ABSTRACT
A new vaccination schedule with one dose of inactivated polio vaccine (IPV) followed by three doses of bivalent oral attenuated live polio vaccine (bOPV) was introduced in China in 2016. Both Sabin IPV (sIPV) and Salk IPV (wIPV) sequentially with bOPV were accepted in the Chinese routine vaccination schedule. We intended to assess the immunogenicity of the current primary schedule (s/wIPV-bOPV-bOPV) and the schedule in the early stage of the switch (tOPV-bOPV-bOPV), and compare immunogenicity between the groups with different polio virus strains. Healthy infants aged 60–89 days were recruited in hospitals in Chongqing. Infants were assigned to one of three treatments (tOPV-bOPV-bOPV, sIPV-bOPV-bOPV or wIPV-bOPV-bOPV) by enrollment time. Polio neutralizing antibody (NA) assays were conducted to assess immunity. 1027 eligible infants were enrolled. Over 95% seroprotection rates against type I poliovirus (PV1) and type III poliovirus (PV3) were observed in all groups. Infants who received tOPV-bOPV-bOPV had higher antibody titers against type II poliovirus (PV2) than did the IPV-bOPV-bOPV. The geometric mean titers (GMTs) of PV2 were only ~20 in the IPV-bOPV-bOPV. GMTs of PV1 were higher than PV3 in s/wIPV-bOPV-bOPV. The primary schedule of s/wIPV-bOPV-bOPV is insufficient to protect children against PV2, and the NA titer to PV3 is lower. Higher antibody responses were induced in sIPV-bOPV-bOPV than that in wIPV-bOPV-bOPV. Supplementary vaccination with one dose of IPV is necessary for children who had no tOPV immune history or had only one IPV to induce higher levels of immunity against PV2 and PV3.
1. Background
During Poliomyelitis elimination, polio vaccines have gone through multiple selections recommended by WHO. Following the elimination of PV2, the Strategic Advisory Group of Experts on Immunization endorsed a globally synchronized switch from tOPV to bOPV1&3.Citation1,Citation2 The Global Polio Eradication Initiative also recommended at least one dose of IPV in routine vaccination schedules when switching from tOPV to bOPV to avoid the potential risk of outbreaks by PV2.Citation3
The use of tOPV was successfully withdrawn from vaccination schedules in China on May 1, 2016, and a new routine schedule was introduced with one IPV dose given at 2 months of age, followed by one bOPV at 3 months, 4 months, and 4 years of age, respectively. Vaccination with sIPV and wIPV were provided to infants as the first dose of polio vaccine routine schedule for free. Although studies have shown that sequential schedules of wIPV with bOPVCitation4–7 and sIPV with tOPVCitation8–10 are sufficient for immunization, the immunogenicity of sequential schedules of different virus strain IPVs with bOPV remains unclear. Differences in the vaccines or the populations might affect immunogenicity.Citation11,Citation12 Since wIPV is no longer the only IPV vaccine for polio eradication due to its higher cost, sIPV has become more popular in low-income and middle-income countries. It is important for policy makers to know whether the immune effect of sIPV sequential with bOPV is the same as wIPV sequentially with bOPV, and whether there is adequate protection for PV2. We assessed the immunogenicity of the routine primary poliovirus vaccination schedule (s/wIPV-bOPV-bOPV) and the schedule in the early stage of the switch (tOPV-bOPV-bOPV) in China, and compared the immunogenicity of sequential vaccination of different strains of polio vaccines to provide scientific evidence for the conversion of polio vaccination procedures.
2. Methods
2.1. Study design and participants
According to per capita disposable income of rural residents in Chongqing, we divided 39 districts into three groups (high/middle/low) by economic level. Two districts in each level were selected by simple random sampling method. Finally, the following 6 areas were selected in the sero-epidemiological survey: (1) Jiangbei district and Fuling district (high level); (2) Hechuan district and Liangping district (middle level); (3) Fengjie district and Pengshui district (low level). In each district, the largest scale hospital was selected as the survey site. Infants aged 60–89 days that came for vaccination of first dose of polio vaccine were invited to participate in our study by convenience sampling. Infants who were immunodeficient or had taken immunosuppressant drugs during the last 2 months and had contraindications to polio vaccine were excluded from our study. Sample size was calculated based on an assumed seroprotection rates of 90%, error margin of±10%, α = 0.05. The sample was further inflated by approximately 20% to account for potential non-response, which resulted in a final sample size of 1140. There were 190 participants in each district and 380 in each level area. Questionnaires were used to collect subjects’ personal information (gender, age, region, vaccination history, etc.) and 3 ml of venous blood was collected from each subject at 4–5 weeks after the third dose of poliovirus vaccine for testing NA to PV.
According to enrollment time, infants were assigned to receive polio vaccine schedules: the first group received one dose of tOPV at age 2 months, followed by two doses of bOPV at ages 3 and 4 months (tOPV-bOPV-bOPV); the second group received one dose of sIPV followed by two doses of bOPV (sIPV-bOPV-bOPV); and the third group received one dose of wIPV followed by two doses of bOPV (wIPV-bOPV-bOPV).
2.2. Procedures
The Sabin IPV (sIPV) used in this study was manufactured by the Chinese Academy of Medical Sciences, it contains at least 15 D-Antigen unit of poliovirus serotype I, 45 D-Antigen unit of poliovirus serotype II, and 45 D-Antigen unit of poliovirus serotype III. The Salk IPV is manufactured by Sanofi Pasteur SA, France, it contains inactivated poliovirus serotype I (Mahoney strain; 40 D-Ag units), poliovirus serotype II (MEF-1 strain; 8 D-Ag units), and poliovirus serotype III (Saukett stain; 32 D-Ag units). The tOPV and bOPV used in this study are manufactured by the China National Pharmaceutical Group Corporation. tOPV is formulated to contain at least 5.8 lgCCID50 per dose of poliovirus serotype I,4.8 lgCCID50 of poliovirus 2,5.3 lgCCID50 of poliovirus III. bOPV is formulated to contain at least 6.0 lgCCID50 per dose of poliovirus serotype I and 5.5 lgCCID50 of poliovirus III.
Blood Samples were immediately placed in ice boxes and transported to the laboratory of local Center for Disease Control and Prevention (CDC). After centrifugation of blood, the serum was separated and then stored in refrigerator under −20°C . The samples were then transported to the polio laboratory of Chinese Academy of Medical Sciences for testing. Microneutralization assay was verified as gold standard by the Global Polio Laboratory Network,Citation13 it was recommended by WHO to measure the presence of type-specific neutralizing antibodies against Sabin-strain poliovirus types 1, 2, and 3. Before testing, each serum sample was inactivated at 56°C for 30 minutes and then diluted from 1:8 to 1:1,024 in two-fold serial dilutions. Each sample was incubated in duplicate wells for 3 hours at 36°C with 50% tissue culture infective doses (TCID50) of poliovirus antigen. After incubation for 7 days, the highest dilution of serum that protected 50% of the cultures was recorded.
2.3. Statistical analyses
Seropositivity rates and GMTs of antibodies were calculated for each group. When the titer of antibody was < 1:8, it was assigned a titer of 1:1, and when the titer was above the upper limit of detection (1/1024),it was assigned a titer of 1/2048. Serum sample with a titer of ≥1:8 for each PV was considered positive.Citation13 Cell controls and a reference serum were included in each test to examine reproducibility of results.
Statistical tests were performed using SPSS 23.0 software. Chi-square test or Fisher’s exact tests were used to determine the difference of antibody sero-positivity among demographic characteristics (schedule & region). Kruskal-Wallis and Wilcoxon test were used to compare neutralizing antibody titers. P-values less than 0.05 was considered to be significant.
3. Result
A total of 1040 children were eligible and assigned to three treatment groups, 1027 (98.75%) of 1040 participants completed all study vaccinations, and their blood samples were tested. Of the respondents (1027), 335(32.62%) were assigned to wIPV-bOPV-bOPV group, 358(34.86%) were assigned to sIPV-bOPV-bOPV group and 334 (32.52%) were assigned to tOPV-bOPV-bOPV group.
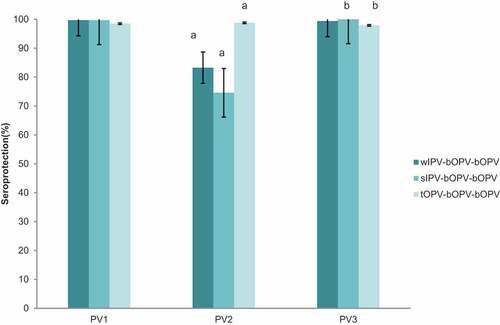
The seroprotection rates and GMTs of PV antibodies were measured at 5 to 11 months of age after completion of different schedules of primary polio vaccination series. The overall seroprotection rates of 1027 subjects in primary series ranged from 98.5 to 99.7% for PV1, from 74.58% to 98.8% for PV2, and from 97.9 to 100% for PV3 () . For PV1, there was no significant difference among different schedule groups. For PV2, the seroprotection rate in sIPV-bOPV-bOPV group was significantly lower than that in other two groups, the seroprotection rates between each two groups differed significantly (P < .05). For PV3, the seroprotection rate in sIPV-bOPV-bOPV group was significantly higher than that in tOPV-bOPV-bOPV(P = .01). The GMTs ranged from 781 to 3244 for PV1, from 15 to 771 for PV2, and from 274 to 1199 for PV3. GMTs of PV2 were only ~ 20 in both wIPV-bOPV-bOPV and sIPV-bOPV-bOPV, which were quite lower than GMTs of PV1 and PV3. And GMT of PV3 in tOPV-bOPV-bOPV was lower than that of PV1 and PV2. GMTs in every serotype significantly differed by pairwise comparison, except for GMTs of PV2 and PV3 between wIPV-bOPV-bOPV and sIPV-bOPV-bOPV ().
Table 1. GMTs of antibody against type I, II and III poliovirus after routine immunization among children of three groups
Figure 1. Seroprotection against type I, II and III poliovirus after different routine vaccination schedules. PV1 = type I poliovirus;PV2 = type II poliovirus;PV3 = type III poliovirus; IPV = Sabin strain inactivated polio vaccine; wIPV = Salk strain inactivated polio vaccine; bOPV = bivalent oral polio vaccine; tOPV = trivalent oral polio vaccine. error bar: standard error.(a)Significant difference of seroprotection in PV2 between each two groups;(b)Significant difference of seroprotection in PV3 between sIPV-bOPV- bOPV and tOPV-bOPV- bOPV
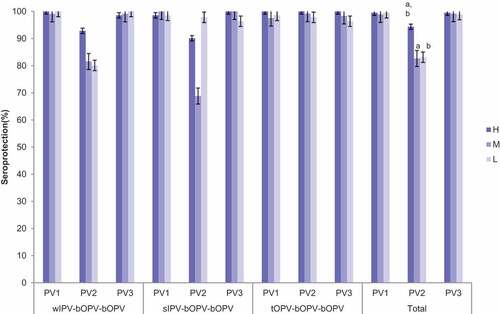
According to different regional economical level, the subjects were divided into three levels(high, middle, low). Of the respondents (1027), 213(20.74%) were from high economic-level areas(H), 375(36.51%) were from middle economic-level areas(M) and 439 (42.75%) were from low economic-level areas(L). In H area, the seroprotection rates ranged from 98.59% to 100% for PV1, from 90.14 to 100% for PV2, and from 98.57 to 100% for PV3. In M area, the seroprotection rates ranged from 97.56 to 100% for PV1, from 68.84 to 99.19% for PV2, and from 98.37 to 100% for PV3. In L area, the seroprotection rates ranged from 98.56 to 100% for PV1, from 72.48 to 97.84% for PV2, and from 96.40 to 100% for PV3. Totally, there was no significant difference of seroprotection among areas for PV1 and PV3, except for the seroprotection rates of PV2 among three areas (PH-M = 0.000,PH-L = 0.000). The differences were mainly observed in sIPV-bOPV-bOPV and wIPV-bOPV-bOPV by layout of areas () . There were significant differences among three areas for GMTs in PV2 and PV3, respectively(Ppv2 = 0.000, Ppv3 = 0.011) . For PV2, the GMT in H area was significantly higher than that in the other two areas(PH-M = 0.000,PH-L = 0.000). For PV3, the GMT in H area was significantly higher than that in L area (P = .008) ().
Table 2. GMTs against type I, II and III poliovirus after different primary immunization schedules among children in areas with different economical levels
Figure 2. Seroprotection against type I, II and III poliovirus after routine vaccination of three schedules in different areas. PV1 = type I poliovirus;PV2 = type II poliovirus;PV3 = type III poliovirus;; sIPV = Sabin strain inactivated polio vaccine; wIPV = Salk strain inactivated polio vaccine; bOPV = bivalent oral polio vaccine; tOPV = trivalent oral polio vaccine. error bar: standard error. There were no significant differences of seroprotection among areas in PV1 and PV3. (a)Significant difference of seroprotection in PV2between high economic level area and middle economic level area. (b) Significant difference of seroprotection in PV2 between high economic level area and low economic level area
4. Discussion
The Polio Eradication and Endgame Strategic Plan’s globally synchronized switch from tOPV to bOPV was accomplished on May 1st, 2016 in all OPV-using countries to mitigate the potential risk of outbreaks by type 2 poliovirus. And a new polio routine vaccination schedule was introduced with one IPV dose. It is important for policy makers to know whether current polio schedule is adequate for type 2 protection in countries with one dose of IPV, and to be informed of the immunological effect of sequential schedules with different strains IPV followed by bOPV.
Our study showed that all three sequential schedules resulted in high antibody titers against PV1 and PV3. However, the immune effect in different economical level areas were different, it might be attributed to complicated factors such as individual differences, cold storage condition of vaccine, the direction of needles, interference from maternal antibodies and circulation of some poliovirus in the environment, etc.
Our study have found that the seroprotection rates of PV1 and PV3 were above 95%, but the schedules with IPV only elicited higher antibody titers against PV1 and PV3 than the schedule with no IPV, that was the same with Saleem AF and Wright PF’s finding.Citation14,Citation15 The result also showed that sIPV-bOPV-bOPV induced stronger immunity than wIPV-bOPV-bOPV against PV1. This phenomenon might be explained as that Sabin strain was used to detect neutralizing antibodies in our NA assays.Citation16 Production of sIPV and bOPV were all using the same vaccine strain (Sabin-strain), so the immune effect of sequential schedule with the same strain vaccines might be better than schedule with different strains(Salk-strain and Sabin-strain) . There were few studies reported the immune effect of sequential schedule with different poliovirus-strains vaccines. Further studies are needed to verify the assumptions.
For PV2, the IPV-bOPV-bOPV groups did not induce a strong immunity comparing with tOPV-bOPV-bOPV group. The seroprotection rates of PV2 were below 85% in two IPV-bOPV-bOPV groups, and it was even lower of 74.58% in sIPV-bOPV-bOPV in some studies.Citation4,Citation7,Citation9,Citation14 GMTs of PV2 in two IPV-bOPV-bOPV groups were also lower than that in tOPV-bOPV-bOPV group, very close to the clinically protective level (1:8). Although there was only one dose of live polio vaccine containing type 2 virus in tOPV-bOPV-bOPV group, the seroprotection rate of PV2 was nearly 100% and GMT of PV2 was up to 1:674. The results showed that the sequential schedule of tOPV- bOPV- bOPV had significantly better effect than IPV- bOPV-bOPV on immunity of PV2.Compared to one dose of IPV, one dose of tOPV expressed a great antigenicity immunogenicity. It indicated that only one dose of IPV followed by bOPV cannot produce enough antibodies of PV2. Although IPV at the first dose can reduce the risk of paralytic polio from OPV or wild poliovirus, but it was less immunogenic for poliovirus type 2.
The presence of type 2 maternally derived antibodies are reported to be prominent at 36 weeks of age and are associated with lower rate of seroprotection in infants who received a single dose of IPV. Therefore, most countries or researchers would adopt at least two IPVs followed by OPVsCitation8,Citation12,Citation17–28 to offset the interference.Citation22 We believe that adding a second IPV dose will lead to higher sero-conversion rates and will induce higher antibody titers against type 2 poliovirus. We suggested that more than one dose of IPV should be brought into polio vaccination schedule and supplementary doses of IPVs should be given to the children who only received one IPV before.
The trial in Chile using IPV followed by bOPV sequential schedules (wIPV-bOPV1&3-bOPV1&3) demonstrated that bOPV could enhance antibody level against type 2 polio virus, and there are some cross-booster effect against PV2 by the other two serotypes included in bOPV.Citation4,Citation29 However, we didn’t observe the same result above in our study. Further studies on cross-reinforcing effect by bOPV on PV2 should be studied.
The immunity level of PV3 is the lowest among all types of poliovirus in many studies, it can be explained by a reduced antigenicity immunogenicity of PV3 when compared to PV1 and PV2.Citation30–32 It would be further studied in future vaccine development and optimization of immunity to PV3 is a matter of urgency.Citation33 Therefore, continuing polio vaccination campaigns (IPV or bOPV1&3) are as important as monitoring the population’s immunity to sustain the present polio-free situation.
This study also has limitations. Firstly, we did not collect blood sample before the first poliovirus vaccine to assess maternal poliovirus neutralizing antibodies pre-vaccination. Though interference of maternal antibodies with infant immune responses to polio vaccination appeared to be one potential barrier to children’s polio sero-conversion rates after vaccinationCitation34, we think this effect could be minimized by giving three doses of polio vaccines during the first year of life with optimal spacing. Secondly, due to the insufficient supply of IPV and bOPV in the beginning of vaccination schedules switch, it was difficult to administer the polio routine vaccination at 2,3,4 month timely, but all subjects finished primary routine vaccination before 7 month.
5. Conclusion
One dose of IPV (Sabin or Salk) followed by two doses of bOPV is insufficient to protect population against type 2 poliovirus. A second dose of IPV has already been recommended into routine vaccination schedules in China on January 1, 2020.Citation35 But there are risks of infecting type 2 poliovirus for the children with only one IPV vaccination history, we suggest that supplementary vaccination with one IPV for these children as soon as possible.
Abbreviations
| PV | = | Poliovirus |
| GMTs | = | Geometric mean titers |
| NA | = | Neutralizing antibodies |
| WPV | = | Wild poliovirus |
| GPEI | = | Global Polio Eradication Initiative |
| EPI | = | Expanded Immunization Program |
| OPV | = | Oral attenuated polio vaccine |
| bOPV | = | Bivalent oral polio vaccine |
| tOPV | = | Trivalent oral polio vaccine |
| IPV | = | Inactivated polio vaccine |
| sIPV | = | Sabin strain IPV |
| wIPV | = | Salk strain IPV |
| SIAs | = | Supplementary immunization activities |
| TCID | = | Tissue culture infective doses |
| CDC | = | Center for Disease Control and Prevention |
| CI | = | Confidence interval |
Authors’ contributions
Qing Wang, Rong Rong and Wenge Tang planned the study. Jiawei Xu, Yuanyuan Zhang and Shanshan Kuang were in charge of data collection and blood samples collection. Jiawei Xu carried out the immunoassays and performed the statistical analysis. Jiawei Xu drafted and Yuanyuan Zhang edited the manuscript. Yuanyuan Zhang and Xiaojuan Fu participated in data analyzed . All authors read and approved the final manuscript.
Availability of data and materials
Data used for the analysis may be found at: http://www.chinacdc.cn/jkzt/ymyjz/
Disclosure of potential conflicts of interest
The authors declare that they have no competing interests.
Ethics approval and consent to participate
All procedures involving human in this study was granted by the Ethical Committee of Chongqing CDC. Written informed consent was obtained from the legal guardians of the children before enrolment.
Acknowledgments
Thanks to the staff for conducting volunteer enrollment and specimen collection in the district CDCs of Chongqing. We also thank the laboratory staff for conducting the respective testing in Chinese Academy of Medical Sciences.
Additional information
Funding
References
- WHO. Meeting of the strategic advisory group of experts on immunization, November 2013—conclusions and recommendations. Wkly Epidemiol Rec. 2014;89:1–20.
- Global Polio Eradication Initiative. The polio endgame strategy 2019–2023. [accessed 2019 Aug 28]. http://polioeradication.org/wp-content/uploads/2019/06/English-polio-endgame-strategy.pdf.
- Van den Ent M, Swift RD, Anaokar S, Hegg LA, Eggers R, Cochi SL. Contribution of global polio eradication initiative funded personnel to the strengthening of routine immunization programs in the 10 focus countries of the Polio Eradication and Endgame Strategic Plan. J Infect Dis. 2017;216(suppl 1):S244–49. doi:10.1093/infdis/jiw567.
- O’Ryan M, Bandyopadhyay AS, Villena R, Espinoza M, Novoa J, Weldon WC, Oberste MS, Self S, Borate BR, Asturias EJ, et al. Inactivated poliovirus vaccine given alone or in a sequential schedule with bivalent oral poliovirus vaccine in Chilean infants: a randomised, controlled, open-label, phase 4, non-inferiority study. Lancet Infect Dis. 2015;15:1273–82. doi:10.1016/S1473-3099(15)00219-4.
- Asturias EJ, Bandyopadhyay AS, Self S, Rivera L, Saez-Llorens X, Lopez E, Melgar M, Gaensbauer JT, Weldon WC, Oberste MS, et al. Humoral and intestinal immunity induced by new schedules of bivalent oral poliovirus vaccine and one or two doses of inactivated poliovirus vaccine in Latin American infants: an open-label randomised controlled trial. Lancet. 2016;388:158–69. doi:10.1016/S0140-6736(16)00703-0.
- Saez-Llorens X, Clemens R, Leroux-Roels G, Jimeno J, Clemens SAC, Weldon WC, Oberste MS, Molina N, Bandyopadhyay AS. Immunogenicity and safety of a novel monovalent high-dose inactivated poliovirus type 2 vaccine in infants: a comparative, observer-blind, randomised, controlled trial. Lancet Infect Dis. 2016;16:321–30. doi:10.1016/S1473-3099(15)00488-0.
- Qiu J, Yang Y, Huang L, Wang L, Jiang Z, Gong J, Wang W, Wang H, Guo S, Li C, et al. Immunogenicity and safety evaluation of bivalent types 1 and 3 oral poliovirus vaccine by comparing different poliomyelitis vaccination schedules in China: a randomized controlled non-inferiority clinical trial. Hum Vaccin Immunother. 2017;13:1–10. doi:10.1080/21645515.2017.1288769.
- Li RC, Li CG, Wang HB, Luo H-M, Li Y-P, Wang J-F, Ying Z-F, Yu W-Z, Shu JD, Wen N, et al. Immunogenicity of two different sequential schedules of inactivated polio vaccine followed by oral polio vaccine versus oral polio vaccine alone in healthy infants in China. J Pediatric Infect Dis Soc. 2016;5:287–96. doi:10.1093/jpids/piv017.
- Sutter RW, Bahl S, Deshpande JM, Verma H, Ahmad M, Venugopal P, Rao JV, Agarkhedkar S, Lalwani SK, Kunwar A, et al. Immunogenicity of a new routine vaccination schedule for global poliomyelitis prevention: an open-label, randomised controlled trial. Lancet. 2015;386:2413–21. doi:10.1016/S0140-6736(15)00237-8.
- Liao G, Li R, Li C, Sun M, Jiang S, Li Y, Mo Z, Xia J, Xie Z, Che Y, et al. Phase 3 trial of a Sabin strain-based inactivated poliovirus vaccine. J Infect Dis. 2016;214:1728–34. doi:10.1093/infdis/jiw433.
- Okayasu H, Sein C, Hamidi A, Bakker WA, Sutter RW. Development of inactivated poliovirus vaccine from Sabin strains: a progress report. Biologicals. 2016;44:581–87. doi:10.1016/j.biologicals.2016.08.005.
- Lu L, Li X, Zhang H, Liu D, Zhang Z, Wang H, Liu F, Ning Z, Li J, Pang X, et al. Immunogenicity and persistence from different 3-dose schedules of live and inactivated polio vaccines in Chinese infants. Vaccine. 2015;33:4653–58. doi:10.1016/j.vaccine.2014.08.091.
- Weldon WC, Oberste MS, Pallansch MA. Standardized methods for detection of poliovirus antibodies. Methods Mol Biol. 2016;1387:145–76.
- Saleem AF, Mach O, Yousafzai MT, Khan A, Weldon WC, Steven Oberste M, Zaidi SS, Alam MM, Quadri F, Sutter RW, et al. Immunogenicity of different routine poliovirus vaccination schedules: a randomized,controlled trial in Karachi, Pakistan. J Infect Dis. 2018;217:443–50. doi:10.1093/infdis/jix577.
- Wright PF, Connor RI, Wieland-Alter WF, Hoen AG, Boesch AW, Ackerman ME, Oberste MS, Gast C, Brickley EB, Asturias EJ, et al. Vaccine-induced mucosal immunity to poliovirus: analysis of cohorts from an open-label, randomised controlled trial in Latin American infants. Lancet Infect Dis. 2016;16:1377–84. doi:10.1016/S1473-3099(16)30169-4.
- Albrecht P, Enterline JC, Boone EJ, Klutch MJ. Poliovirus and polio antibody assay in HEp-2 and Vero cell cultures[J]. J Biol Stand. 1983;11(2):91–97. doi:10.1016/S0092-1157(83)80031-6.
- Li XM, Zhang ZJ, Wang HH, Liu F, Zhang L-W, Chu P, Xu Y, Zhang H-R, Li J, Liu D-L, et al. Immunogenicity and safety of a booster dose of inactivated polio vaccine[J]. Zhonghua Yu Fang Yi Xue Za Zhi. 2013;47(10):905–09.
- Faden H, Duffy L. Effect of concurrent viral infection on systemic and local antibody responses to live attenuated and enhanced-potency inactivated poliovirus vaccines[J]. Am J Dis Child. 1992;146(11):1320–23. doi:10.1001/archpedi.1992.02160230078023.
- Faden H, Modlin JF, Thoms ML, McBean AM, Ferdon MB, Ogra PL. Comparative evaluation of immunization with live attenuated and enhanced-potency inactivated trivalent poliovirus vaccines in childhood: systemic and local immune responses[J]. J Infect Dis. 1990;162(6):1291–97. doi:10.1093/infdis/162.6.1291.
- Halsey NA, Blatter M, Bader G, Thoms ML, Willingham FF, O’Donovan JC, Pakula L, Berut F, Reisinger KS, Meschievitz C, et al. Inactivated poliovirus vaccine alone or sequential inactivated and oral poliovirus vaccine in two-, four- and six-month-old infants with combination Haemophilus influenzae type b/hepatitis B vaccine[J]. Pediatr Infect Dis J. 1997;16(7):675–79. doi:10.1097/00006454-199707000-00010.
- Asturias EJ, Dueger EL, Omer SB, Melville A, Nates S, Laassri M, Chumakov K, Halsey N. Randomized trial of inactivated and live polio vaccine schedules in Guatemalan infants[J]. J Infect Dis. 2007;196(5):692–98. doi:10.1086/520546.
- Gaensbauer JT, Gast C, Bandyopadhyay AS, O’Ryan M, Saez-Llorens X, Rivera L, Lopez-Medina E, Melgar M, Weldon WC, Oberste MS, et al. Impact of maternal antibody on the immunogenicity of inactivated polio vaccine in infants immunized with bivalent oral polio vaccine: implications for the polio eradication endgame[J]. Clin Infect Dis. 2018;67(S1):S57–65. doi:10.1093/cid/ciy649.
- Faden H, Duffy L, Sun M, Shuff C. Long-term immunity to poliovirus in children immunized with live attenuated and enhanced-potency inactivated trivalent poliovirus vaccines[J]. J Infect Dis. 1993;168(2):452–54. doi:10.1093/infdis/168.2.452.
- Guérin N, Bregère P, Caudrelier P, Raynaud O. Neutralizing antibody response to oral poliovirus vaccine after primary immunization with inactivated poliovirus vaccine[J]. Eur J Clin Microbiol Infect Dis. 1998;17(11):815–16. doi:10.1007/s100960050198.
- Laassri M, Lottenbach K, Belshe R, Wolff M, Rennels M, Plotkin S, Chumakov K. Effect of different vaccination schedules on excretion of oral poliovirus vaccine strains[J]. J Infect Dis. 2005;192(12):2092–98. doi:10.1086/498172.
- Abraham R, Minor P, Dunn G, Modlin JF, Ogra PL. Shedding of virulent poliovirus revertants during immunization with oral poliovirus vaccine after prior immunization with inactivated polio vaccine [J]. J Infect Dis. 1993;168(5):1105–09. doi:10.1093/infdis/168.5.1105.
- Ogra PL, Faden HS, Abraham R, Duffy LC, Sun M, Minor PD. Effect of prior immunity on the shedding of virulent revertant virus in feces after oral immunization with live attenuated poliovirus vaccines[J]. J Infect Dis. 1991;164(1):191–94. doi:10.1093/infdis/164.1.191.
- Minor PD, Dunn G, Ramsay ME, Brown D. Effect of different immunisation schedules on the excretion and reversion of oral poliovaccine strains[J]. J Med Virol. 2005;75(1):153–60. doi:10.1002/jmv.20250.
- Parent Du Chatelet I, Merchant AT, Fisher-Hoch S, Luby SP, Plotkin SA, Moatter T, Agboatwalla M, Mc Cormick JB. Serological response and poliovirus excretion following different combined oral and inactivated poliovirus vaccines immunization schedules[J]. Vaccine. 2003;21:1710–18. doi:10.1016/S0264-410X(02)00523-6.
- Wicker S, Rabenau H, Gottschalk R, Doerr HW, Allwinn R. seroprotection rates of vaccine-preventable and blood-transmissible viral infections (measles,mumps, rubella, VZV, polio, HBV, HCV, HIV) in medical students[J]. Med Microbiol Immunol. 2007;196:145–50. doi:10.1007/s00430-007-0036-3.
- Diedrich S, Schreier E. The German Health Interview and examination survey for children and adolescents: state of immunity against poliomyelitis in German children[J]. Bundesgesundheitsblatt. 2007;50(5–6):771–74. doi:10.1007/s00103-007-0239-1.
- Pires de Miranda M, Carmo Gomes M, Rebelo de Andrade H. seroprotection rates of antibodies to poliovirus in individuals living in Portugal, 2002[J]. Euro Surveill. 2007;12(6):E7–E8. doi:10.2807/esm.12.06.00717-en.
- Reinheimer C, Friedrichs I, Rabenau HF, Doerr HW. Deficiency of immunity to poliovirus type 3: alurking danger? [J]. BMC Infect Dis. 2012;12:24. doi:10.1186/1471-2334-12-24.
- James TG, Chris G, Ananda SB, Miguel O’, Xavier Saez-Llorens, Luis R, Eduardo Lopez-Medina, Mario M, William CW, Steven OM, et al. Impact of maternal antibody on the immunogenicity of inactivated polio vaccine in infants immunized with bivalent oral polio vaccine: implications for the polio eradication endgame. Clinc Infectious Diseases 2018;67 (Suppl 1):S57–65.
- Ning W, Zhijie A, Hong Y, et al. Considerations and suggestions for polio vaccination strategies in China [J]. Chin J Vaccines Immun. 2018;24:349–53.
