ABSTRACT
Influenza virus is a life-threatening pathogen that infects millions of people every year, with annual mortality in the hundreds of thousands. The scenario for controlling infection has worsened with increasing numbers of vaccine hesitancy cases reported worldwide due to objections on safety, religious and other grounds. Uses of haram (impermissible) and mashbooh (doubtful) ingredients in vaccine production has raised doubts among Muslim consumers and consequently stimulated serious vaccine hesitancy. To address this major problem, we have reviewed and recommended some alternatives appropriate for manufacturing cell-based influenza vaccine which comply with Islamic laws and consumers’ needs. Intensive assessments of current influenza vaccine production in both scientific and Islamic views have led to the identification of four main ingredients deemed impermissible in novel sharia-compliant (approved by Islamic laws) vaccine manufacturing. Only some of these impermissible components could be replaced with halal (permissible) alternatives, while others remain impermissible due to unavailability and unsuitability.
Introduction
A vaccine is a biopharmaceutical product which is used to prevent infection for a communicable disease throughout the world. It contains antigenic substances which can activate the immune system in the body to protect against a certain disease. Vaccination, which initially started in England, began to be implemented globally as part of a community immunization program in the 1800s.Citation1,Citation2 The acceptance of vaccination around the world, however, has shown a declining trend recently due to vaccine hesitancy, particularly in Muslim countries like Malaysia, Afghanistan, Saudi Arabia, and Pakistan.Citation3,Citation4 Vaccine hesitancy and rejection among Muslims is based on assumptions and beliefs that such a practice and the ingredients used are “doubtful (mashbooh)”, and they would prefer to abstain to maintain their sanctity.
In Islamic laws, the consumption of dead meat (carcasses), blood, and swine flesh which has been summoned with the name other than Allah are strictly prohibited and classified as al-najasat (ritually impure, sing. najs) and therefore haram (impermissible) unless in darurat (dire conditions) as stated in the Quran (al-Ma’idah: 3).Citation5 Outside of dire conditions, those things cannot be eaten, drunk, or used for medicinal purposes as proscribed by the Prophet Muhammad in the hadith (prophetic traditions), “Allah has not placed a cure for your diseases in things that He has forbidden for you.”Citation6
A vaccine, however, is permissible for Muslims’ consumption, even if some of its ingredients are made of porcine or other impermissible substances, based on the Islamic concept al-darurat tubih al-mahzurat (dire necessity renders the impermissible to be permissible).Citation6 The opinion, however, varies among scholars from different legal schools, who have distinctive opinions in defining the exact meaning of darurat. Some scholars classify vaccinations as a dire need, while some of them consider it otherwise because of the unguaranteed effects of vaccines in preventing and curing diseases. To solve the intricate arguments and, at the same time, to avoid any destructive consequences, sharia-compliant or generally known as halal (permissible) vaccines that are purely free of any ritually impure ingredients are needed.
The production of halal vaccines could be enabled through the establishment of sharia-compliant vaccine manufacturing facilities according to Islamic jurisprudence. Fundamentally, sharia-compliant means any procedures and/or materials that obey sharia laws, the commands of Allah concerning the conduct of human beings who are accountable.Citation7 The establishment of the sharia-compliant vaccine manufacturing facilities are expected to fulfill halal vaccine demands for more than 1.8 billion Muslim consumers around the world with an estimated market value USD 50 USD billion.Citation8
Influenza vaccine is one of the most common vaccine taken annually to prevent influenza infection which cause three to 5 million cases of severe infection and approximately 300–650 thousand death worldwide every year.Citation9 Yearly vaccination against the disease has been recommended in most countries in the world. The application of influenza vaccine is very important for Muslim community as well, particularly for pilgrimage purposes in Saudi Arabia, since respiratory illnesses related to influenza have been reported to be the most common disease infecting pilgrims every year.Citation10
To fulfil the yearly demands of influenza vaccine, the manufacturing method of the influenza vaccine has been shifting from egg-based to cell-based. In the cell-based vaccine production, various types of impermissible ingredients employed which incite serious concerns among Muslim consumers. In current publications, there are very minimal studies conducted to analyze the halal status of ingredients used in the cell-based vaccine production. Therefore, this study was conducted with objectives: 1) to review the current production of cell-based influenza vaccine, 2) to identify the impermissible ingredients utilized in the current production, and 3) to search for suitable alternatives of the impermissible ingredients which are applicable for use in sharia-compliant cell-based influenza vaccine manufacturing. The goal of this paper is to serve as a useful and reliable reference to initiate and accelerate the research and development (R&D) of sharia-compliant manufacturing, especially for the production of a halal influenza vaccine, which is considered as the communal obligation (fard kifayah) of Muslims.
Methods
The overview of the current production of a cell-based influenza vaccine was summarized based on inputs gathered from industrial visits to Biomedical Research Center, Northwest Minzu University, Lanzhou and Guan Jie Biotechnology Co. Ltd., Jilin, China. Additional information relevant to the production process were searched from Google and Google Scholar databases using search terms [cell-based influenza vaccine], [upstream process for influenza vaccine production], [downstream production of vaccine], [purification of vaccine], [formulation of vaccine], and [vaccine component]. The specific names of vaccine components were also searched to identify its origins and constituents.
The halal status assessment for the cell-based influenza vaccine production was conducted following guidelines published in Malaysian Standard for Halal Pharmaceuticals (MS 2424:2019).Citation7 Additional opinions of scholars and sharia laws relevant with the context were searched from Google and Google Scholar databases using search terms [halal pharmaceutical], [halal and haram in biotechnology], [halal and haram in food], and [vaccine component and Islamic view]. All search terms used also were searched in the Malay language to diverse related information. In this study, only religiously impermissible ingredients were reviewed in details and their alternatives were suggested based on Islamic jurisprudences.
Results
Current production of a cell-based influenza vaccine
The current production of an inactivated cell-based influenza vaccine involves at least four stages of complex processes starting with upstream processes and followed by downstream processes. The downstream processes comprise of separation and purification processes, virus inactivation process, and vaccine formulation, fill and finish processes. depicts typical process units involved throughout the production of a cell-based influenza vaccine.
Figure 1. Generic production process of a cell-based influenza vaccine. 1) The upstream process involves replication of host cells grown in a bioreactor containing formulated growth media. Sufficient host cells are then inoculated with influenza virus with trypsin addition to assist internalization of the virus into the host cells. 2) After a few days of incubation, the culture solution containing influenza virus undergoes separation and purification processes using various types of centrifuge and filtration units. 3) Subsequently, the purified virus is exposed to formaldehyde to inactivate its virulence. 4) The aseptically filtered inactivated influenza virus is later formulated with excipients before being packaged as a powdered or liquid influenza vaccine
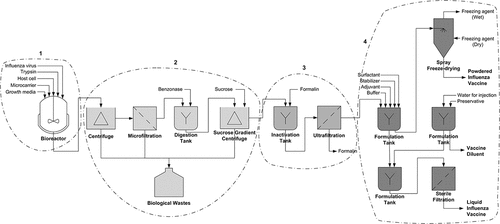
In upstream processes of the cell-based influenza vaccine production, a dog-kidney-derived cell line, Madin-Darby Canine Kidney (MDCK), has been widely used as a substrate for influenza virus propagation.Citation11 The MDCK cells can be grown either adherently on microcarriers or in suspension using serum-containing or serum-free media.Citation12–14 Cultivation of suspension MDCK cells in serum-free media is currently favored due to lower labor and processing costs and is proven to be effective enough for high-yield production of influenza vaccine.Citation12 Antibiotics such as neomycin, streptomycin, polymyxin B, and amphotericin B might be added to the culture media to prevent bacterial contamination during the manufacturing process.Citation15,Citation16 The MDCK cells are cultivated in the culture media until their concentration adequate for influenza virus inoculation. Prior to influenza virus inoculation, trypsin is added to facilitate effective internalization of influenza virus into MDCK cells.Citation17 The culture is then incubated for a few days to propagate a sufficient amount of influenza virus before being harvested.
The harvested influenza viruses are subsequently recovered from mixed culture media through downstream processes. In general, influenza viruses can be separated and purified through combination of various methods, such as precipitation, flocculation, centrifugation, extraction, microfiltration, ultrafiltration, and by using bead-based or membrane-based chromatography.Citation18,Citation19 In common practices, mixed culture media containing influenza viruses are initially centrifuged to separate solid and highly dense wastes such as microcarriers, host cells, cell debris, organelles, and insoluble impurities from the mixture.Citation18 After that, the resulting supernatant is further purified through repeated membrane filtration and ultracentrifugation. Ultrafiltration is the preferred and most economical method to concentrate and purify viruses, as it effectively and efficiently separates viruses from soluble biopolymers such as proteins, ribonucleic acid (RNA), deoxyribonucleic acid (DNA), and lipids.Citation19 Besides that, nuclease treatment using benzonase and additional polishing steps using chromatography unit operations may also be added to enhance the purity of recovered influenza viruses from nucleic acid residuals and contaminations, as suggested by Wolff and Reichl.Citation19
For producing an inactivated influenza vaccine, the purified influenza viruses are then treated with inactivating agents such as formaldehyde, β-propiolactone and glutaraldehyde to render it nonvirulent.Citation15,Citation16 Formaldehyde is the most common inactivating agent used in vaccine production; however, it is known to be carcinogenic to humans.Citation15,Citation16 To ensure the safety of influenza vaccine produced, complete removal of formaldehyde from the solution using ultrafiltration following the inactivation process must be held effectively prior to formulation with excipients. Additional chemical treatments to split influenza viruses may also be added after the inactivation process to produce more purified inactivated influenza vaccine.Citation16
In the last stage of influenza vaccine production, highly purified inactivated influenza viruses are formulated with excipients to improve the immunogenicity and stability of the produced influenza vaccines.Citation20 The excipients may be comprised of one or more of the following components: adjuvant(s), stabilizer(s), surfactant(s), buffering agent(s), diluents (e.g., saline solution, sterile water, or protein-based fluids), and preservative(s).Citation21 Currently, adjuvants made of aluminum salts and squalene-based oil-in-water emulsions (e.g., MF59 and AS03) are widely used in influenza vaccine formulation and are proven to be effective at enhancing the immunogenicity of vaccine antigens.Citation22 Furthermore, the addition of surfactants (e.g., polysorbate, tween, pluronic) and stabilizers either from sugar-based (e.g., sucrose, trehalose, dextrose, mannitol, sorbitol), protein-based (e.g., human albumin, gelatine), or amino acid-based stabilizers (e.g., arginine, glycine, glutamate) can prevent the rapid degradation of protein antigens caused by mechanical stresses and temperature changes, especially during the drying process and vaccine transportation, distribution, and storage.Citation20,Citation21,Citation23
The formulated influenza vaccine can be produced in either liquid or powdered form. The liquid influenza vaccine is generated directly after the formulation process and filtered aseptically before being packaged in a single-vial vaccine. On the other hand, to generate the powdered influenza vaccine, the formulated solution must undergo aseptic freeze-drying using cooling agents such as dry ice vapor, liquid nitrogen, or ethanolCitation23 to make it powdered before being packaged in two individual vials containing lyophilized vaccine and vaccine diluent, respectively. Preservatives such as phenol, 2-phenoxyethanol, and thimerosal (disuse in certain countries) may be added to the liquid diluent or vaccine to prevent bacterial and fungal contamination.Citation15,Citation16
Impermissible ingredients used in influenza vaccine production and their alternatives based on Islamic jurisprudence
Analyses of current cell-based influenza vaccine production indicated some serious concerns regarding the state of ingredients and unit operations used during the manufacturing process. According to the authors’ judgments based on Islamic rules, most of the unit operations used in influenza vaccine production seem to be free of any impermissible ingredients, except for membrane separation and purification units such as microfiltration, ultrafiltration, nanofiltration, and chromatography units. Those operations units are probably made of, coated with, or linked to gelatin,Citation24,Citation25 heparin,Citation26 or animal bone charcoal that originate from non-halal animals (e.g., pigs and non-slaughtered cows).Citation27 However, reviews undertaken by Cassano and BasileCitation28 and Wolff and ReichlCitation19 showed that most of the membrane filters or resins used in vaccine production are made of natural polymers (e.g., cellulose, agarose, dextran), synthetic polymers (e.g., polysulfone, polyethersulfone (PES), polytetrafluoroethylene (PTFE)), ceramics other than bone charcoal (e.g., silica, glass, alumina), or sintered metal.
Therefore, the authors believe there is no serious concern or prohibition regarding the use of any unit operation in vaccine production, as sharia-compliant and contaminant-free membrane separation units with significantly good efficiency are available for use instead, but more precise investigations should be conducted to confirm this hypothesis. In terms of vaccine ingredients and excipients, there are four main types of ingredients that are clearly judged as najs, either completely haram or mashbooh according to Islamic laws. These ingredients exist in the form of host cells, growth media, proteolytic enzymes, and gelatin.
Host cells for virus production
To date, the MDCK cell line, which is derived from a dog kidney, had been used widely in the manufacturing of influenza vaccine as the most ideal host cell line for propagating the influenza virus.Citation11,Citation29 In Islam, dogs are haram to be consumed because they are categorized as a carnivorous animal with claws and fangs.Citation30 All Sunni schools of thought except Maliki consider dog, in whole or in part, as najs, while Shafi’i classifies them precisely as wholly najs mughallazah (severely impure), which requires a special ritual cleaning procedure.Citation31 Hence, the use of the MDCK cell line to generate vaccines is forbidden in Islam, as it cannot be eaten or injected into bodies and could implicate arduous treatments or ritual cleaning to ensure the “purity” of the production equipment and the end products based on the Islamic definition.
Even though separation and purification processes could efficiently sieve out enormous amounts of residual host cells, the end products usually would not be 100% free of residual host-cell proteins. Based on the requirements set by authorities such as the U.S. Food and Drug Administration (FDA)Citation32 and the World Health Organization (WHO),Citation33 the maximum residual host-cell DNA in each dose of vaccine must be less than 10 ng, and, if possible, must be less than 10 pg. If vaccine manufacturers merely followed the standards set by the authorities, the vaccines we use today would probably contain a miniscule amount of najs, as the residuals cannot be eliminated completely. Concerns regarding the effects of residual host cells on human health have also been described in a few previous studies.Citation34–37
To address these problems, both in terms of safety and Islamic concerns, a better alternative host cell line, specifically for influenza vaccine manufacturing, must be chosen. Other than the MDCK cell line, various types of cell lines originated from a human (e.g., HEK293Citation38 and PER.C6Citation39), hamster (e.g., BHK-21Citation40), monkey (e.g., VeroCitation14), avian (e.g., EB66,Citation41,Citation42 AGE1.CR,Citation43 DuckCelt-T17,Citation44 and PBS-1Citation45), and insect (e.g., Sf9Citation46) have been tested as candidates for large-scale production of influenza virus. Considering the ethics, norms, and religious aspects, as listed in , the avian cell lines from either duck (EB66, AGE1.CR, or DuckCelt-T17) or chick embryo (PBS-1) seem to be the best options available to replace the MDCK cell line. Human-derived products or cell lines are totally rejected in Islam because the human is a pure and respected organism, and its exploitation is haram.Citation47
Table 1. Opinion of Islam on various types of host cells used for influenza virus propagation
In the Islamic perspective, a duck or chick slaughtered in the name of Allah (otherwise defined in this review as a non-slaughtered animal) is considered as halal meat and absolutely permissible for consumption by Muslims.Citation48 Although the established avian cell lines are not extracted from a religiously slaughtered duck or chick and deemed as carcasses, the authors have confidence that the selection is the next closest option to halal ingredients. In term of production suitability, avian cell lines such as PBS-12SF, DuckCelt-T17, EB66, and AGE1.CR are known to be free of adventitious agents, can proliferate to high densities in suspension forms using serum-free media in various production modes, are highly permissive to a broad range of influenza strains and are capable of producing a high yield of influenza virus efficiently.Citation42–45 In brief, avian cell lines of either duck or chick origin have great potential to be used as alternative substrates for the production of sharia-compliant influenza vaccines.
Growth media for host-cell consumption
To propagate a sufficient amount of influenza virus for vaccine production, high densities of host cells must be obtained first. Like other living organisms, the viability and growth rate of host cells depends directly on the supplemented nutrients. Serum-containing media is the most common and effective synthetic media used for cultivating high densities of assorted cell culture types. This media involves the addition of mammalian serum usually extracted from human, bovine, or equine blood, which is then mixed with established basal media.Citation49–51 Serum is a naturally complex, ill-defined, and varied biological component rich in active substances such as hormones, growth factors, binding and transport proteins, attachment and spreading factors, natural fatty acids, lipids, amino acids, vitamins, and trace elements necessary for the survival and growth of cell and tissue cultures.Citation50–52
As stated in the Quran, blood is strictly forbidden for consumption by Muslims,Citation5 thus, the application of blood in vaccine production should be avoided. Uses of human-derived and animal-derived components, especially serum extracted from bovine fetuses, incites not only religious concerns but also ethical and safety concerns.Citation53,Citation54 For example, draining of blood from bovine fetuses using cardiac puncture causes severe suffering to the fetuses, and those practices are absolutely inhumane and against ethical procedures.Citation52,Citation53 In terms of safety, the transmission of variant Creutzfeldt-Jakob disease (vCJD) and bovine spongiform encephalopathy (BSE) between species through infectious proteins or prions causes serious apprehension among researchers, manufacturers, and consumers.Citation52,Citation54
Fortunately, with advancements in life sciences, the numbers of animal components used in cell cultures are gradually being reduced, and a few types of serum-free media are already applicable for certain cell types. Although the use of serum-free media could eliminate supplementation with ill-defined serum, some essential proteins and hormones originating from human or animal sources such as insulin, transferrin, and albumin are still needed to maintain healthy and stable cell growth.Citation49,Citation50 Surprisingly, chemically defined media which categorized under serum-free media and generally claimed as animal-component-free media also may actually contain recombinant proteins, which are often categorized as non-animal components.Citation54 According to sharia laws, recombinant proteins or any genetically modified products expressed using DNA, RNA, plasmid or any related materials derived from human, forbidden animals (e.g., porcine), or non-slaughtered animals (e.g., bovine) are stringently forbidden and regarded as najs unless halal materials are used instead.Citation55,Citation56
Apart from that, the basic components of synthetic media known as basal media, such as Minimum Essential Medium (MEM), Dulbecco’s Modified Eagle Medium (DMEM) and Roswell Park Memorial Institute (RPMI 1640), which are usually supplemented with serum or serum-substitutes, typically constitute at least 13 essential amino acids, 8 water-soluble vitamins, 6 inorganic salts, and glucose to support lasting cell growth.Citation57 Toward the establishment of serum-free media, more complex basal media containing wider types of amino acids, vitamins, minerals, and metabolites are used to compensate essential components of serum.Citation58 Aligned with safety and religious concerns raised by Ermis,Citation59 Fletcher and HarrisCitation54 and Karahalil,Citation60 amino acids used in basal media most likely contain animal-derived residues resulting from amino acid extraction from animal tissues, enzymatic processes, or microbial fermentation.Citation61 Some of the amino acids can be synthesized chemically, but most of the organic components exist abundantly in animal tissues or are naturally synthesized by microorganisms presumably fed with animal-sourced media during the fermentation process. Vitamins such as myo-inositol,Citation62 riboflavin,Citation63 and pantothenic acidCitation64 may also be produced similarly to amino acids, thus similar concerns should be evaluated.
Disappointingly, limited access to information due to proprietary issues and ambiguous origins of components constituting commercialized cell culture media impede comprehensive evaluations and selection of sharia-compliant growth media. Until now, the authors believed that each of the established growth media available today, including so-called animal-component-free media, still contained at least one component as fine as DNA that originated from humans, forbidden animals, or non-slaughtered animals, which are considered as impermissible ingredients in Islam. Besides that, purified blood-derived components like albumin, transferrin, and hydrocortisoneCitation49 may still be in use in most of the growth media to imitate the innate growth environment of cells and tissues, which contradict sharia laws that prohibit the utilization of najs including blood, carrion, and pus.Citation30 Additional opinions of Islam regarding various origins of growth media components are further described in .
Table 2. Components of growth media and their corresponding opinion of Islam
As there are no alternative growth media that are 100% free of impermissible ingredients and compliant with sharia laws, chemically defined and animal-component-free media is the best option available for selection. However, the suitability of growth media for host cells must be considered. Auspiciously, avian cell lines such as PBS-12SF, DuckCelt-T17, EB66, and AGE1.CR were successfully adapted to grow in serum-free media.Citation42–45 If those cell lines can be adapted in chemically defined media without supplementation with any animal-derived components or consist solely of plant-based ingredients, a better alternative growth media usable for sharia-compliant vaccine processing could be created in the future.
Proteolytic enzymes
In cell culture technology, most naturally isolated cells or primary cells tend to grow adherently by forming protein bridges on the surface of culture flasks or microcarriers. Disruption of the protein bridges using proteolytic enzymes, also called proteases, is thus necessary to detach and dissociate the cells during passaging. Trypsin, a serine protease that specifically cleaves the peptide bonds at the C-terminal end of positively charged lysine and arginine side chains, has been proven to detach and dissociate cells effectively from culture flasks and microcarriers.Citation65
Beside been used solely as a cell detachment agent, trypsin is also useful for mediating efficient propagation of influenza virus in host cells. The addition of trypsin prior to influenza virus infection could assist the internalization process of influenza virus into the host cells through cleavage of the virion surface glycoprotein known as hemagglutinin (HA) precursor HA0 into HA1 and HA2 subunits.Citation17 The presence of trypsin also interferes in the pathogen defense mechanisms of host cells, which significantly promotes high titer production of influenza virus.Citation66 Although trypsin plays important roles in the successful production of influenza vaccines, its origin is frequently disputed mainly by Muslims. Most of the commercialized trypsin applicable to cell culture studies and vaccine productions today were extracted from porcine pancreas,Citation4,Citation55 and some of it originated from bovine pancreas.Citation65 The use of porcine trypsin in sharia-compliant processing is clearly forbidden by Islamic laws, while the use of bovine trypsin is only permissible if it has been extracted from a religiously slaughtered bovine as explained in .
Table 3. Opinion of Islam on different types of commercialized proteolytic enzymes
To meet the demand for animal-free products, a recombinant fungal protease, namely trypLE, has been generated through the fermentation process and is currently commercialized as a trypsin substitute with similar functions to trypsin.Citation67,Citation68 TrypLE has superior effectiveness as a cell detachment agent for various cell types with greater stability and lower toxicity effects compared with traditional animal-derived trypsin.Citation67–70 The origin of trypLE, however, cannot be traced meticulously due to proprietary issues. Impermissible genetic materials sourced from forbidden animals might be used during production of the recombinant protease, thus it is prone to be excluded from sharia-compliant processing unless additional information proves otherwise.
In order to eliminate any doubts, accutase, a trypsin replacement prominently claimed by suppliers as a non-bacterial and mammalian-free protease and collagenase mixtureCitation71,Citation72 extracted from marine lifeCitation73 or specifically from an invertebrate speciesCitation74 such as a crustaceanCitation67 seems the best option available. The selection was made based on sharia laws allowing the consumption of all kinds of marine life merely living in water except disgusting, poisoning, and intoxicating organisms.Citation48,Citation55 In terms of performance, the capacity of accutase to detach and dissociate viable cells is lower than that of trypsin, but a few days after trypsinization, accutase-treated cells successfully recovered more cells and rapidly grow better than trypsin-treated cells.Citation75 The cellular behavior of accutase-treated cells also remained unchanged, and they could proliferate longer than cells dissociated with trypsin.Citation76 Addition of accutase prior to influenza virus infection also slightly improved virus propagation in the host cells (MDCK), although its effectiveness was incomparable to that of trypsin.Citation77 With those abilities, accutase could be a potential alternative to be used in sharia-compliant influenza vaccine production.
Gelatin
In vaccine production, maintaining the properties and structures of the active ingredients, including protein antigen(s), in vaccines is very important to avoid degradation and denaturation of components, which can lead to decreased effectiveness and potency of the vaccines. For the preparation of freeze-dried vaccine, a stabilizer is added prior to the lyophilization process to provide a matrix that can contain and protect vaccine components from the harsh conditions of the process.Citation16 Gelatin and human serum albumin (HSA) are frequently used stabilizers for live attenuated and inactivated vaccines (including influenza vaccine), which contain high complexity, intrinsic instability, and environmental stress sensitivity of active components.Citation16,Citation21,Citation78 In terms of safety, both materials could transmit adventitious agents, but the application of HSA involves stricter requirements from authorities, and the source can only be certain certified manufacturers.Citation16 In contrast, gelatin is a more feasible option with a more readily available supply.
Gelatin is a biopolymer protein partially hydrolyzed from collagen abundantly found in animal skins, bones, cartilages, and connective tissues.Citation79–81 Out of 108 vaccines analyzed and listed by Cardoso, Petrovajová, and Horňáková,Citation21 24 contain stabilizers made from gelatin, 15 from HSA, and 5 from both HSA and gelatin. The origins of the gelatins were not specified, and only six of them were specified as porcine-derived gelatin. The unknown gelatins were most likely of porcine origin, as nearly half of the global production of gelatin is sourced from pig skin (46%), followed by bovine hides (29.4%), pork and cattle bones (23.1%), and other sources (1.5%) such as fish and poultry skins and bones.Citation80,Citation81 Economical production, a shorter pre-treatment process, and less generation of wastewater have become the main reasons why porcine gelatin is produced on a massive scale compared with bovine gelatin.Citation82
Although some Muslim scholars allow the use of gelatins sourced from any type of animal, including pigs, which they believe have been through an istihalah (transformation) process,Citation6,Citation83,Citation84 gelatins sourced from halal animals must be prioritized to avoid any argument. Nowadays, halal bovine gelatin is already manufactured and commercialized for Muslim consumption, but the supplies are limited, and the product niche is not as large as that of other types of gelatin.Citation8,Citation48,Citation81 To satisfy the requirement of sharia-compliant processing, other than using halal bovine gelatin, fish gelatin,Citation79,Citation81 plant-based alternative of gelatin,Citation48 and other types of sugar alcohols, such as sorbitol, lactose, inulin, and trehalose,Citation21 have great potential to be used as vaccine stabilizers instead of HSA, porcine, and bovine gelatins with unknown status. The invention of bacterial cellulose as gelatin substitute in future also might be another great option available to be used.Citation85
The application of gelatin is not only limited as a vaccine stabilizer but is also useful as a coating material or ligand for microcarriers.Citation86–88 Microcarriers, bead-like materials used to grow adherent cells in suspension form, are regularly encapsulated with gelatin to improve their biocompatibility through imitation of innate conditions favorable for cell adhesion and growth. In nature, cells tend to adhere to each other or to the extracellular matrix made up of collagens, proteoglycans, fibronectin, laminin, elastin, or chondronectin to nurture efficient cell functions, particularly for cell metabolism and differentiation.Citation87 Most of the commercial microcarriers, such as Cytodex 3, Cultispher S, Cultispher G, FACT III, Gelibeads, and Ventregel, have surface coatings made of porcine gelatin;Citation86–88 therefore, those microcarriers are barred from sharia-compliant processing. As alternatives, ionic microcarriers made up of cellulose, dextran, or synthetic polymers (e.g., polyacrylamide, polystyrene, and polyurethane) treated and substituted with ionic materials (e.g., diethylaminoethanol (DEAE)) can be used instead.Citation87,Citation88 The charge density overlaid on the surface of the ionic microcarriers will promote cell attachment and spreading. Cytodex 1, Cytoline 2, and Cytopore 2 are exemplars of commercial ionic microcarriers that have been well studied and used for scale-up production of viral vaccineCitation86 and suitably implemented in sharia-compliant processing.
Recommendations for sharia-compliant cell-based influenza vaccine manufacturing
Based on the findings collected throughout this study, a few alternative suggestions, as summarized in , could be implemented in the manufacturing process of sharia-compliant cell-based influenza vaccines. The alternative suggestions for host cells and growth media may not obey sharia laws fully, but they are the best and closest options available today. Better options that obey sharia laws might be reserved in future if more studies related to this development can be carried out. The effectiveness of each alternative proteolytic enzyme, microcarrier, and gelatine stabilizer need to be tested to verify the suitability of those alternatives for producing safe, effective, and profitable influenza vaccines.
Table 4. Impermissible materials used in current production of cell-based influenza vaccines and their corresponding sharia-compliant alternatives
In selecting those suggestions, chemical-based, plant-based, and animal-component-free materials are prioritized over animal-based materials to avoid intricate verification and possible fraudulent claims of raw material status. All human-based materials also must be avoided at all costs in sharia-compliant vaccine manufacturing.
Discussion
Based on the results, the current production of cell-based influenza vaccine does involve the use of impermissible or haram ingredients in the form of host cells, growth media, proteolytic enzymes, and gelatin. Other ingredients classified as mashbooh are not discussed in details in this study as the alternatives of those ingredients are available and optionally used by manufacturers. The authors would like to emphasize that the determination of the halal status of ingredients used in the cell-based influenza vaccine production and the selection of corresponding sharia-compliant alternatives as discussed in this paper were mostly made based on guidelines published in the world first halal pharmaceutical standard, MS 2424:2019.Citation7 The halal definition and requirements as stated in the standard and applied in this study may differ from other countries or Muslim communities as they follow different schools of thought like Hanafi, Hanbali, Maliki, and Shafi’i.Citation89–91 In future studies, other standards certified by other countries or certifying bodies should be considered in defining the exact definition and requirements of halal and sharia-compliant production. The creation of a global halal standard is essential for the establishment of halal products including vaccines which are well accepted by all Muslim communities.
Besides the absence of the global halal standardCitation55,Citation89,Citation90 and halal-listing pharmacopoeia,Citation92 long duration of R&D stage, high cost production and operation, as well as stringent requirements for safety and halal aspects of the whole manufacturing process become the major constraints for the establishment of the halal vaccine industry.Citation93 In particular, the current production of influenza vaccine has a relatively low-profit margin due to its low product selling price. Toward the establishment of the sharia-compliant influenza vaccine manufacturing, changes of the production process and raw materials as recommended in the Results section will increase the overall production costs and create time-consuming regulatory challenges such as the need for clinical trials to confirm the effectiveness and safety of the produced vaccine and the lengthy procedures of halal certification. Inaccessible and shortage of halal raw materials supplies such as gelatinCitation8 and lack of human capital who are competent in both manufacturing fields and sharia lawsCitation55 also contribute to other challenges for manufacturers. Due to those constraints, manufacturers will be reluctant to implement the new sharia-compliant process. Although the establishment of the sharia-compliant or halal-only facilities for vaccine production seem impractical and not the best action plan for the meantime, the production of halal vaccine is expected to bring additional profits to manufacturers in future, as there are huge potential market and increasing demands for halal pharmaceutical products.Citation8
Granted that there are a lot of hurdles toward the establishment of the sharia-compliant vaccine manufacturing, Muslims must keep working on this establishment for the sake of Muslims well-being. With the creation of a halal vaccine, the demands of halalopathyCitation94 and ethnocentric medicinesCitation95 among Muslims and non-Muslims can be attained, as some similar vaccine components, are also forbidden in other religions, as described by Grabenstein.Citation84 The successful establishment of sharia-compliant vaccine manufacturing in the future will contribute immensely to the expansion of the halal pharmaceutical industry, which is estimated to be worth USD 131 USD billion by the end of 2023.Citation8
This study has several limitations. Owing to the proprietary nature of vaccine development and production details, this review might not capture all related issues accurately. As some information for this study was collected through industrial visits, there might be some bias in the analysis as those issues that could be ascertained during the observation and interview process are less proprietary hence discoverable, whereas the most proprietary information was not revealed during the observation and interview process and thus could not become part of this study and analysis.
Conclusions
Increasing vaccine hesitancy cases due to religious and safety grounds cannot be ignored. When provided with better vaccine alternatives, consumers, especially Muslims, might change their minds and perceptions of vaccines. Unfortunately, the establishment of sharia-compliant vaccine production cannot become a reality in just a few years. The complexity of vaccine processing itself and inaccessible supplies of sharia-compliant materials has hindered, for the most part, this endeavor. High infectivity and rapid mutation of the influenza virus complicate this endeavor even more as Muslims desperately need sharia-compliant influenza vaccine supplies each year to protect themselves.
As presented above, at this moment, the establishment of sharia-compliant influenza vaccine production still cannot be realized fully. Host cells essentially needed for influenza virus propagation are mostly derived from human, dog, monkey, insect, or avian carcasses, which are prohibited from use in sharia-compliant processing. Blood, human-, porcine-, and non-halal bovine-derived proteins, as well as mashbooh fermentation and recombinant products used in the preparation of growth media applicable to host cell propagation are currently irreplaceable and represent the greatest challenge to be resolved. Impermissible proteolytic enzymes and gelatin application in influenza vaccine production, fortunately, can be replaced with permissible ingredients, although the ingredients are not equally effective. All alternative ingredients suggested in this review were generated solely on the basis of combined information gathered from previous studies, and those ingredients might be impractical for use in real production of influenza vaccine unless they are tested on the laboratory and industrial scales. To verify and uphold those suggestions, more intensive and scientific studies should be done in the near future. New inventions of sharia-compliant cell lines and applicable growth media may also resolve existing problems with sharia-compliant processing.
Although most parts of this review focused on the establishment of sharia-compliant vaccine processing, the outputs might be useful for befitting the creation of safer animal-free vaccines that meet strict regulation policies. In sharia-compliant or halal processing, all ingredients employed for food and medicine production must be sourced from safe, clean, and nutritious supplies, notably involving animal-derived products that compulsorily originated from religiously slaughtered animals and passed high safety screening as exercised under the concept of halalan toyyiban. This review may also be used as a reference for amending more specific and standard guidelines for halal vaccine certification. The authors hope that this review could be a precursor and great help to other researchers who have the same interests in developing sharia-compliant vaccine processing.
Declaration of interest statement
The authors declare that they have no conflicts of interest.
Acknowledgments
Acknowledgements are extended to Department of Chemical and Process Engineering, UKM for their support, and also to Biomedical Research Centre, Northwest Minzu University and Jilin Guan Jie Biotechnology Co. Ltd. for their kind assists and shared knowledge during our visits.
Additional information
Funding
References
- Riedel S. Edward Jenner and the history of smallpox and vaccination. Baylor Univ Med Cent Proc. 2005;18(1):21–25. PMID: 16200144. doi:10.1080/08998280.2005.11928028.
- Greenwood B. The contribution of vaccination to global health: past, present and future. Philos Trans R Soc L B Biol Sci. 2014;369(1645):20130433. PMID: 24821919. doi:10.1098/rstb.2013.0433.
- Ahmed A, Lee KS, Bukhsh A, Al-Worafi YM, Sarker MMR, Ming LC, Khan TM. Outbreak of vaccine-preventable diseases in Muslim majority countries. J Infect Public Heal. 2018;11(2):153–55. PMID: 28988775. doi:10.1016/j.jiph.2017.09.007.
- Khoo YSK, Ghani AA, Navamukundan AA, Jahis R, Gamil A. Unique product quality considerations in vaccine development, registration and new program implementation in Malaysia. Hum Vaccin Immunother. 2020;16(3):530–38. PMID: 31652090. doi:10.1080/21645515.2019.1667206.
- Zarif MMM, Murad A, Yusof AM. The use of forbidden materials in medicinal products: an Islamic perspective. Middle-East J Sci Res. 2013;13(Approachesof Halal and Thoyyib for Society, Wellness and Health):5–10. doi:10.5829/idosi.mejsr.2013.16.s.10022.
- Padela AI, Furber SW, Kholwadia MA, Moosa E. Dire necessity and transformation: entry-points for modern science in Islamic bioethical assessment of porcine products in vaccines. Bioethics. 2014;28(2):59–66. doi:10.1111/bioe.12016.
- Department of Standards Malaysia (STANDARDS MALAYSIA). Halal pharmaceuticals - general requirements (first revision) MS2424:2019. Cyberjaya (Malaysia): Department of Standards Malaysia; 2019.
- Thomson Reuters, Dinar Standard. An inclusive ethical economy: state of the global Islamic economy report 2018/19. Dubai (UAE): Thomson Reuters; 2018. [accessed 2019 Sep 29]. https://haladinar.io/hdn/doc/report2018.pdf
- World Health Organization (WHO). Influenza (Seasonal). 2018 Nov 6 [accessed 2019 Aug 26]. https://www.who.int/en/news-room/fact-sheets/detail/influenza-(seasonal)
- Balkhy HH, Memish ZA, Bafaqeer S, Almuneef MA. Influenza a common viral infection among Hajj pilgrims: time for routine surveillance and vaccination. J Travel Med. 2004;11(2):82–86. PMID: 15109471. doi:10.2310/7060.2004.17027.
- Gregersen JP, Schmitt HJ, Trusheim H, Bröker M. Safety of MDCK cell culture-based influenza vaccines. Futur Microbiol. 2011;6(2):143–52. PMID: 21366415. doi:10.2217/fmb.10.161.
- Huang D, Peng WJ, Ye Q, Liu XP, Zhao L, Fan L, Xia-Hou K, Jia HJ, Luo J, Zhou LT, et al. Serum-free suspension culture of MDCK cells for production of influenza H1N1 vaccines. PLoS One. 2015;10(11):e0141686. PMID: 26540170. doi:10.1371/journal.pone.0141686.
- Hu AY, Tseng YF, Weng TC, Liao CC, Wu J, Chou AH, Chao HJ, Gu A, Chen J, Lin SC, et al. Production of inactivated influenza H5N1 vaccines from MDCK cells in serum-free medium. PLoS One. 2011;6(1): e14578. PMID: 21283675. doi:10.1371/journal.pone.0014578.
- Genzel Y, Dietzsch C, Rapp E, Schwarzer J, Reichl U. MDCK and Vero cells for influenza virus vaccine production: a one-to-one comparison up to lab-scale bioreactor cultivation. Appl Microbiol Biotechnol. 2010;88(2):461–75. PMID: 20617311. doi:10.1007/s00253-010-2742-9.
- Offit PA, Jew RK. Addressing parents’ concerns: do vaccines contain harmful preservatives, adjuvants, additives, or residuals? Pediatrics. 2003;112(6 Pt 1):1394–97. PMID: 14654615. doi:10.1542/peds.112.6.1394.
- Finn TM, Egan W. Vaccine additives and manufacturing residuals in vaccines licensed in the United States. In: Plotkin SA, Orenstein WA, Offit PA, Edwards KM, editors. Plotkin’s vaccines. 7th ed. Philadelphia (PA): Elsevier; 2018. p. 75–83.e2. doi:10.1016/B978-0-323-35761-6.00007-9
- Eisfeld AJ, Neumann G, Kawaoka Y. Influenza A virus isolation, culture and identification. Nat Protoc. 2014;9(11):2663–81. PMID: 25321410. doi:10.1038/nprot.2014.180.
- Besnard L, Fabre V, Fettig M, Gousseinov E, Kawakami Y, Laroudie N, Scanlan C, Pattnaik P. Clarification of vaccines: an overview of filter based technology trends and best practices. Biotechnol Adv. 2016;34(1):1–13. PMID: 26657051. doi:10.1016/j.biotechadv.2015.11.005.
- Wolff MW, Reichl U. Downstream processing of cell culture-derived virus particles. Expert Rev Vaccines. 2011;10(10):1451–75. PMID: 21988309. doi:10.1586/erv.11.111.
- Josefsberg JO, Buckland B. Vaccine process technology. Biotechnol Bioeng. 2012;109(6):1443–60. PMID: 22407777. doi:10.1002/bit.24493.
- Cardoso FMC, Petrovajová D, Horňáková T. Viral vaccine stabilizers: status and trends. Acta Virol. 2017;61(3):231–39. PMID: 28854787. doi:10.4149/av_2017_301.
- Soema PC, Kompier R, Amorij JP, Kersten GF. Current and next generation influenza vaccines: formulation and production strategies. Eur J Pharm Biopharm PMID: 26047796. 2015;94:251–63. doi:10.1016/j.ejpb.2015.05.023.
- Amorij JP, Huckriede A, Wilschut J, Frijlink HW, Hinrichs WL. Development of stable influenza vaccine powder formulations: challenges and possibilities. Pharm Res. 2008;25(6):1256–73. PMID: 18338241. doi:10.1007/s11095-008-9559-6.
- Hankaniemi MM, Laitinen OH, Stone VM, Sioofy-Khojine A, Määttä JAE, Larsson PG, Marjomäki V, Hyöty H, Flodström-Tullberg M, Hytönen VP. Optimized production and purification of Coxsackievirus B1 vaccine and its preclinical evaluation in a mouse model. Vaccine. 2017;35(30):3718–25. PMID: 28579231. doi:10.1016/j.vaccine.2017.05.057.
- Ersahin ME, Ozgun H, Dereli RK, Ozturk I, Roest K, van Lier JB. A review on dynamic membrane filtration: materials, applications and future perspectives. Bioresour Technol PMID: 22534368. 2012;122:196–206. doi:10.1016/j.biortech.2012.03.086.
- Zhao M, Vandersluis M, Stout J, Haupts U, Sanders M, Jacquemart R. Affinity chromatography for vaccines manufacturing: finally ready for prime time? Vaccine. 2019;37(36):5491–503. PMID: 29627235. doi:10.1016/j.vaccine.2018.02.090.
- Salleh MMM, Deuraseh N, Subri IM, Rahman SA, Mustafa S, Jamaludin MA, Safian YHM. The use of ceramic product derived from non-ḥalal animal bone: is it permissible from the perspective of Islamic law? Int J Asian Soc Sci. 2017;7(3):192–98. doi:10.18488/journal.1/2017.7.3/1.3.192.198.
- Cassano A, Basile A. Membranes for industrial microfiltration and ultrafiltration. In: Basile A, Nunes SP, editors. Advanced membrane science and technology for sustainable energy and environmental applications. Cambridge (UK): Woodhead Publishing; 2011. p. 647–79. doi:10.1533/9780857093790.5.647.
- World Health Organization. Cell culture as a substrate for the production of influenza vaccines: memorandum from a WHO meeting. Bull World Heal Organ. 1995;73(4): 431–35. PMID: 7554013.
- Ab Halim MAB, Kashim MIABM, Salleh MMM, Nordin NB, Husni ABM. Halal pharmaceuticals. The Soc Sci. 2015;10(4):490–98. doi:10.3923/sscience.2015.490.498.
- Tuan Sidek TM, Ridzwan A. Aplikasi najis mughallazah dalam penetapan halal semasa di Malaysia menurut perspektif Maqasid al-Shariah [Applications of severe najs in determining the current halal in Malaysia according to Maqasid al-Shariah perspective]. J AL-ANWAR, Persat Bek Mhs Islam Timur. 2018; 5(1):1–21. [accessed 2019 Jun 18]. https://umexpert.um.edu.my/file/publication/00002816_165228_77194.pdf.
- Food and Drug Administration (FDA). Guidance for industry: characterization and qualification of cell substrates and other biological materials used in the production of viral vaccines for infectious disease indications. 2010 Feb [accessed 2019 Jun 20]. http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/default.htm
- World Health Organization (WHO). Recommendations for the evaluation of animal cell cultures as substrates for the manufacture of biological medicinal products and for the characterization of cell banks. Annex 3. In: WHO Expert Committee on Biological Standardization. Sixty first report. WHO technical report series. No. 978. Geneva (Switzerland): WHO Press; 2010. [accessed 2019 Jun 20]. https://www.who.int/biologicals/Cell_Substrates_clean_version_18_April.pdf
- Hess RD, Weber F, Watson K, Schmitt S. Regulatory, biosafety and safety challenges for novel cells as substrates for human vaccines. Vaccine. 2012;30(17):2715–27. PMID: 22342707. doi:10.1016/j.vaccine.2012.02.015.
- Sheng-Fowler L, Lewis AM, Peden K. Issues associated with residual cell-substrate DNA in viral vaccines. Biologicals. 2009;37(3):190–95. PMID: 19285882. doi:10.1016/j.biologicals.2009.02.015.
- Peden K, Sheng L, Pal A, Lewis A. Biological activity of residual cell-substrate DNA. Dev Biol (Basel). 2006;123:45–53. PMID: 16566436.
- Wang X, Hunter AK, Mozier NM. Host cell proteins in biologics development: identification, quantitation and risk assessment. Biotechnol Bioeng. 2009;103(3):446–58. PMID: 19388135. doi:10.1002/bit.22304.
- Le Ru A, Jacob D, Transfiguracion J, Ansorge S, Henry O, Kamen AA. Scalable production of influenza virus in HEK-293 cells for efficient vaccine manufacturing. Vaccine. 2010;28(21):3661–71. PMID: 20347632. doi:10.1016/j.vaccine.2010.03.029.
- Pau MG, Ophorst C, Koldijk MH, Schouten G, Mehtali M, Uytdehaag F. The human cell line PER.C6 provides a new manufacturing system for the production of influenza vaccines. Vaccine. 2001;19(17–19):2716–21. PMID: 11257414. doi:10.1016/s0264-410x(00)00508-9.
- van Wielink R, Kant-Eenbergen HCM, Harmsen MM, Martens DE, Wijffels RH, Coco-Martin JM. Adaptation of a Madin-Darby canine kidney cell line to suspension growth in serum-free media and comparison of its ability to produce avian influenza virus to Vero and BHK21 cell lines. J Virol Methods. 2011;171(1):53–60. PMID: 20933017. doi:10.1016/j.jviromet.2010.09.029.
- Genzel Y. Designing cell lines for viral vaccine production: where do we stand? Biotechnol J. 2015;10(5):728–40. PMID: 25903999. doi:10.1002/biot.201400388.
- White KM, Ayllon J, Mena I, Potenski A, Krammer F, García-Sastre A. Influenza B virus reverse genetic backbones with improved growth properties in the EB66® cell line as basis for vaccine seed virus generation. Vaccine. 2018;36(9):1146–53. PMID: 29395518. doi:10.1016/j.vaccine.2018.01.050.
- Lohr V, Rath A, Genzel Y, Jordan I, Sandig V, Reichl U. New avian suspension cell lines provide production of influenza virus and MVA in serum-free media: studies on growth, metabolism and virus propagation. Vaccine. 2009;27(36):4975–82. PMID: 19531390. doi:10.1016/j.vaccine.2009.05.083.
- Petiot E, Proust A, Traversier A, Durous L, Dappozze F, Gras M, Guillard C, Balloul JM, Rosa-Calatrava M. Influenza viruses production: evaluation of a novel avian cell line DuckCelt®-T17. Vaccine. 2018;36(22):3101–11. PMID: 28571695. doi:10.1016/j.vaccine.2017.03.102.
- Coussens PM, Smith KA, Weber PSD, Colvin CJ. Immortalized chick embryo cell line adapted to serum-free growth conditions and capable of replicating human and reassortant H5N1 influenza strains for vaccine production. Vaccine. 2011;29(47):8661–68. PMID: 21911025. doi:10.1016/j.vaccine.2011.08.122.
- Cox MMJ, Anderson DK. Production of a novel influenza vaccine using insect cells: protection against drifted strains. Influenza Other Respi Viruses. 2007;1(1):35–40. PMID: 19453478. doi:10.1111/j.1750-2659.2006.00007.x.
- Jamaludin MA, Ramli MA, Rahman SA Panduan makanan halal haram menurut perspektif al-Quran: analisis terhadap isu-isu makanan semasa [Guidelines of halal haram food according to al-Quran perspective: analysis on current food issues]. In: International Seminar on Wahyu Asas Tamadun 2; 2011; Nilai, Malaysia. p. 1–15.
- Regenstein JM, Chaudry MM, Regenstein CE. The kosher and halal food laws. Compr Rev Food Sci Food Saf. 2003;2(3):111–27. doi:10.1111/j.1541-4337.2003.tb00018.x.
- Yao T, Asayama Y. Animal-cell culture media: history, characteristics, and current issues. Reprod Med Biol. 2017;16(2):99–117. PMID: 29259457. doi:10.1002/rmb2.12024.
- van der Valk J, Brunner D, De Smet K, Fex Svenningsen Å, Honegger P, Knudsen LE, Lindl T, Noraberg J, Price A, Scarino ML, et al. Optimization of chemically defined cell culture media – replacing fetal bovine serum in mammalian in vitro methods. Toxicol Vitr. 2010;24(4):1053–63. doi:10.1016/j.tiv.2010.03.016.
- Gstraunthaler G. Alternatives to the use of fetal bovine serum: serum-free cell culture. ALTEX. 2003;20(4):275–81. PMID: 14671707.
- Brunner D, Frank J, Appl H, Schöffl H, Pfaller W, Gstraunthaler G. Serum-free cell culture: the serum-free media interactive online database. ALTEX. 2010;27(1):53–62. doi:10.14573/altex.2010.1.53.
- Jochems CEA, Van der Valk JBF, Stafleu FR, Baumans V. The use of fetal bovine serum: ethical or scientific problem? ATLA Altern to Lab Anim. 2002;30(2):219–27. PMID: 11971757. doi:10.1177/026119290203000208.
- Fletcher T, Harris H. Safety drives innovation in animal-component-free cell-culture media technology. BioPharm Int. 2016; 29(9):22–27. [accessed 2019 Mar 14]. http://www.biopharminternational.com/safety-drives-innovation-animal-component-free-cell-culture-media-technology.
- Mohezar S, Zailani S, Tieman M. Tapping into the halal pharmaceutical market: issues and challenges. In: Ab. Manan SK, Abd Rahman F, Sahri M, editors. Contemporary issues and development in the global halal industry. Singapore: Springer Singapore; 2016. p. 531–41. doi:10.1007/978-981-10-1452-9_48.
- Kashim MIAM, Jamsari EA, Safiai MH, Adnan NIM, Safri LS. Genetic modified organisms (GMOs) from the perspective of science and Maqasid Shari‘ah. Int J Civ Eng Technol. 2018; 9(8):1381–93. [accessed 2020 Feb 14]. http://www.iaeme.com/ijciet/issues.asp?JType=IJCIET&VType=9&IType=8.
- Eagle H. Amino acid metabolism in mammalian cell cultures. Science (80-). 1959;130(3373):432–37. PMID: 13675766. doi:10.1126/science.130.3373.432.
- Freshney RI. Defined media and supplements. In: Freshney RI, editor. Culture of animal cells. 5th ed. Hoboken (NJ): John Wiley & Sons; 2005. p. 115–28. doi:10.1002/0471747599.cac009
- Ermis E. Halal status of enzymes used in food industry. Trends Food Sci Technol. 2017;64:69–73. doi:10.1016/j.tifs.2017.04.008.
- Karahalil E. Principles of halal-compliant fermentations: microbial alternatives for the halal food industry. Trends Food Sci Technol. 2020;98:1–9. doi:10.1016/j.tifs.2020.01.031.
- D’Este M, Alvarado-Morales M, Angelidaki I. Amino acids production focusing on fermentation technologies – A review. Biotechnol Adv. 2018;36(1):14–25. PMID: 28888551. doi:10.1016/j.biotechadv.2017.09.001.
- Lu Y, Wang L, Teng F, Zhang J, Hu M, Tao Y. Production of myo-inositol from glucose by a novel trienzymatic cascade of polyphosphate glucokinase, inositol 1-phosphate synthase and inositol monophosphatase. Enzyme Microb Technol PMID: 29499774. 2018;112:1–5. doi:10.1016/j.enzmictec.2018.01.006.
- Lim SH, Choi JS, Park EY. Microbial production of riboflavin using riboflavin overproducers,Ashbya gossypii, Bacillus subtilis, and Candida famate: an overview. Biotechnol Bioprocess Eng. 2001;6(2):75–88. doi:10.1007/BF02931951.
- Acevedo-Rocha CG, Gronenberg LS, Mack M, Commichau FM, Genee HJ. Microbial cell factories for the sustainable manufacturing of B vitamins. Curr Opin Biotechnol PMID: 30138794. 2019;56:18–29. doi:10.1016/j.copbio.2018.07.006.
- Mótyán JA, Tóth F, Tőzsér J. Research applications of proteolytic enzymes in molecular biology. Biomolecules. 2013;3(4):923–42. PMID: 24970197. doi:10.3390/biom3040923.
- Seitz C, Isken B, Heynisch B, Rettkowski M, Frensing T, Reichl U. Trypsin promotes efficient influenza vaccine production in MDCK cells by interfering with the antiviral host response. Appl Microbiol Biotechnol. 2012;93(2):601–11. PMID: 21915610. doi:10.1007/s00253-011-3569-8.
- Rourou S, van der Ark A, van der Velden T, Kallel H. A microcarrier cell culture process for propagating rabies virus in Vero cells grown in a stirred bioreactor under fully animal component free conditions. Vaccine. 2007;25(19):3879–89. doi:10.1016/j.vaccine.2007.01.086.
- Nestler L, Evege E, Mclaughlin J, Munroe D, Tan T, Wagner K, Stiles B. TrypLE TM Express: A temperature stable replacement for animal trypsin in cell dissociation applications. QUEST. 2004; 1(1):42–47. [accessed 2019 Feb 20]. https://pdfs.semanticscholar.org/6c6b/b1d2772ffccd0f004135dbf07bc1750feecd.pdf.
- Manira M, Khairul Anuar K, Seet WT, Ahmad Irfan AW, Ng MH, Chua KH, Mohd Heikal MY, Aminuddin BS, Ruszymah BHI. Comparison of the effects between animal-derived trypsin and recombinant trypsin on human skin cells proliferation, gene and protein expression. Cell Tissue Bank. 2014;15(1):41–49. PMID: 23456438. doi:10.1007/s10561-013-9368-y.
- Tsuji K, Ojima M, Otabe K, Horie M, Koga H, Sekiya I, Muneta T. Effects of different cell-detaching methods on the viability and cell surface antigen expression of synovial mesenchymal stem cells. Cell Transplant. 2017;26(6):1089–102. PMID: 28139195. doi:10.3727/096368917x694831.
- Nacalai Tesque. Cell dissociation reagents (accutase). [accessed 2019 Jul 9]. https://www.nacalai.co.jp/global/reagent/research/Accutase.html
- Sigma-Aldrich. Accutase ® solution. 2019 [accessed 2019 Jul 9]. https://www.sigmaaldrich.com/content/dam/sigma-aldrich/docs/Sigma/Datasheet/6/a6964dat.pdf
- Corning. Accutase ® cell detachment solution. 2015 [accessed 2019 Jul 4]. www.corning.com/lifesciences
- Biological Industries. Accutase. 2014 Jan [accessed 2019 Jul 9]. https://www.bioind.com/media/wysiwyg/product/cell-culture-reagents/Accutase-cell-dissociation.pdf
- Skog M, Sivlér P, Steinvall I, Aili D, Sjöberg F, Elmasry M. The effect of enzymatic digestion on cultured epithelial autografts. Cell Transplant. 2019;28(5):638–44. PMID: 30983404. doi:10.1177/0963689719833305.
- Bajpai R, Lesperance J, Kim M, Terskikh AV. Efficient propagation of single cells accutase-dissociated human embryonic stem cells. Mol Reprod Dev. 2008;75(5):818–27. doi:10.1002/mrd.20809.
- Azmi MAI Kesan enzim proteolitik berbeza terhadap infektiviti virus influenza B ke atas kultur sel mamalia untuk penghasilan vaksin patuh syariah [Effect of different proteolytic enzymes on the infectivity of influenza B virus in mammalian cell culture for production of sharia-compliance virus vaccines] [BEng. thesis]. Bangi (Malaysia): Universiti Kebangsaan Malaysia; 2019.
- Kumru OS, Joshi SB, Smith DE, Middaugh CR, Prusik T, Volkin DB. Vaccine instability in the cold chain: mechanisms, analysis and formulation strategies. Biologicals. 2014;42(5):237–59. PMID: 24996452. doi:10.1016/j.biologicals.2014.05.007.
- Batu A, Regenstein JM, Doğan İS. Gelatin issues in halal food processing for Muslim societies. Int Period Lang Lit Hist Turkish or Turkic. 2015;10(14):37–52. doi:10.7827/TurkishStudies.8928.
- Gomez-Guillen MC, Gimenez B, Lopez-Caballero ME, Montero MP. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011;25(8):1813–27. doi:10.1016/j.foodhyd.2011.02.007.
- Rakhmanova A, Khan ZA, Sharif R, Lü X. Meeting the requirements of halal gelatin: a mini review. MOJ Food Process Technol. 2018;6(6):477–82. doi:10.15406/mojfpt.2018.06.00209.
- Gelatin Manufacturers Institute of America (GMIA). Gelatin handbook. 2019 [accessed 2020 Feb 13]. http://www.gelatin-gmia.com/uploads/1/1/8/4/118450438/gmia_gelatin_manual_2019.pdf
- Kashim MIAM. Istihalah dan kesannya kepada makanan menurut perspektif Islam [The effect of istihalah on food according to Islamic perspective]. J Soc Sci Humanit. 2017; 12(2):102–11. [accessed 2019 Mar 20]. http://ejournal.ukm.my/ebangi/article/viewFile/20398/6436.
- Grabenstein JD. What the world’s religions teach, applied to vaccines and immune globulins. Vaccine. 2013;31(16):2011–23. PMID: 23499565. doi:10.1016/j.vaccine.2013.02.026.
- Abd Rahman N, Kamarudin NS, Esa F, Kalil MS, Kamarudin SK. Bacterial cellulose as a potential hard gelatin capsule. J Kejuruter. 2019;SI 2(1):151–56. doi:10.17576/jkukm-2019-si2(1)-19.
- Whitford WG, Fairbank A. Considerations in scale-up of viral vaccine production. Bioprocess Int. 2011; 9(8):16–28. [accessed 2019 Jan 29]. https://bioprocessintl.com/manufacturing/monoclonal-antibodies/considerations-in-scale-up-of-viral-vaccine-production-320990/.
- Nilsson K. Microcarrier cell culture. Biotechnol Genet Eng Rev. 1988;6(1):403–39. PMID: 3071374. doi:10.1080/02648725.1988.10647854.
- Li B, Wang X, Wang Y, Gou W, Yuan X, Peng J, Guo Q, Lu S. Past, present, and future of microcarrier-based tissue engineering. J Orthop Transl. 2015;3(2):51–57. PMID: 30035040. doi:10.1016/j.jot.2015.02.003.
- Hassan WMW. Globalising halal standards: issues and challenges. Halal J. 2007;38–40. [accessed 2020 Oct 14]. https://www.academia.edu/243214/Globalising_Halal_Standards_Issues_and_Challenges
- Mahyeddin MS, Afifi M, Halim A, Mahyeddin M, Salleh M. The possibility of uniformity on halal standards in organization of Islamic countries (OIC) country. World Appl Sci J. 2012; 17(Towardsthe Traceability of Halal and Thoyyiban Application):6–10. [accessed 2020 Oct 14]. http://ddms.usim.edu.my:80/jspui/handle/123456789/15369.
- Kashim MIAM, Majid LA, Adnan AHM, Husni ABM, Nasohah Z, Samsudin MA, Yahaya MZ. Principles regarding the use of haram (forbidden) sources in food processing: a critical Islamic analysis. Asian Soc Sci. 2015;11(22):17–25. doi:10.5539/ass.v11n22p17.
- Norazmi MN, Lim LS. Halal pharmaceutical industry: opportunities and challenges. Trends Pharmacol Sci. 2015;36(8):496–97. PMID: 26187623. doi:10.1016/j.tips.2015.06.006.
- Abdullah AB. Challenges facing halal vaccine industry. New Straits Times. 2017 Apr 21. [accessed 2018 Aug 13. https://www.nst.com.my/opinion/columnist/2017/04/232513/challenges-facing-halal-vaccine-industry.
- Alzeer J. Halalopathy: A science of trust in medicine. J Integr Med. 2019;17(3):150–54. PMID: 30948352. doi:10.1016/j.joim.2019.03.005.
- Basari H. New frontiers in halal pharmaceuticals. Int J Res Pharm Sci. 2019;10(SPL1). doi:10.26452/ijrps.v10ispl1.1687.
