ABSTRACT
This study consisted of two rounds of cross-sectional observations designed to evaluate the persistence of immune protection induced high antigen content hepatitis B (HB) vaccine at 60 μg/1.0 ml formulations administered at a three-dose schedule (Days 0, 28, and 56) in non-responders to routine HB vaccination. In the original phase 3 study, we enrolled 1091 healthy participants (16–60 years old) seronegative for antibody against HB surface antigen (anti-HBs) after primary vaccination. Participants were randomized (2:2:1) to receive three booster doses of HB vaccine containing 60 μg, 30 μg, or 10 μg of antigen per dose 28 days apart. In the group receiving the 60 μg HB vaccine, 428 participants’ serum samples were available at pre-vaccination and 28 days after each vaccine dose and were included in immunogenicity analysis. With two written informed consents, we collected blood samples from 276 (67.2%) participants in 2014 and 239 (58.2%) in 2019, who had completed the full course of revaccination and reached the seropositive (anti-HBs≥10 mIU/ml) standard in the 60 μg vaccine group of the original phase 3 study. The HBV seropositive rate was found to decrease from 96.0% in 28 days after receiving the third dose of 60 μg HB vaccine, to 48.2% in 2014, and to 40.6% in 2019, with anti-HBs GMC of seropositive individuals was 584.0 mIU/ml, 142.4 mIU/ml, and 169.1 mIU/ml, respectively. Analysis of 181 vaccinees who had serologic test results available both in 2014 and in 2019, and results revealed a dynamic trend in anti-HBs titer similar to that for the whole immune persistence cohort. Of paramount importance, the serologic test results found that 24.9% (45/181) participants had higher anti-HBs concentrations in 2019 than in 2014, this could be interpreted as natural boosters, secondary to HBV exposure without infection because protected. In conclusion, protective antibody persists about 11 years after immunization of Chinese non-responders with 3 doses of 60 μg HB vaccine. Booster doses of vaccine do not seem necessary to ensure long-term protection.
1. Introduction
Hepatitis B is a potentially life-threatening liver infection caused by the hepatitis B virus (HBV). HBV infection is a major public health problem worldwide, which can cause both acute and chronic disease, and puts people at high risk of death from cirrhosis and liver cancer.Citation1,Citation2 Globally, in 2015, an estimated 257 million people were living with chronic hepatitis B infection (defined as hepatitis B surface antigen-positive), and over 887 000 deaths are caused by the virus every year. To combat hepatitis, the World Health Assembly approved the global health sector strategy to eliminate viral hepatitis as a public health threat by 2030, with a target of reducing new infections by 90% and mortality by 65%.Citation3 According to a 2018 report by the International Agency for Research on Cancer, China has the 9th highest rate of liver cancer worldwide.Citation4 But in the size of her population (1.4 billion, 2019), China would certainly confront the largest number of liver cancer patients in the world, and the absolute number of HBV-infected people is about 70.5 million.Citation5 Therefore, China has the largest burden of HBV infection in the world and will be a major contributor to the global elimination of hepatitis B disease by 2030. The country has made good progress in reducing the incidence of HBV infection in the past several decades. However, China still faces challenges in achieving its target of 65% reduction in mortality from hepatitis B by 2030.Citation6
Hepatitis B (HB) vaccine is recognized as the first vaccine to prevent cancer in the world and numerous studies have shown that HB vaccine is a critical intervention for the elimination of HBV epidemics.Citation6 Wider provision of the existing, safe, and effective HB vaccine will dramatically reduce new HBV infections, reducing rates of chronic illness and death.Citation3 By 2015, the global prevalence of the hepatitis B surface antigen (HBsAg) has decreased from 4.7% to 1.3% in children under 5 years of age, as a result of worldwide vaccination programs; however, the prevalence remains high in non-vaccinated people.Citation2 In China, the HBsAg prevalence rate in the general population dropped from 9.75% in 1992 to 7.18% in 2006 and 2.64% in 2014 (1–29 years old),Citation7 thanks to a national program for HBV immunization instituted in 1992, in conjunction with completely free HBV vaccination for all newborns since 2005.Citation8 However, the actual protective effect after vaccination varies from person to person due to the variability of vaccine-mediated immune response. The concentration of hepatitis B surface antibody above 10 mIU/ml is considered a reliable marker of protection against HBV infection 1 to 3 months after completion of the final dose of the basic immunization program with the HB vaccine.Citation9,Citation10 In the general population, the reported non-response rate after HB vaccine ranges from 10% to 30%, and these non-responders remain susceptible to HBV.Citation11–13 Based on targets of the World Health Organization’s Global health sector strategy on viral hepatitis 2016–2021, we emphasize the need to reduce the incidence of non-response in China.
In October 2005, Kangtai Biological Products Co., Ltd. (Shenzhen, Guangdong Province) obtained the Drug Clinical trial approval issued by the State Food and Drug Administration (SFDA). Then, from 2006 to 2008, a phase 3, double-blind, controlled clinical trial was conducted in Ganyu and Lianshui County, Jiangsu Province of China. In the original phase 3 study, we enrolled healthy participants (16–60 years old) seronegative for antibody against HB surface antigen (anti-HBs) after primary vaccination, who had anti-HBs titers <10 mlU/ml at 28 days following routine vaccination with licensed HB vaccine containing 10 μg of antigen. Participants were randomized (2:2:1) to receive three booster doses of HB vaccine formulations containing 60 μg, 30 μg, or 10 μg of antigen per dose 28 days apart. Blood samples were obtained pre-vaccination and 28 days after each dose to assess immunogenicity. Reactogenicity and safety were evaluated up to 28 days after each vaccine dose. The original phase 3 study showed that the HB vaccine containing 60 μg of antigen appears to be significantly more immunogenic in terms of seroconversion rates and GMCs than conventional vaccine in non-responders to conventional vaccines containing 10 μg of antigen with a similar safety profile. These findings supported the administration of this HB vaccine containing 60 μg of antigen in Chinese non-responders.Citation14
In 2010, the State Food and Drug Administration (SFDA) approved the HB vaccine containing 60 μg of antigen. Currently, about 1 million doses of the 60 μg HB vaccine have been distributed around 31 provinces of China and administrated to the non-responders to the standard primary immunization of 10 μg dose HB vaccines. However, the duration of protection after HB vaccination is not exactly known. Therefore, the purpose of this study was to evaluate the persistence of immune protection induced by three doses (Days 0, 28, and 56) of high antigen content HB vaccine at 60 μg/1.0 ml formulations administered to non-responders after routine primary vaccination.
2. Methods
2.1. Study design
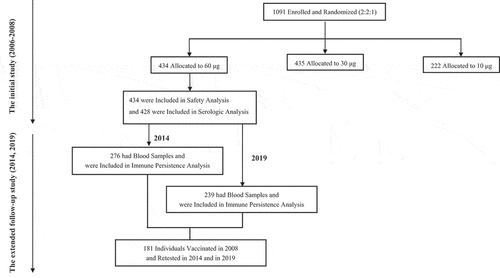
This study contained two rounds of cross-sectional observations based on a phase 3, double-blind, controlled clinical trial conducted in China (NCT01203319).Citation14 shows the overall study design. In the original phase 3 study, we recruited 1091 healthy participants aged 16 to 60 years and seronegative for anti-HBs after primary vaccination, who had anti-HBs titers <10 mlU/ml at 28 days following routine vaccination with licensed 10 μg HB vaccine. Participants were randomized (2:2:1) to receive three booster doses (Days 0, 28, and 56) of HB vaccine formulations containing 60 μg, 30 μg, or 10 μg of antigen at 28-day intervals. Further immunogenicity analyses, we included 428 (221 males, 207 females) participants in the immunogenicity cohort who received 3 doses 60 μg HB vaccine on a 0-1-2 month schedule and had serologic test results available at baseline and 28 days after each vaccine dose. To evaluate the persistence of immunogenicity, 411 of the 428 vaccinees who had reached 10 mIU/ml seropositive standard were the subjects of this study. A blood sample was taken 6 years (2014) and 11 years (2019) after the third dose of 60 μg HB vaccine from 276 (67.2%; 137 males, 139 females) and 239 (58.2%; 131 males, 108 females) vaccinees and assayed for anti-HBs antibody, respectively. Of these, 181 vaccinees (99 males, 82 females) had serologic test results available both in 2014 and in 2019.
2.2. Endpoint measurement
A blood sample of 5.0 mL was obtained from every individual at enrollment to measure the concentration of anti-HBs. Anti-HBs GMC was detected by using the chemiluminescence assay Architect i2000 (Abbott, Chicago, IL, USA). If anti-HBs >1000 mIU/ml, an arbitrary value of 1000 mIU/ml was assigned to allow for calculation of the Anti-HBs GMC. All tests were conducted according to the manufacturers’ instructions. According to the titer of anti-HBs after immunization of HB vaccine, the body’s response to HB vaccine can be divided into four states: <10 mIU/ml = non-responders; 10–100 mIU/ml = low-responders; 100–1000 mIU/ml = moderate responders; ≥1000 mIU/ml = high-responders.Citation15 The primary endpoint of the study was the percentages of participants with protective levels of anti-HBs in two rounds of cross-sectional observations. Since an anti-HBs titer greater than or equal to 10 mIU/ml was considered protective against HBV infection,Citation16,Citation17 subjects in this study will be classified as seropositive when the anti-HBs titer was ≥10 mIU/ml. The secondary endpoint was the anti-HBs GMC.
3. Statistical analysis
We collected the basic information including sociodemographic information (e.g., name, sex, birth date, or place of birth), HB vaccination history, and history of HB through face to face interview with the study subject. Anti-HBs titers were compared by use of the non-parametric Mann–Whitney U test. We tested differences in frequency with the χ2 test and regarded p <.05 as significant. Logistic regression analysis was used to determine the possible impact on the immune persistence of the candidate vaccine. Statistical calculations were undertaken with SPSS Statistics standard 23.0.
4. Results
The comparison of demographic characteristics between the follow-up group and the lost group in 2014 and 2019 is shown in . For the whole immune persistence cohort, the HBV seropositive rate was found to decrease from 96.0% in 2008, to 48.2% in 2014, and to 40.6% in 2019 (P <.0001; ), with anti-HBs GMC of seropositive individuals was 584.0 mIU/ml (95% CI: 547.7–620.3 mIU/ml), 142.4 mIU/ml (95% CI: 99.7–185.0 mIU/ml), and 169.1 mIU/ml (95% CI: 113.1–225.1 mIU/ml) (P <.0001; ), respectively. Our data provide evidence that the titer of anti-HBs drops sharply about 6 years after vaccination and then drops slowly between 6 and 11 years of vaccination. Analysis of 181 vaccinees who had serologic test results available both in 2014 and in 2019, and results revealed a dynamic trend in anti-HBs titer similar to that for the whole immune persistence cohort ().
Table 1. Comparison of demographic characteristics between the follow-up group and the lost group in 2014 and 2019
Table 2. Seropositive rates and anti-HBs concentrations (mIU/ml) in 2008, 2014, and 2019
Table 3. Seropositive rates and anti-HBs concentrations (mIU/ml) in 181 individuals vaccinated in 2008 and retested in 2014 and in 2019
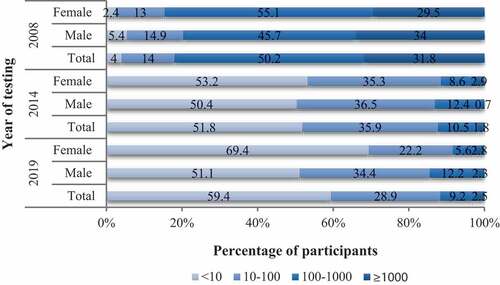
Twenty-eight days after administration of the third dose of 60 μg HB vaccine, the proportions of subjects with antibody titer of <10 mIU/ml, 10–100 mIU/ml, 100–1000 mIU/ml, and ≥1000 mIU/ml were 4%, 14%, 50.2%, and 31.8%, respectively. Six years after the third dose of 60 μg HB vaccine, 48.2% vaccinees examined still had protective levels of anti-HBs (10–100 mIU/ml: 35.9%; 100–1000 mIU/ml: 10.5%; ≥1000 mIU/ml: 1.8%), while 133 (51.8%) vaccinees had antibody <10 mIU/ml with three of them with undetectable antibody. Eleven years after vaccination, 97 out of 239 (40.6%) vaccinees examined still had protective levels of anti-HBs (10–100 mIU/ml: 28.9%; 100–1000 mIU/ml: 9.2%; ≥1000 mIU/ml: 2.5%) ().
Figure 2. Classification of participants based on their serum concentration of anti-HBs antibody in 2008, 2014 and 2019. 2008 = 28 days after receiving the third dose of 60 μg HB vaccine; 2014 = 6 years after receiving the third dose of 60 μg HB vaccine; 2019 = 11 years after receiving the third dose of 60 μg HB vaccine. According to the titer of anti-HBs, the body’s response to HB vaccine can be divided into four states: <10 mIU/ml = non-responders; 10–100 mIU/ml = low-responders; 100–1000 mIU/ml = moderate responders; ≥1000 mIU/ml = high-responders
In order to analyze factors affecting seropositive rate of 60 μg hepatitis B vaccine after 11 years, whether anti-HBs level ≥10 mIU/ml was used as a dependent variable, and independent variables used to conduct logistic regression analyses include sex and age. The final analysis is shown in . Sex was the possible impact on the immune persistence of 60 μg HB vaccine.
Table 4. Logistic regression analysis of seropositive rate of anti-HBs in 2019 with the baseline characters
5. Discussion
The results of the original phase 3 study showed that the GMCs after one dose of 60 μg recombinant HB vaccine were in a similar range to those after two doses of 30 μg or three doses of 10 μg HB vaccine. This was also seen for seroconversion rates. These findings thus support the administration of this high antigen content vaccine in Chinese non-responders.Citation14 Two rounds of cross-sectional observations found that six and eleven years after vaccination, the seropositive rate of anti-HBs among vaccinees remained about 40% (48.2%, 40.6%), similar to the 47.4% seropositive rate of anti-HBs in the general population (immunization status: 13.8%-Yes, 68.8%-No, 17.4%-Unknown) aged 15–59 in China.Citation18 Therefore, we can infer that the seropositive rate of anti-HBs after 11 years of vaccination has been reduced to a relatively low level. However, the serologic test results found that 24.9% (45/181) participants had higher anti-HBs concentrations in 2019 than in 2014, this could be due to the natural infection of the HBV, so as to stimulate the GMCs for anti-HBs rise again. Serum samples collected at different time periods were not simultaneously tested, and thus we cannot exclude that variation in antibody titers could be due to laboratory variables. However, we have always employed the same technology for anti-HBs antibody titration, make such a hypothesis unlikely. These data suggest the existence of protective antibody. Thus, our finding shows that booster doses of vaccine do not seem necessary to ensure long-term protection after 11 years of vaccination.
Loss of antibody does not necessarily imply loss of protection, because the long incubation period of HBV could allow time for the immunological memory to protect them against acute disease or the development of chronic carriage.Citation19 In a study in the United States, 49% of subjects had anti-HBs titers <10 mIU/ml during 30 years follow-up, but 88% of persons without seroprotective levels of anti-HBs had a rapid rise in titer after a booster, which confirmed the existence of immune memory.Citation20 So far, many studies on the long-term protective effect of HB vaccine have shown that immune memory outlasted the presence of detectable circulating antibodies.Citation15,Citation21,Citation22 A studyCitation23 in Hong Kong demonstrated that after a routine three-dose HBV vaccination, approximately 90% of the subjects who received the vaccine remained protected for more than 30 years regardless of the anti-HBs antibody titer. Therefore, most researchers have proposed that due to the strong immune memory after vaccination, the immunity can be maintained for more than 10 years even if the anti-HBs drops to an undetectable level.Citation24–27
Some limitations of our study should be taken into consideration. Firstly, as in most analogous research,Citation16,Citation27,Citation28 serological surveys were not conducted annually, and the occurrence of a booster due to natural HBV infection could not be detected and eventually could not be excluded from the current analysis. From this point, the persistence of immunity demonstrated in this study might not be only due to vaccine immunization, but also to natural exposure to HBV. Secondly, because there are no personal immunization records for adults, we can only collect the subjects’ history of booster immunization through questionnaires, this memory-based immunization information may have recall bias. Finally, in this study, only from the perspective of humoral immune antibody level, the single evaluation index is not enough to systematically evaluate the immune persistence of HB vaccine.
In light of our findings, the use of routine booster doses of HB vaccine does not seem necessary to maintain long-term protection in Chinese non-responders (16–60 years old). Clearly, this observation is specific to the 11-year checkpoint. Additional follow-up is warranted to establish whether a primary course of vaccination in non-responders may confer life-long protection or whether boosters may be needed at a later point in life.
6. Conclusions
Protective antibody persists about 11 years after immunization of Chinese non-responders (16–60 years old) with 3 doses of 60 μg HB vaccine. Booster doses of vaccine do not seem necessary to ensure long-term protection. These findings might play an important role in its clinical application and decisions about mass administration of 60 μg HB vaccine for controlling hepatitis B epidemics in the future.
Disclosure of potential conflicts of interest
Jingshan Zheng, Huayu Li, Jianhui Gan are employed by Shenzhen Kangtai Biological Products Co., Ltd. All other authors have no conflicts.
Additional information
Funding
Notes on contributors
Fengcai Zhu
Fengcai Zhu and Jianhui Gan designed the trial and the study protocol. Jingshan Zheng, Qi Liang and Huayu Li led and participated in the site work. Juan Li, Fanyue Meng, and Li Zhang contributed to the data collection, data management, and statistical analysis and wrote the paper. Jingxin Li contributed to lead and participate in the revising of the manuscript. Each author listed on the manuscript has seen and approved the submission of this version of the manuscript.
References
- Li H, Yan L, Shi Y, Lv D, Shang J, Bai L, Tang H. Hepatitis B virus infection: overview. Adv Exp Med Biol. 2020;1179:1–16. doi:10.1007/978-981-13-9151-4_1.
- Seto WK, Lo YR, Pawlotsky JM, Yuen MF. Chronic hepatitis B virus infection. Lancet. 2018 Nov 24;392(10161):2313–24. doi:10.1016/S0140-6736(18)31865-8.
- World Health Organization. Global health sector strategy on viral hepatitis 2016–2021. Geneva: World Health Organization; 2016. [accessed 2020 Jul 8]. http://www.who.int/hepatitis/strategy2016-2021/ghss-hep/en/.
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018 Nov;68(6):394–424. doi:10.3322/caac.21492.
- Liu J, Zhang S, Wang Q, Shen H, Zhang M, Zhang Y, Yan D, Liu M. Seroepidemiology of hepatitis B virus infection in 2 million men aged 21-49 years in rural China: a population-based, cross-sectional study. Lancet Infect Dis. 2016 Jan;16(1):80–86. doi:10.1016/S1473-3099(15)00218-2.
- Liu J, Liang W, Jing W, Liu M. Countdown to 2030: eliminating hepatitis B disease, China. Bull World Health Organ. 2019 Mar 1;97(3):230–38. doi:10.2471/BLT.18.219469.
- Wang ZZ, Li MQ, Wang P, Yang ZX, Wei L, Zeng Y, Li YP, Yan L, Liu XE, Zhuang H. Comparative immunogenicity of hepatitis B vaccine with different dosages and schedules in healthy young adults in China. Vaccine. 2016 Feb 17;34(8):1034–39. doi:10.1016/j.vaccine.2016.01.018.
- Xiao J, Wang F, Wong NK, He J, Zhang R, Sun R, Xu Y, Liu Y, Li W, Koike K, et al. Global liver disease burdens and research trends: analysis from a Chinese perspective. J Hepatol. 2019 Jul;71(1):212–21. doi:10.1016/j.jhep.2019.03.004.
- Jack AD, Hall AJ, Maine N, Mendy M, Whittle HC. What level of hepatitis B antibody is protective? J Infect Dis. 1999 Feb;179(2):489–92. PMID: 9878036. doi:10.1086/314578.
- Wang J, He Y, Jin D, Liu J, Zheng J, Yuan N, Bai Y, Yan T, Yang Y, Liu Y, et al. No response to hepatitis B vaccine in infants born to HBsAg(+) mothers is associated to the transplacental transfer of HBsAg. Infect Dis (Lond). 2017 Aug;49(8):576–83. doi:10.1080/23744235.2017.1292541.
- Yen YH, Chen CH, Wang JH, Lee CM, Changchien CS, Lu SN. Study of hepatitis B (HB) vaccine non-responsiveness among health care workers from an endemic area (Taiwan). Liver Int. 2005 Dec;25(6):1162–68. doi:10.1111/j.1478-3231.2005.01157.x.
- Joukar F, Mansour-Ghanaei F, Naghipour MR, Asgharnezhad M. Immune responses to single-dose versus double-dose hepatitis B vaccines in healthcare workers not responding to the primary vaccine series: a randomized clinical trial. Hepat Mon. 2016 Feb 27;16(2):e32799. doi:10.5812/hepatmon.32799.
- Chen X, Gui X, Zhang L, Huang F, Zhong H, Pang Z, Wang S, Tang L, Fu L, Peng Y, et al. Maternal anti-HBVs suppress the immune response of infants to hepatitis B vaccine. J Viral Hepat. 2016 Dec;23(12):955–60. doi:10.1111/jvh.12572.
- Pan HX, Zeng Y, Song XF, Zhang YJ, Xu K, Liang ZL, Zhu FC. Immune response to hepatitis B vaccine with high antigen content in non-responders after standard primary vaccination in Chinese adults. Vaccine. 2014 Jun 17;32(29):3706–12. doi:10.1016/j.vaccine.2014.02.094.
- Isolani AP, Sversuti CS, Sell AM, Moliterno RA. Protection against hepatitis B by the Butang recombinant vaccine in newborn children in South Brazil. Mem Inst Oswaldo Cruz. 2006 Aug;101(5):551–53. doi:10.1590/s0074-02762006000500012.
- Wu W, Lv J, Liu J, Yan B, Feng Y, Xu A, Zhang L. Persistence of immune memory among adults with normal and high antibody response to primary hepatitis B vaccination: results from a five-year follow-up study in China. Hum Vaccin Immunother. 2018;14(10):2485–90. doi:10.1080/21645515.2018.1477911.
- Pileggi C, Papadopoli R, Bianco A, Pavia M. Hepatitis B vaccine and the need for a booster dose after primary vaccination. Vaccine. 2017 Nov 1;35(46):6302–07. doi:10.1016/j.vaccine.2017.09.076.
- Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, Zhang Y, Liu J, Gong X, Chen Y, et al. Epidemiological serosurvey of hepatitis B in China–declining HBV prevalence due to hepatitis B vaccination. Vaccine. 2009 Nov 5;27(47):6550–57. doi:10.1016/j.vaccine.2009.08.048.
- Zanetti AR, Mariano A, Romanò L, D’Amelio R, Chironna M, Coppola RC, Cuccia M, Mangione R, Marrone F, Negrone FS, et al. Study Group. Long-term immunogenicity of hepatitis B vaccination and policy for booster: an Italian multicentre study. Lancet. 2005 Oct 15–21;366(9494):1379–84. doi:10.1016/S0140-6736(05)67568-X
- Bruce MG, Bruden D, Hurlburt D, Zanis C, Thompson G, Rea L, Toomey M, Townshend-Bulson L, Rudolph K, Bulkow L, et al. Antibody levels and protection after hepatitis B vaccine: results of a 30-year follow-up study and response to a booster dose. J Infect Dis. 2016 Jul 1;214(1):16–22. doi:10.1093/infdis/jiv748.
- Poovorawan Y, Chongsrisawat V, Theamboonlers A, Crasta PD, Messier M, Hardt K. Long-term anti-HBs antibody persistence following infant vaccination against hepatitis B and evaluation of anamnestic response: a 20-year follow-up study in Thailand. Hum Vaccin Immunother. 2013 Aug;9(8):1679–84. doi:10.4161/hv.24844.
- Bagheri-Jamebozorgi M, Keshavarz J, Nemati M, Mohammadi-Hossainabad S, Rezayati MT, Nejad-Ghaderi M, Jamalizadeh A, Shokri F, Jafarzadeh A. The persistence of anti-HBs antibody and anamnestic response 20 years after primary vaccination with recombinant hepatitis B vaccine at infancy. Hum Vaccin Immunother. 2014;10(12):3731–36. doi:10.4161/hv.34393.
- Lin AW, Wong KH. Long-term protection of neonatal hepatitis B vaccination in a 30-year cohort in Hong Kong. J Hepatol. 2013 Dec;59(6):1363–64. doi:10.1016/j.jhep.2013.08.021.
- Spradling PR, Kamili S, Xing J, Drobeniuc J, Hu DJ, Middleman AB. Response to challenge dose among young adults vaccinated for hepatitis B as infants: importance of detectable residual antibody to hepatitis B surface antigen. Infect Control Hosp Epidemiol. 2015 May;36(5):529–33. doi:10.1017/ice.2015.6.
- Yao J, Shan H, Chen Y, Jiang ZG, Dai XW, Ren JJ, Xu KJ, Ruan B, Yang SG, Li Q. The one year effects of three doses of hepatitis B vaccine as a booster in anti-HBs-negative children 11-15 years after primary immunization; China, 2009-2011. Hum Vaccin Immunother. 2015;11(5):1114–19. doi:10.4161/21645515.2014.987001.
- Coppola N, Corvino AR, De Pascalis S, Signoriello G, Di Fiore E, Nienhaus A, Sagnelli E, Lamberti M. The long-term immunogenicity of recombinant hepatitis B virus (HBV) vaccine: contribution of universal HBV vaccination in Italy. BMC Infect Dis. 2015 Mar 25;15:149. doi:10.1186/s12879-015-0874-3.
- Zhao YL, Han BH, Zhang XJ, Pan LL, Zhou HS, Gao Z, Hao ZY, Wu ZW, Ma TL, Wang F, et al. Immune persistence 17 to 20 years after primary vaccination with recombination hepatitis B vaccine (CHO) and the effect of booster dose vaccination. BMC Infect Dis. 2019 May 30;19(1):482. doi:10.1186/s12879-019-4134-9.
- Zanetti AR, Romanò L, Giambi C, Pavan A, Carnelli V, Baitelli G, Malchiodi G, Valerio E, Barale A, Marchisio MA, et al. study group. Hepatitis B immune memory in children primed with hexavalent vaccines and given monovalent booster vaccines: an open-label, randomised, controlled, multicentre study. Lancet Infect Dis. 2010 Nov;10(11):755–61. doi:10.1016/S1473-3099(10)70195-X.