ABSTRACT
Background: The first Chinese domestic 13-valent pneumococcal conjugate vaccine (WoAnxin®, PCV-13) is available for children aged 2 months to 5 years and is more economical than import vaccine with equal safety and immunogenicity. However, the cost-effectiveness of this new PCV-13 for children <5 years in mainland China is not clear. Methods: In the present study, we developed a Markov model under societal perspective to evaluate the incremental cost-effectiveness ratios (ICERs) of five birth cohorts of 100,000 Chinese infants across four alternative vaccination programs:1) no vaccination; 2) vaccinate 4 doses of new PCV-13 for children aged 2 to 6 months; 3) vaccinate 3 doses of new PCV-13 for children aged 7 to 11 months; 4) vaccinate 2 doses of new PCV-13 for children aged 12 to 23 months; 5) vaccinate 1 dose of new PCV-13 for children aged 2 to 5 years. We conducted one-way and probability sensitivity analysis to determine the uncertainty of the model findings. Results: We found that with awillingness-to-pay (WTP) threshold of three-times Chinese per-capita gross domestic product (GDP) all vaccination programs were cost-effective compared to no vaccination and children aged 2 to 5 years received 1 dose of new PCV-13 would incur the lowest additional cost of US$2417 per quality-adjusted-life-years (QALYs) compare with other vaccination programs ($15394/QALYs for 4 doses program, $9292/QALYs for 3 doses program, $4445/QALYs for 2 doses program). Conclusions: According to our results, China should give priority to incorporating new PCV-13 into its national immunization program.
Introduction
Streptococcus Pneumoniae (SP) is the main cause of bacterial pneumonia, meningitis, acute otitis media (AOM), and bacteremia in children under 5 years of age.Citation1 In 2015, there were about 294,000 children died of pneumococcal diseases worldwide and the mortality of SP infection in children under 5 years of age was near 45/100,000, and most children who died of pneumococcus (81%) were presented with pneumonia.Citation2 China ranks 4th in the countries with the highest burden of pneumococcal diseases in children under 5 years of age, which accounts for 3% of the total number of cases in the world.Citation2 With the increasing antibiotic resistance of SP, there is an urgent need to use vaccines to control pneumococcal diseases.Citation3,Citation4 World Health Organization (WHO) suggests that all countries should incorporate pneumococcal-combined vaccine (PCV) into their own National Immunization Programs (NIP), especially those countries where the mortality among children under 5 years of age >50‰.Citation5 As of 2019, there are 149 countries that have integrated PCVs into their NIP except China.Citation6
There are only two vaccines available in China to prevent pneumococcal diseases, 13-valent pneumococcal conjugate vaccine (PCV-13) and 23-valent pneumococcal polysaccharide vaccine (PPV-23). Since PPV-23 cannot be applied to children <2 years, we did not consider this vaccine in our study.Citation7 Although the previous cost-effectiveness analysis of PCV-13 has found that integrated PCVs into NIP might be cost-effective, PCV-13 is still working as acategory II vaccine with alow coverage rate in China since 2016.Citation8–11 Citation11 For instance, the coverage rate of PCV-13 (Prevenar®) in children under 5 years vaccinated at least three doses in Ningbo, a city with high levels of economic development, was only 8.66% in 2017–2018, which is lower than that of developed countries. Citation9Moreover, the previous cost-effectiveness analysis of PCV-13 was based on imported vaccines with a single vaccination procedure (3p+1 schedule, children receive at 2, 3, 4 months of age with one booster dose at 12–15 months of age) and cites a large numbers of non-local epidemiological data, the reliability of effectiveness might be decreased.
The first Chinese PCV-13 has been licensed by China National Medical Products Administration (NMPA) in December2019 for all children aged 2 months to 5 years at 556 Chinese Yuan (US$81) per dose. Previous clinical trials have shown the safety and immunogenicity of new PCV-13 are non-inferior to imported vaccines (Prevnar®, PCV7).Citation12 However, before introducing the new PCV-13 into the NIP, a cost-effectiveness analysis is necessary. Therefore, this study evaluated the cost-effectiveness of Chinese new PCV-13 for children aged 2 months to 5 years at the present prices by using the most updated and comprehensive data.
Methods
Model overview and analytic framework
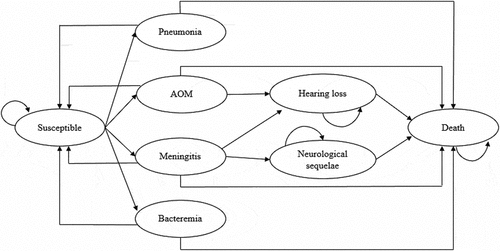
In this article, we used the decision tree and Markov model to estimate the cost-effectiveness of four vaccination programs compared to no vaccination over the first 5 years of life and the lifetime effects.Citation11 The model estimated the clinical and economic impact of vaccination strategies from asocietal perspective in five hypothetical cohorts of 100,000 infants by decision analysis software Treeage Pro 2020. The model based on the natural history of diseases was presented in . Every susceptible infant entered into the model was assumed to be healthy but exposed to the risk for SP pneumonia, SP meningitis, SP bacteremia, SP acute otitis media (AOM) and the risk of each disease condition was dependent on their natural incidence and vaccine efficacy (). In this research, the costs of long-term sequelae such as hearing loss and neurodevelopmental impairment were considered over the remaining lifetimes of the patients (life expectancy 77 years). The length of each Markov state cycled is 1 year and the half-cycle correction is used in this model.
Table 1. Parameters used in this model
Intervention strategies
We defined “no vaccination” as control status, children received flowing four immunization programs as intervention status, and the results were presented as incremental cost-effectiveness ratio (ICER) in dollar cost per quality-adjusted life-year (QALY) gained. We assume that the new PCV-13 would be funded by the government and reach 90% coverage as other NIP vaccines.Citation33,Citation34 A total of five intervention strategies were included in our study: (1) no vaccination; (2) vaccinate four doses of new PCV-13 for children aged 2 to 6 months; (3) vaccinate three doses of new PCV-13 for children aged 7 to 11 months; (4) vaccinate 2 doses of new PCV-13 for children aged 12 to 23 months; (5) vaccinate 1 dose of new PCV-13 for children aged 2 to 5 years.
Epidemiological data
Model parameters were based on the latest published literature, statistical yearbooks, and field surveys. Local data were adopted with priority in our model, and we only justified data from other places when the local data were not available. The model parameters with sensitivity analysis range and distribution were presented in . The age-specific mortality rates for the general population were obtained from the latest Health Statistics Yearbook.Citation20 Because there is no surveillance system for bacteremia in mainland China, the bacteremia data in Taiwan were cited in this study due to demographic homogeneity.Citation10The data on the incidence of AOM came from asystematic evaluation of the literature.Citation15–19 The age-specific incidence rate of all-cause pneumonia came from our previous population-based investigation in Baiyin, Gansu province, during 2015–2016.Citation13 The mortality rate of pneumonia was cited from the Health Statistics Yearbook.Citation20 The incidence and mortality rate of SP meningitis were obtained from acute meningitis and encephalitis syndrome (AMES) surveillance in China.Citation14,Citation21 From this surveillance, the proportion of meningitis cases with neurological sequelae and hearing impairment were 19.08% and 6.87%, respectively.Citation21 There are no mortality data for hearing loss and AOM in China, thus the mortality rates of hearing impairment and otitis media are assumed to be consistent with that of healthy children. The SP solation rates of pneumonia were cited from asystematic review of the etiology of community-acquired pneumonia in China.Citation24 The SP solation rates of AOM are 50.0% which were also obtained from the Chinese population.Citation25
Costs and utilities
The analysis was conducted from societal perspectives. The cost of pneumococcal disease per case was reported in , which were derived from the previously published economic analysis studies.Citation10,Citation13,Citation21 The cost of neurological sequelae and hearing loss of meningitis was considered over the remaining lifetime.Citation21 According to the China Education Statistical Yearbook,Citation23 65% of children with neurological sequelae and hearing impairment need special education, and the investment in special education is 3666 USD per year. Therefore, we assumed that 65% of children with sequelae need special education for up to 9 years. The costs used in this analysis were adjusted to 2019 based on Chinese per-capita GPD and CPI at the national level. The health outcomes of this study were measured by QALYs, of which 0 represents death and 1 represents complete health. QALYs for each state were cited from published studies.Citation22,Citation26–28 We assumed the discount rate as 5% for both costs and utilities in our analysis.
Immunization cost and vaccine cost
Based on the redundant capabilities of the existing immunization planning system, additional manpower and fixtures can be avoided. Therefore, in this study, incremental immunization planning costs for one dose of the vaccine only included vaccine costs ($81) and management costs ($2).Citation22
Vaccine efficacy
We assumed that the vaccine efficacy including both direct and indirect protection would be obtained 1 month after basic immunization.Citation35 For example, for children aged 2 to 6 months, the vaccine efficacy is calculated at 9 months of age, assuming that children were vaccinated at the median age (4 months) and the protection would be obtained 1 month after three doses of basic immunization. Direct protection includes vaccine effectiveness against invasive diseases and pneumonia. We used 93% (CI: 76%-98%) as the level of vaccine effectiveness against invasive pneumococcal disease (IPD) and pneumonia.Citation29 IPD includes both meningitis and bacteremia. And 69.9% (CI: 29.8%-87.1%) was used as the level of vaccine efficacy against AOM.Citation29 Referring to similar studies, herd effects in unvaccinated populations accounted for 20% of direct effects, hence we used 18.6% of indirect effects for IPD and pneumonia.Citation30 Herd effects of PCV-13 against AOM were assumed to be zero due to the lack of conclusive data.
Cost-effectiveness analysis
There is no official policy on the cost-effectiveness of health care decisions in China, ICER was used to compare the incremental cost and QALYs for five alternative strategies. According to WHO recommendation standard, 3-time Chinese per-capita gross domestic product (GDP) in 2019 was used as athreshold for incremental cost-effectiveness analysis: the vaccination strategies can be considered to be “cost-effective” or “highly cost-effective” if the ICER is 1–3 time per-capita GDP or less than 1-time per-capita GDP. The Chinese per-capita GDP was 10276 USD in 2019.
Sensitivity analyses
One-way sensitivity analysis was performed for all parameters, and the range of plausible values for model parameters was based on the literature, while other parameters were individually varied at abase level of 25%. For probability sensitivity analysis, generated parameters were performed by Monte Carlo simulations (number of samples = 100,000) to evaluate the probability of being cost-effective for each immunization programs compare with others.
Results
Averted cases and death
The cumulative number of reductions in pneumococcal disease and death based on birth cohorts following new PCV-13 vaccination strategies are presented in . Within the hypothetical cohort, under avaccination coverage of 90%, the new PCV-13 vaccination at 2 to 6 months could avert 1196 pneumonia cases, 16 meningitis cases, 13214 AOM cases, 20 bacteremia cases, 12 deaths, 3 meningitis sequelae cases and 1 hearing loss cases associated with SP compared with no vaccinations. In theory, the new PCV-13 used for children aged 7 to 11 months would avert 1154 pneumonia cases, 14 meningitis cases, 12431 AOM cases, 18 bacteremia cases, 11 deaths, 3 meningitis sequelae cases and 1 hearing loss case. For pneumonia, vaccination at 12 to 23 months or 2 to 5 years old would avert 916 and 509 cases, respectively. Vaccination at 12 to 23 months could reduce 12 meningitis cases, 10213 AOM cases, 17 bacteremia cases, 9 deaths, 1 sequelae case and 1 hearing loss case. Vaccination at 2 years old would reduce 7 meningitis cases, 5776 AOM cases, 10 bacteremia cases, 5 deaths and 1 sequelae cases.
Table 2. The cumulative number of reductions in pneumococcal disease and death based on birth cohorts following new PCV-13 vaccination strategies
Cost-effectiveness
The cost of each health status following the PCV-13 vaccination programs was compared with non-vaccination and are shown in . In comparison to the non-vaccination strategy, the cost of obtaining 1 QALY for children who received one to four doses of new PCV-13 was 2417, USD 4445, USD 9292, USD 15394, USD respectively, which were less than 2-time Chinese per-capita in 2019.
Table 3. Projected costs and effectiveness of PCV programs in five cohorts of Chinese infants
Sensitivity analyses
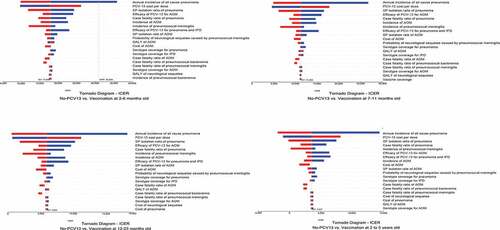
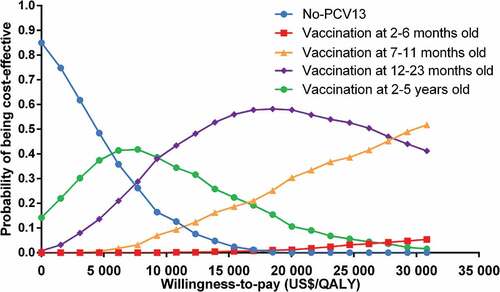
The results of the one-way sensitivity analyses were shown as tornado diagrams to identify the most sensitivity parameters (). The main factors affecting the results of ICER were the annual incidence of all-cause pneumonia and the PCV-13 cost per dose. Cost-effectiveness acceptability curve was shown in , based on the probabilistic sensitivity analyses, under the threshold of 3-time Chinese per-capita GDP the probability of cost-effective for no PCV-13 and children received 1–4 doses of new PCV-13 were 0, 1.6%, 43.4%, 50% and 5%, respectively.
Discussion
In consistent with other similar studies, diseases considered in this model include pneumonia, bacteremia, meningitis, its sequelae and AOM which are the most important pneumococcal diseases.Citation29,Citation36 We found that under the model cycled in yearly increments for 5 years, the ICER of primary infant PCV programs for children under 1 year of age and catch-up programs for children 1 to 5 years was less than 2 times or 1-time Chinese per-capita GDP in 2019, respectively. And receiving one dose of new PCV-13 for children at 2 to 5 years of age is the most cost-effective strategy.
PCV-13 (Prevnar®) has been shown to be safe and have both direct and indirect effects against pneumococcal disease covered by PCV-13 serotypes and is used worldwide.Citation5 Most of the cost-effectiveness analyses of PCV-13(Prevnar®) are based on the primary infant PCV programs. PCV-13 (Prevnar®) is probably cost-effective in low- and middle-income countries with 3-doses or 4-doses immunization schedules, and the use of PCV-13 (Prevnar®) has significant benefits in saving lives and avoiding disability especially in Africa and Asia.Citation37,Citation38 Recent research shows that under the 2 + 1 dose schedule, the ICER for PCV-13 (Prevnar®) in Thailand was 2350 USD/QALY, which was lower than the willingness-to-pay threshold of 5100 USD.Citation39 In our study, the ICER for children who accepted four doses of new PCV-13 was 17858 USD/QALY which was less than 2 times the Chinese per-capita GDP in 2019. The results are consistent with previous cost-effectiveness analysis of PCV-13 (Prevnar®) that Chinese infants start vaccination at 2 months of age for a total of four doses, the ICER ranges from 11464 USD/QALY to 20709 USD/QALY, which was also lower than 2 times Chinese per-capita GDP in 2019.Citation8,Citation10,Citation11 Primary infant PCV programs, along with catch-up programs for children 1–5 years are often used in the firstyear of introducing PCV into NIP, which can reduce the IPDs of each age group and accelerate group immunization.Citation40 Recently, a study in Taiwan indicates that implementing a catch-up vaccination program without primary vaccination in infancy might be an effective way to reduce the burden of pneumococcal disease when health funding is limited.Citation41 In Taiwan, IPD incidence in children aged 0–5 years decreased from 18.9/100,000 in 2010–2012 to 9.4/100,000 in 2013–2014, as the result of the catch-up vaccination programs that a single dose of PCV-13 (Prevnar®) is provided free for children 2 to 5 years of age to in 2013, following by an expansion of the National Program to provide vaccinations for children between 12 and 24 months of age in 2014 with 2 doses of PCV-13.Citation41 Our study showed the catch-up programs for older children were highly cost-effective and the ICER was lower than 1 time of Chinese per-capita GDP in 2019.
Routine use of childhood PCVs has substantially changed the epidemiology of the pneumococcal disease in the unvaccinated population age through herd immunity effects.Citation42 The new PCV-13 is not accepted by most Chinese parents due to the high prices even which is cheaper than PCV-13 (Prevnar®). A research in 2019 has shown that more than 70% of Chinese parents will accept PCV-13 when the vaccine prices were below 40 USD/dose, as the prices increase to 100 USD/dose the vaccination willingness rates drop to 20% and only 4% of parents accept PCV-13 when the price is more than 100 USD.Citation43 The price of imported PCV-13 and new PCV-13 per dose is 120 USD and 80 USD respectively, which is not acceptable for most parents. Introducing the new PCV-13 into NIP of China can guarantee vaccination coverage which is significant to reduce the burden of pneumococcal disease.
There are a few limitations in this study. Firstly, previous clinical trials have shown that there is no significant difference in the incidence and severity of new PCV-13 adverse reactions compared with those of PCV-7. The common adverse events following immunization (AEFI) are fever, diarrhea, crying, redness and swelling which are not serious and have alow incidence. Hence, we didn’t consider the impact of adverse events on PCV13 cost-effectiveness.Citation44 Secondly, childhood PCVs can reduce the incidence of pneumococcal disease in unvaccinated populations due to indirect effects. One study indicates that the herd effect gained from vaccination for 10 birth groups was equal to vaccinated in one high-risk groups (≥65 years). Citation45 In our study, we assumed that indirect protection of new PCV-13 would be obtained after 1 month of basic immunization and excluded any herd effect in individuals over 5 years old, which is very conservative and might underestimate the benefits of new PCV-13
Conclusion
All the vaccination programs, especially for children aged 2 to 5 years receiving one dose of new PCV-13, would be cost-effective compared to no vaccination. China should give priority to integrating PCV-13 into the national immunization program. Our study only assessed the economic effects of different vaccination strategies from social perspectives, programmatic factors including timeliness and expected coverage of vaccination should also be taken into account while setting the specific vaccination program
Disclosure of potential conflicts of interest
All authors report no potential conflict of interest.
Additional information
Funding
Notes on contributors
Xuxia Wang
Caixia Wang, Xuxia Wang, Li Su and Xueyan Gu carried out the concepts, designs, literature search, data analysis and manuscript preparation. Qiuling Mu and Xuejun Guo carried out literature search, investigation and manuscript editing. Xuejun Guo and Xuxia Wang performed manuscript review. All authors have read and approved the version to be published and agree to be accountable for all aspects of the work.
References
- Shiri T, Khan K, Keaney K, Mukherjee G, McCarthy ND, Petrou S. Pneumococcal disease: Asystematic review of health utilities, resource use, costs, and economic evaluations of interventions. Value Health. 2019;22(11):1329–44. doi:10.1016/j.jval.2019.06.011.
- Brian W, O’Brien KL, Adena G, Majumder A, Liu L, Chu Y, Lukšić I, Nair H, McAllister DA, Campbell H, etal. Burden of Streptococcus pneumoniae and Haemophilus influenzae type bdisease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000–15. Lancet Global Health. 2018;6(7):e744–e757. doi:10.1016/S2214-109X(18)30247-X.
- Song JH. Advances in pneumococcal antibiotic resistance. Expert Rev Respir Med. 2013;7(5):491–98. doi:10.1586/17476348.2013.816572.
- Lewnard JA, Lo NC, Arinaminpathy N, Frost I, Laxminarayan R. Childhood vaccines and antibiotic use in low- and middle-income countries. Nature. 2020;581(7806):94–99. doi:10.1038/s41586-020-2238-4.
- WHO. Pneumococcal conjugate vaccines in infants and children under 5 years of age: WHO position paper– february 2019.
- World Health Organization. Immunization coverage: 2019,[accessed, Sep 15, 2020]. https://www.who.int/news-room/fact-sheets/detail/immunization-coverage
- Choi MJ, Kang SO, Oh JJ, Park S-B, Kim M-J, Cheong H-J. Cost-effectiveness analysis of 13-valent pneumococcal conjugate vaccine versus 23-valent pneumococcal polysaccharide vaccine in an adult population in South Korea. Hum Vaccin Immunother. 2018;14(8):1914–22. doi:10.1080/21645515.2018.1456602.
- Hua Z, Jinchun H, Bin W, Che D. Cost-effectiveness analysis of routine 13-valent pneumococcal conjugate vaccinations in Chinese infants. Hum Vaccin Immunother. 2018;14(6):1444–52. doi:10.1080/21645515.2018.1438794.
- Lizhi L, Lixia Y, Yi C, Ting F, Liang Z, Hongjun D, Guozhang X. Vaccination coverage of 13-valent pneumococcal conjugate vaccine among children born in 2017-2018 in Ningbo City, Zhejiang Province, China. Clin JBiol. 2020;33(1):50–54.
- ShenK, Wasserman M, Liu D, YangHong Y, Yang J, Guzauskas GF, Wang BCM, Hilton B, Farkouh R. Estimating the cost-effectiveness of an infant 13-valent pneumococcal conjugate vaccine national immunization program in China. PLoS One. 2018;13(7):e0201245. doi:10.1371/journal.pone.0201245
- Maurer KA, Huey-Fe C, Abram LW, Hegde ST, Patel T, Boulton ML, Hutton DW. Cost-effectiveness analysis of pneumococcal vaccination for infants in China. Vaccine. 2016;34(50):6343–49. doi:10.1016/j.vaccine.2016.10.051.
- Jingjing C, Lin Y, Zhen H, Shi NM, Zhao YL, Xia SL, Li GH, Li RC, Li YP, Yang SY, et al. Safety and immunogenicity of anew 13-valent pneumococcal conjugate vaccine versus alicensed 7-valent pneumococcal conjugate vaccine: astudy protocol of arandomized non-inferiority trial in China. BMJOpen. 2016;6(10):e012488. doi:10.1136/bmjopen-2016-012488.
- Guijun N, Xuxia W, Shiwen L, Yuying Z, Bingling Z, Xiaoshu Z, Yixing L, Zhundong Y, Weizhong Y. Retrospective investigation of the disease burden of community acquired pneumonia among children under 5 years old in Baiyin city of Gansu province, 2015-2016. Chin jvacc immun. 2017;23(1):18–21+12.
- Yixing L, Zhujun S, Huanyu W, Guijun N, Junhong L, Zhundong Y. Acute meningitis and encephalitis syndrome surveillance disease burden in four China prefectures. Chin JPrevent Med. 2019;53(2):164–68.
- Investigation of Pre-school Chidren’s Secretory Otitis Media Prevalence in Xiantao City. JAudiol Speech Pathol. 2015;23:12–314.
- JunL. Prevalence of secretory otitis media in some kindergarten children in Tanggu District. Harbin Med. 2013;33:194–200
- Zhinan W, Ping C, Zhongqiang X, Youhua W, Yanling H, Bin Z, Ronghua H, Zhong C,Sunfang Y. The prevalence of otitis media with effusion of kindergarten children in Wuhan city. JClin Otolaryngol Head Neck Surgery. 2009;23(22):1036–1037+1043.
- Yibing D, Meiling W. Recent Investigation on the Occurrence of Ophthalmitis and Otitis media in Preschool Children. Mater Child Health Care China. 2004;7(2):119.
- Hongyan T, Ruidan H, Qing L. Investigation on the prevalence of secretory otitis media in children aged 2–7 in Chengdu. JAudiol Speech Pathol. 2019;27:83–84.
- National Bureau of Statistics of China. China Statistical Yearbooks. Beijing: 2019 [accessed Sep 15, 2019]. http://www.stats.gov.cn/tjsj/ndsj/2019/indexch.htm.
- Wenting L. Study on economic burden of bacterial meningitis in China[dissertation]. Beijing: Chinese Center for Disease Control and Prevention; 2016.
- Guijun N, Zundong Y, Yixing L, Qinghua W, Wenzhong Y. Cost-effectiveness of the Haemophilus influenzae type bvaccine for infants in mainland China. Hum Vaccin Immunother. 2018;14(1):36–44. doi:10.1080/21645515.2017.1385687.
- Huanzhong X, Changwei Q. Educational statistics yearbook of China. 2015. Beijing: China Statistics Press; 2016.
- Guijun N, Xuxia W, Dan W, Yin Z, Li Y, Wang H, Yang W. The etiology of community-acquired pneumonia among children under 5 years of age in mainland China, 2001-2015: Asystematic review. Hum Vaccin Immunother. 2017;13(1):2742–50. doi:10.1080/21645515.2017.1371381.
- Jinjian F, Ling L, Zhuoxin L, Xu S, Lin N, Qin P, Ye X, McGrath E. Etiology of acute otitis media and phenotypic-molecular characterization of streptococcus pneumoniae isolated from children in Liuzhou, China. BMC Infect Dis. 2019;19(1):168. doi:10.1186/s12879-019-3795-8.
- Oostenbrink R, Ha AM, Essink-Bot ML. The EQ-5D and the Health Utilities Index for permanent sequelae after meningitis: A head-to-head comparison. JClin Epidemiol. 2002;55(8):791–99. doi:10.1016/S0895-4356(02)00448-1.
- Oh PI, Maerov P, Pritchard D, Knowles SR, Einarson TR, Shear NH. A cost-utility analysis of second-line antibiotics in the treatment of acute otitis media in children. Clin Ther. 1996;18(1):160-82. doi: 10.1016/s0149-2918(96)80188-3.
- Bennett JE, Sumner W, Downs SM, Jaffe DM. Parents’ utilities for outcomes of occult bacteremia. Arch Pediatr Adolesc Med. 2000;154:43–48.
- Delgleize E, Leeuwenkamp O, Theodorou E, Van de Velde N. Cost-effectiveness analysis of routine pneumococcal vaccination in the UK: acomparison of the PHiD-CV vaccine and the PCV-13 vaccine using a Markov model. BMJ Open. 2016;6(11):e010776. doi:10.1136/bmjopen-2015-010776.
- Marie RG, Yuwei Z, Matthew RM, Cynthia GW, Carlos GG. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med. 2013;369(2):155-63. doi:10.1056/NEJMoa1209165.
- Machao L, Yao W, Hong Z, Yong L, XueJun C, HongWei Y, Ping M, DingCheng W, Bingchang Z, Aiying D, et al. Serotype distribution and clinical characteristics associated with streptococcus pneumoniae among Chinese children and adults with invasive pneumococcal disease: a multicenter observational study. Hum Vaccin Immunother. [Preprint.] 2020 [accessed, Jan 4, 2021]. doi: 10.1080/21645515.2020.1757996.
- Wang J, Liu F, Ao P, Li X, Zheng H, Wu D, Zhang N, Yu J, Yuan J, Wu X, et al. Detection of Serotype Distribution and Drug Resistance of Streptococcus Pneumoniae Isolated From Pediatric Patients. Lab Med. 2017;48(1):39–45. doi:10.1093/labmed/lmw059
- Hu Y, Chen Y, Liang H, Wang Y. Routine vaccination coverage of children aged 1-7 years in Zhejiang province, China. Hum Vaccin Immunother. 2018;14(12):2876–83. doi:10.1080/21645515.2018.1504523
- He Y, Liu Y, Dai B, Zhao L, Lin J, Yang J, Yu H. Assessing vaccination coverage, timeliness, and its temporal variations among children in arural area in China. Hum Vaccin Immunother. 2020;1–9. doi:10.1080/21645515.2020.1772620.
- Zhu F, Hu Y, Li J, Ye Q, Young MM, Liang JZ, Gruber WC, Giardina PC, Scott DA. Immunogenicity and Safety of the 13-Valent Pneumococcal Conjugate Vaccine Administered in a3 + 1 versus 2 + 1 Dose Schedule Among Infants in China. Pediatr Infect Dis J. 2019;38(11):1150–58. doi:10.1097/INF.0000000000002458.
- Rubin JL, McGarry LJ, Strutton DR, Klugman KP, Pelton SI, Gilmore KE, Weinstein MC. Public health and economic impact of the 13-valent pneumococcal conjugate vaccine (PCV13) in the United States. Vaccine. 2010;28(48):7634–43. doi:10.1016/j.vaccine.2010.09.049.
- Saokaew S, Rayanakorn WDB, Wu DBC, Chaiyakunapruk N. Cost Effectiveness of Pneumococcal Vaccination in Children in Low- and Middle-Income Countries: ASystematic Review. Pharm Econom. 2016;34(12):1211–25. doi:10.1007/s40273-016-0439-3.
- Chen C, Francisco CL, Stefan F, Sidharta S, Yoong J, SundaramN, JitM. Effect and cost-effectiveness of pneumococcal conjugate vaccination: aglobal modelling analysis. Lancet Global Health. 2019;7(1):e58–e67. doi:10.1016/S2214-109X(18)30422-4.
- Dilokthornsakul P, Kengkla K, Saokaew S, Permsuwan U, Techasaensiri C, Chotpitayasunondh T, Chaiyakunapruk N. An updated cost-effectiveness analysis of pneumococcal conjugate vaccine among children in Thailand. Vaccine. 2019;37(32):4551–60. doi:10.1016/j.vaccine.2019.06.015.
- Kobayashi M, Bigogo G, Kim L, Mogeni OD, Conklin LM, Odoyo A, Odiembo H, Pimenta F, Ouma D, Harris AM, etal. Impact of 10-Valent Pneumococcal Conjugate Vaccine Introduction on Pneumococcal Carriage and Antibiotic Susceptibility Patterns Among Children Aged <5 Years and Adults With Human Immunodeficiency Virus Infection: Kenya, 2009-2013. Clin Infect Dis. 2020:70(5):814-826. doi:10.1093/cid/ciz285.
- Chun-Yi L, Chuen-Sheue C, Cheng-Hsun C, Wang E-T, Chen -Y-Y, Yao S-M, Chang L-Y, Huang L-M, Lin T-Y, Chou J-H, etal. Successful Control of Streptococcus pneumoniae 19A Replacement With a Catch-up Primary Vaccination Program in Taiwan. Clin Infect Diseas. 2019;69(9):1581–87. doi:10.1093/cid/ciy1127.
- Troeger C, Blacker B, KhalilI A, Rao PC, Cao J, Zimsen SRM, Albertson SB, Deshpande A, Farag T, Abebe Z, etal. Estimates of the global, regional, and national morbidity, mortality, and etiologies of lower respiratory infections in 195 countries, 1990–2016: asystematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018;18(11):1191–210. doi:10.1016/S1473-3099(18)30310-4.
- White Paper on Pneumonia and Vaccine Popularization in Chinese Children.
- Kwan KS, Oh IS, Kim HJ, Song I, Park MS, Shin J-Y. Signal detection of adverse events following pneumococcal vaccines from the Korea adverse event reporting system database, 2005–2016. Yonsei Med J. 2020;61(3):243–50. doi:10.3349/ymj.2020.61.3.243.
- Shiri T, Datta S, Madan J, Tsertsvadze A, Royle P, Keeling MJ, McCarthy ND, Petrou S. Indirect effects of childhood pneumococcal conjugate vaccination on invasive pneumococcal disease: asystematic review and meta-analysis. Lancet Global Health. 2017;5(1):e51–e59. doi:10.1016/S2214-109X(16)30306-0