEs) –adult and toddler cohort
In total, 106 (88.33%) infant participants reported at least one AE within 28 days following any vaccination, 19 (95.00%) in the low-dose group, 18 (90.00%) in the intermediate-dose group, 19 (95.00%) in the high-dose group, 26 (86.67%) in placebo 1 group and 24 (80.00%) in the placebo 2 group. At least one solicited AE was recorded in 79 infants (65.83%) among the whole group. 12 infants (60.00%) from the low-dose group, 15 infants (75.00%) from the intermediate-dose group, 16 infants (80.00%) from the high-dose group, 17 infants (56.67%) from the placebo1 group and 19 infants (63.33%) from the placebo 2 group reported at least one solicited AE during the 14-day postvaccination follow-up period. At least one unsolicited AE was recorded in 78 infants (65.00%) in the whole group. There were 13 infants (65.00%) in the vaccine groups, 21 infants (70.00%) from the placebo 1 group and 18 infants (60.00%) from placebo 2. There were five SAEs in the infant cohort, including one case of acute laryngitis in the low-dose group, one case of acute asthmatic bronchitis and one case of upper respiratory infection in the intermediate-dose group, and two cases of bronchopneumonia in the placebo 1 group, which were not adjudged to be related to the vaccine. A total of seven participants refused the subsequent vaccine dose due to an AE ().
Table 5. Summary of adverse enents (AEs) –infant cohort
AEs of special interest are shown in . Fever (50.00% in low-dose, 50.00% in intermediate-dose, 70.00% in high-dose, 40.00% in placebo 1 and 50.00% in placebo 2) and diarrhea (15.00% in low-dose, 40.00% in intermediate-dose, 40.00% in high-dose, 30.00% in placebo 1 and 23.33% in placebo 2) were the most common solicited AEs among all the whole groups. There was no statistically significant difference between the five groups with respect to the incidences of fever, diarrhea, vomiting, anorexia, irritability/abnormal crying, drowsiness, or allergy.
Table 6. Summary of solicited adverse enents (AEs) –infant cohort
3.3 Stool sample analyses: vaccine- strain rotavirus shedding
Stool specimens were collected from the infant participants every day during the 14-day post-vaccination follow-up period. Virus shedding was observed in 1 subject in the low-dose group on days 5 and 9 after the administration of the first dose. Two subjects in the high-dose group had virus shedding on days 1 and 2 after the administration of the first dose. No rotavirus shedding was observed in the intermediate-dose group or placebo groups. The average percentage of rotavirus vaccine shedding in the infant vaccine groups was 5.00% (3/60). Sequencing results showed that the rotaviruses detected in stools samples were vaccine strains.
3.4 Immunogenicity
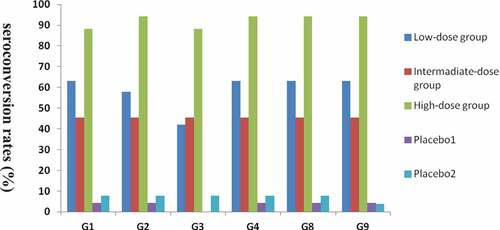
Seroconversion rates for IgA antibody to each rotavirus serotype in the vaccine were significantly higher in the vaccine groups than in the placebo groups. The anti-rotavirus IgA seroconversion rate of the high-dose group reached approximately 90.00%. The seroconversion rate of the intermediate-dose group was 45.45% for all rotavirus serotypes, and that of the low-dose group ranged from 42.11% (G3) to 63.16% (G1, G2, G4, G8, and G9). The seroconversion rates for IgA antibody in the high-dose group were higher than those in the low-dose group and intermediate-dose group, and no significant difference was found between the low-dose group and the intermediate-dose group .
Figure 2. Seroconversion rates in serum at 28 days after the last vaccination in vaccine and placebo groups (infants)
The baseline GMCs of anti-rotavirus IgA were comparable between the five infant groups. The GMC was higher than 153.46 U/ml (except G3) in the high-dose group, ranged from 31.23 U/ml (G3) to 49.46 U/ml (G4) in the intermediate-dose group, ranged from 25.90 U/ml (G3) to 41.88 U/ml (G9) in the low-dose group, whereas it was approximately 10.0 U/ml in the placebo1 and placebo 2 groups (). The post-dose 3 (PD3) GMCs of anti-rotavirus IgA among vaccine recipients were generally higher than among placebo recipients; additionally, the GMCs of high-dose group were higher than the low-dose group and intermediate-dose group, and a significant difference was not observed between the low-dose group and the intermediate-dose group.
Table 7. Summary of IgA response to serotypes G1, G2, G3, G4, G8 and G9 in the per-protocol immunogenicity popolation–infant cohort
4. Discussion
This phase I clinical study is the first to evaluate the safety and immunogenicity of a novel hexavalent (G1, G2, G3, G4, G8, and G9) rotavirus vaccine in healthy adults, toddlers, and infants. According to relevant regulations,Citation17 vaccination for children and infants in the target population should be conducted in the order of adults, children, and infants due to their low tolerance to adverse reactions. The safety of the vaccine was first evaluated in healthy adults and toddlers to provide sufficient safety data to allow the study to proceed in infant subjects. The rotavirus vaccine is not indicated for adults and toddlers, therefore, only a single high dose of vaccine was assigned to evaluate the safety of the vaccine. In the adult and toddler groups, the same incidence of AEs was observed in the vaccine group as that in the placebo group. Consistent with other studies in adults,Citation12,Citation18–20 no grade 3 AEs or SAEs occurred in any group, and no clinically significant changes in the hematological, biochemical, or urine parameters were observed in any subjects.
In the infant cohort, an important target population, we not only evaluated the safety but also conducted immunogenicity evaluations. In analyses of all AEs, there was no statistically significant difference between the vaccine groups (the high-dose, intermediate-dose, and low-dose groups) and placebo groups (the placebo 1 and placebo 2 groups) with respect to the incidences of AEs. Moreover, most AEs resolved shortly after their onset, which is in accordance with the results of a study by Vibhu et. al.Citation21 The rate of a least one recorded solicited AE was 80.00% in the high-dose group, a value that was higher than the rates observed in the other groups, but the difference was not statistically significant. Fever was the most frequently solicited AE in all groups, accounting for more than 66.67% of reports. Five subjects (low-dose group: 1, intermediate-dose group: 2, placebo 1: 2) reported SAEs, and no SAE was considered vaccine-related in vaccine recipients. Consistent with the results of safety evaluations of other rotavirus vaccines,Citation22–30 the incidence of AEs in the vaccine group was not higher than the placebo group. Correspondingly, no cases of intussusception or death were reported in the study.
In the present study, two placebo controls were designed. Placebo 2 contained antacids that were not present in placebo 1. The purpose was to assess the safety and tolerance of placebo 1 and placebo 2. The results showed that there was no significant difference in solicited AE and unsolicited AE rates in placebo 1 and 2 in the adult, children, and infant groups in the phase I clinical study. Placebo 2 with an antacid was safe and well tolerated in subjects.
Although serum IgA is not a correlate of protection, it acts as the best available measure of seroconversion so far and has been used to measure vaccine immunogenicity of candidate rotavirus vaccines.Citation12,Citation31,Citation32 The immunogenicity of two rotavirus vaccines (Rotarix and RotaTeq) have been evaluated in numerous phase I, II, and III studies in Chinese infants.Citation8,Citation33 Rong-cheng Li et al. showed that the anti-RV IgA seroconversion rate was 74.7% with RIX4414 rotavirus vaccine,Citation33 meanwhile, the study by Zhaojun Mo showed that the anti-RV IgA seroconversion rate was 89.4% with pentavalent rotavirus vaccine (RotaTeqTM).Citation8 In our study, the anti-rotavirus IgA seroconversion rate of the high-dose group reached approximately 90.00%. The seroconversion rate of the intermediate-dose group was 45.45% for all rotavirus serotypes, and that of the low-dose group ranged from 42.11% (G3) to 63.16% (G1, G4, G8, and G9). The recipients of the highest antigen concentration (the high-dose vaccine group) displayed the maximum seroconversion rate for IgA antibodies, whereas the placebo group showed the minimum seroconversion rate, and no difference was found between the low-dose group and the intermediate-dose group. The inability to establish a positive dose-response for the IgA antibodies may be due to the limited sample size. However, the main purpose of a phase I clinical trial is to observe the safety and preliminary immunogenicity, and the immunogenicity of the vaccine will be further assessed in a phase II clinical trial.
As a live vaccine, transmission of vaccine virus strains from vaccinated children to unvaccinated contacts harbors the potential for herd immunity, but also the risk of vaccine-derived disease in immunocompromised contacts.Citation34 In terms of fecal shedding, it has been previously reported that for RotaTeq, vaccine-virus shedding occurs in approximately 10% of the recipients after the first dose and very rarely thereafter.Citation21,Citation34 In this study, shedding of vaccine-related rotavirus was detected in 5.00% (3/60) of vaccine recipients after the first dose, and no virus shedding was detected after the second and third doses, which is in accordance with the results of a study by Vesikari et.al.Citation24 Nevertheless, vaccination should be encouraged since the risk of vaccine transmission and subsequent vaccine-derived diseases is much lower in immunocompromised contacts than in wild-type rotavirus diseases.Citation34
The study had some limitations. Although the subjects had been required to immune the other vaccines interval 2 weeks since oral the study vaccine, overlap of phenomenons might still occur. Coadministration of the study vaccine with other vaccines could have affected to assess the safety of the study vaccine, because some reactogenicity could be due to the other vaccines. As indicated above, the other limitation was the inability to establish a positive dose-response about the IgA antibodies due to the limited sample size, meanwhile the limited sample size substantially reduced the probability of capturing rare adverse AEs. Meanwhile, a phase III trial of the novel hexavalent rotavirus vaccine is underway to evaluate its safety, immunogenicity, and efficacy.
In conclusion, the results of this phase I clinical trial of the novel hexavalent rotavirus vaccine in adults, toddlers, and infants support the further development and testing of the platform. The hexavalent rotavirus vaccine was safe and immunogenic, with evidence that it might provide protection against rotavirus disease in infants.
Disclosure of potential conflicts of interest
Qing-Liang Li, Kai Duan, Zhi-Jun Jiang, Xuan Bai, and Ge-Lin Xu are currently employees of Wuhan Institute of Biological Products Co., Ltd. Xiao-Ming Yang is currently an employee of China National Biotec Group Co., Ltd., Beijing, China. The other authors declare they have no conflicts of interest.
Acknowledgments
We greatly appreciate all the researchers who were involved in this clinical trial and the participants who participated this study and their parents or legal guardians.
Additional information
Funding
References
- Tate JE, Burton AH, Boschi-Pinto C, Parashar UD. World health organization-coordinated global rotavirus surveillance n. global, regional, and national estimates of rotavirus mortality in children <5 years of age, 2000–2013. Clin Infect Dis: Off Publ Infect Dis Soc Am. 2016;62:S96–S105.
- World Health Organization. Rotavirus vaccines. WHO Position Paper, February 1, 2013.
- Jin H, Wang B, Fang Z, Duan Z, Gao Q, Liu N, Zhang L, Qian Y, Gong S, Zhu Q, et al. Hospital-based study of the economic burden associated with rotavirus diarrhea in eastern China. Vaccine. 2011;29:7801–06. doi:10.1016/j.vaccine.2011.07.104.
- Zhang J, Duan Z, Payne DC, Yen C, Pan X, Chang Z, Liu N, Ye J, Ren X, Tate JE, et al. Rotavirus-specific and overall diarrhea mortality in chinese children younger than 5 years: 2003 to 2012. Pediatr Infect Dis J. 2015;34:e233–7. doi:10.1097/INF.0000000000000799.
- WHO. Meeting of the immunization strategic advisory group of experts. 2009 April;84(23):220–36.
- Burke RM, Tate JE, Kirkwood CD, Steele AD, Parashar UD. Current and new rotavirus vaccines. Curr Opin Infect Dis. 2019;32:435–44. doi:10.1097/QCO.0000000000000572.
- Burnett E, Parashar UD, Tate JE. Global impact of rotavirus vaccination on diarrhea hospitalizations and deaths among children <5 years old: 2006–2019. J Infect Dis. 2020 October;222(10):1731–39. doi:10.1093/infdis/jiaa081.
- Mo Z, Ma X, Luo P, Mo Y, Kaplan SS, Shou Q, Zheng M, Hille DA, Arnold BA, Liao X, et al. Immunogenicity of pentavalent rotavirus vaccine in Chinese infants. Vaccine. 2019;37:1836–43. doi:10.1016/j.vaccine.2019.02.018.
- Kapikian AZ, Simonsen L, Vesikari T, Hoshino Y, Morens DM, Chanock RM, La Montagne JR, Murphy BR. A hexavalent human rotavirus-bovine rotavirus (UK) reassortant vaccine designed for use in developing countries and delivered in a schedule with the potential to eliminate the risk of intussusception. J Infect Dis. 2005;192(Suppl 1):S22–9.
- Gutierrez G. [The National Program to Control Diarrheal Diseases: its impact on health and health services]. Salud Publica Mex. 1994;36:127–28.
- Midthun K, Greenberg HB, Hoshino Y, Kapikian AZ, Wyatt RG, Chanock RM. Reassortant rotaviruses as potential live rotavirus vaccine candidates. J Virol. 1985;53:949–54. doi:10.1128/JVI.53.3.949-954.1985.
- Paul A, Babji S, Sowmyanarayanan TV, Dhingra MS, Ramani S, Kattula D, Kang G. Human and bovine rotavirus strain antigens for evaluation of immunogenicity in a randomized, double-blind, placebo-controlled trial of a single dose live attenuated tetravalent, bovine-human-reassortant, oral rotavirus vaccine in Indian adults. Vaccine. 2014;32:3094–100. doi:10.1016/j.vaccine.2014.03.013.
- Clements-Mann ML, Dudas R, Hoshino Y, Nehring P, Sperber E, Wagner M, Stephens I, Karron R, Deforest A, Kapikian AZ, et al. Safety and immunogenicity of live attenuated quadrivalent human-bovine (UK) reassortant rotavirus vaccine administered with childhood vaccines to infants. Vaccine. 2001;19:4676–84. doi:10.1016/S0264-410X(01)00242-0.
- Clements-Mann ML, Makhene MK, Mrukowicz J, Wright PF, Hoshino Y, Midthun K, Sperber E, Karron R, Kapikian AZ. Safety and immunogenicity of live attenuated human-bovine (UK) reassortant rotavirus vaccines with VP7-specificity for serotypes 1, 2, 3 or 4 in adults, children and infants. Vaccine. 1999;17:2715–25. doi:10.1016/S0264-410X(98)00497-6.
- Vesikari T, Karvonen AV, Majuri J, Zeng SQ, Pang XL, Kohberger R, Forrest B, Hoshino Y, Chanock R, Kapikian A, et al. Safety, efficacy, and immunogenicity of 2 doses of bovine-human (UK) and rhesus-rhesus-human rotavirus reassortant tetravalent vaccines in Finnish children. J Infect Dis. 2006;194:370–76. doi:10.1086/505151.
- Fujii Y, Doan YH, Wahyuni RM, Lusida MI, Utsumi T, Shoji I, Katayama K. Improvement of rotavirus genotyping method by using the semi-nested multiplex-PCR with new primer set. Front Microbiol. 2019 March;10:647.
- Nationa Medical Products Administration. https://www.nmpa.gov.cn/index.html
- Li RC, Li YP, Mo ZJ, Luo D, Huang T, Kong JL, Wang L-H, Song N-S, Liu A, Zhang H, et al. Reactogenicity and safety of a liquid human rotavirus vaccine (RIX4414) in healthy adults, children and infants in China: randomized, double-blind, placebo-controlled Phase I studies. Hum Vaccin Immunother. 2013;9:1638–42. doi:10.4161/hv.25076.
- Dhingra MS, Kundu R, Gupta M, Kanungo S, Ganguly N, Singh MP, Bhattacharya MK, Ghosh R, Kumar R, Sur D, et al. Evaluation of safety and immunogenicity of a live attenuated tetravalent (G1-G4) Bovine-Human Reassortant Rotavirus vaccine (BRV-TV) in healthy Indian adults and infants. Vaccine. 2014;32(Suppl 1):A117–23. doi:10.1016/j.vaccine.2014.03.069.
- Anil K, Desai S, Bhamare C, Dharmadhikari A, Madhusudhan RL, Patel J, Kulkarni PS. Safety and tolerability of a liquid bovine rotavirus pentavalent vaccine (LBRV-PV) in adults. Vaccine. 2018;36:1542–44. doi:10.1016/j.vaccine.2018.02.024.
- Kanchan V, Zaman K, Aziz AB, Zaman SF, Zaman F, Haque W, Khanam M, Karim MM, Kale S, Ali SK, et al. A randomized Phase I/II study to evaluate safety and reactogenicity of a heat-stable rotavirus vaccine in healthy adults followed by evaluation of the safety, reactogenicity, and immunogenicity in infants. Hum Vaccin Immunother. 2020 Mar 3;16(3):693–702. doi:10.1080/21645515.2019.1664239. Epub 2019 Oct 29.
- Kawamura N, Tokoeda Y, Oshima M, Okahata H, Tsutsumi H, Van Doorn LJ, Muto H, Smolenov I, Suryakiran PV, Han HH, et al. Efficacy, safety and immunogenicity of RIX4414 in Japanese infants during the first two years of life. Vaccine. 2011;29:6335–41. doi:10.1016/j.vaccine.2011.05.017.
- Iwata S, Nakata S, Ukae S, Koizumi Y, Morita Y, Kuroki H, Tanaka Y, Shizuya T, Schödel F, Brown ML, et al. Efficacy and safety of pentavalent rotavirus vaccine in Japan: a randomized, double-blind, placebo-controlled, multicenter trial. Hum Vaccin Immunother. 2013;9:1626–33. doi:10.4161/hv.24846.
- Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, Dallas MJ, Heyse JF, Goveia MG, Black SB, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354:23–33. doi:10.1056/NEJMoa052664.
- Mo Z, Mo Y, Li M, Tao J, Yang X, Kong J, Wei D, Fu B, Liao X, Chu J, et al. Efficacy and safety of a pentavalent live human-bovine reassortant rotavirus vaccine (RV5) in healthy Chinese infants: A randomized, double-blind, placebo-controlled trial. Vaccine. 2017;35:5897–904. doi:10.1016/j.vaccine.2017.08.081.
- Kulkarni PS, Desai S, Tewari T, Kawade A, Goyal N, Garg BS, Kumar D, Kanungo S, Kamat V, Kang G, et al. A randomized Phase III clinical trial to assess the efficacy of a bovine-human reassortant pentavalent rotavirus vaccine in Indian infants. Vaccine. 2017;35:6228–37. doi:10.1016/j.vaccine.2017.09.014.
- Bhandari N, Rongsen-Chandola T, Bavdekar A, John J, Antony K, Taneja S, Goyal N, Kawade A, Kang G, Rathore SS, et al. Efficacy of a monovalent human-bovine (116E) rotavirus vaccine in Indian infants: a randomised, double-blind, placebo-controlled trial. Lancet. 2014;383:2136–43. doi:10.1016/S0140-6736(13)62630-6.
- Zade JK, Kulkarni PS, Desai SA, Sabale RN, Naik SP, Dhere RM. Bovine rotavirus pentavalent vaccine development in India. Vaccine. 2014;32(Suppl 1):A124–8. doi:10.1016/j.vaccine.2014.03.003.
- Narang A, Bose A, Pandit AN, Dutta P, Kang G, Bhattacharya SK, Datta S, P.V. S, Delem A, Han HH, et al. Immunogenicity, reactogenicity and safety of human rotavirus vaccine (RIX4414) in Indian infants. Hum Vaccin. 2009;5:414–19. doi:10.4161/hv.5.6.8176.
- Bhandari N, Sharma P, Taneja S, Kumar T, Rongsen-Chandola T, Appaiahgari MB, Mishra A, Singh S, Vrati S. A dose-escalation safety and immunogenicity study of live attenuated oral rotavirus vaccine 116E in infants: a randomized, double-blind, placebo-controlled trial. J Infect Dis. 2009;200:421–29. doi:10.1086/600104.
- Franco MA, Angel J, Greenberg HB. Immunity and correlates of protection for rotavirus vaccines. Vaccine. 2006;24:2718–31. doi:10.1016/j.vaccine.2005.12.048.
- Jiang B, Gentsch JR, Glass RI. The role of serum antibodies in the protection against rotavirus disease: an overview. Clin Infect Dis: Off Publ Infect Dis Soc Am. 2002;34:1351–61. doi:10.1086/340103.
- Li RC, Huang T, Li Y, Wang LH, Tao J, Fu B, Si G, Nong Y, Mo Z, Liao X, et al. Immunogenicity and reactogenicity of the human rotavirus vaccine, RIX4414 oral suspension, when co-administered with routine childhood vaccines in Chinese infants. Hum Vaccin Immunother. 2016;12:785–93. doi:10.1080/21645515.2015.1085143.
- Anderson EJ. Rotavirus vaccines: viral shedding and risk of transmission. Lancet Infect Dis. 2008;8:642–49. doi:10.1016/S1473-3099(08)70231-7.