ABSTRACT
In Norway, the incidence of invasive meningococcal disease (IMD) is higher among 16–19-year-olds than in the general population. Most IMD cases among teenagers are caused by serogroup Y. Since 2011, one dose of meningococcal ACWY conjugate vaccine (MCV4) has been recommended for teenagers with out-of-pocket payment. The teenagers are usually vaccinated through the school health service at age 18. This study aimed to estimate costs and health gains of introducing MCV4 to Norwegian teenagers through the national immunization program (NIP). A Markov model was used to analyze the cost-effectiveness of universal MCV4 vaccination of either 15-year-olds or 18-years-olds. Occurrences of IMD were simulated from 15 until 23 years of age. Costs were estimated from a healthcare perspective. Sensitivity analyses evaluated the impact of vaccine price, vaccination uptake, IMD incidence and discount rate. Compared to today’s practice of vaccinating 18-year-olds with out-of-pocket payment, introducing MCV4 to 15-year-olds in a NIP-setting, with 90% vaccine uptake and 50% rebate on vaccine price, prevented 3.2 hospitalizations, 0.20 sequelae and 0.47 deaths among 15–23-year-olds, annually. Total costs were reduced by €30,000 and 9.7 quality-adjusted life-years (QALYs) were gained per birth cohort. The probability of cost-effectiveness was 99.0%, assuming a willingness-to-pay threshold of €86,000/QALY for severe diseases in Norway. Cost-effectiveness was highly dependent on vaccine price. Vaccination of 18-year-olds in a NIP-setting was also cost-effective, but less than NIP-vaccination of 15-year-olds. Introduction of MCV4 to the 15-year-olds in the Norwegian NIP is likely to be cost-effective given a rebate on the vaccine price.
Introduction
Neisseria meningitidis, the meningococcus, is a common human commensal that in rare occasions cause invasive meningococcal disease (IMD). Despite antibiotic and supportive treatment, mortality from IMD remains high and many patients suffer sequelae influencing their quality of life (QoL).Citation1,Citation2 Serogroups A, B, C, W, X, and Y are the most common disease-causing variants.Citation3 Incidence and serogroup distribution varies by region and age. IMD primarily affects infants, adolescents, and young adults.Citation4 Serogroup B predominates in the general population in Europe.Citation4,Citation5
Rarity of the disease and unexpected affection of previously healthy individuals make the risk of IMD at an individual level unpredictable. Influence of long-term sequelae on the patient, as well as indirect effects on family members and society, are difficult to capture fully.Citation6,Citation7 In addition, the emergence of hypervirulent clonesCitation8,Citation9 and unforeseen outbreaks with cases spread over timeCitation10,Citation11 also predicts disease burden challenging at a public health level.
Both meningococcal ACWY conjugate vaccines (MCV4)Citation12,Citation13 and meningococcal B protein vaccines (MenB)Citation14,Citation15 have been shown to induce protective immunity. Some European countries have a general recommendation for MCV4 vaccination of adolescents, but adolescent MenB vaccination free of charge is currently not implemented in any national immunization program (NIP) in Europe.Citation16 In Norway, meningococcal vaccines are not implemented in the NIP, and are currently only recommended for various risk groups, including the 16–19-year-olds.
After a peak of 5.3 cases of IMD per 100,000 in 2010, the mean incidence in Norwegian 15–19-year-olds has decreased to 1.2 per 100,000 in 2017–2019.Citation17 Between 2017 and 2019 the mean incidence was 0.8 per 100,000 in children <5 years, and 0.3 per 100,000 in both the 5–14-year-olds and adults >19 years. Serogroup Y (75%) has predominated in the 15–19-year-olds, with no cases of serogroup B observed in this age group since 2014. In children <5 years, serogroup B (83%) has dominated, whereas serogroups W and Y are most prominent among the 5–14-year-olds (serogroups B 17%, C 17%, W 33%, Y 33%). Serogroups B, W, and Y were most common among adults >19 years (serogroups B 31%, C 4%, W 27%, Y 38%).
The majority of IMD cases in Norwegian teenagers has occurred among students taking part in the “russ” celebration, a tradition among upper secondary school graduates (mostly aged 18–19 years) including a month-long period of binge-drinking and partying with peers.Citation18 Due to an observed increase in IMD among teenagers involved in the “russ” celebration from 2009, a national recommendation for MCV4 vaccination was initiated from 2011 for the 17–19-year-olds and expanded to the 16–19-year-olds in 2012. Vaccination is arranged through the school health service. Despite the national recommendation, MCV4 is not implemented for all teenagers in the national immunization program, and vaccine cost is financed with students paying out-of-pocket. Currently, several municipalities and counties are sponsoring MCV4 for one cohort (mostly 18–19-year-old graduates) in upper secondary school. In some areas, the vaccination uptake has increased from around 40% to 80% among graduates when vaccination is given free of charge and administered on school grounds,Citation19 indicating that purchasing power and accessibility may influence MCV4 uptake among Norwegian teenagers. In 2019, 48% of the 18–19-year-olds were vaccinated with MCV4 nationally.Citation19 This practice might have contributed to the observed recent decline in incidence of IMD in teenagers.Citation17
Despite the currently low incidence of IMD, there is an ongoing debate in Norway whether MCV4 should be implemented free of charge as part of the NIP for teenagers, to ensure equal access to vaccination and reduce inequality in health. This study aimed to perform an explorative evaluation of costs and health effects from a healthcare perspective to find out if introducing one dose of MCV4 to either 15-year-olds or 18-year-olds through the Norwegian NIP could be cost-effective.
Materials and methods
Vaccination strategies
Three different vaccination strategies were explored (): 1) vaccinating 18-year-olds as a risk group outside the NIP with out-of-pocket payment (Current practice); 2) vaccinating 15-year-olds free of charge as part of the NIP (Universal 15) or 3) vaccinating 18-year-olds free of charge as part of the NIP (Universal 18). Vaccination of 18-year-olds was chosen since this is when most Norwegian teenagers currently are vaccinated and engage in the “russ” celebration. Vaccination at 15 years of age was chosen as an alternative strategy, as most teenagers in Norway start upper secondary school at 15–16 years of age.
Table 1. Overview of vaccination strategies used to model cost-effectiveness
Model
We created a probabilistic Markov cohort model in R version 3.6.2 simulating the impact of teenage MCV4 vaccination on survival, health-related QoL, and resource use from age 15 until 105 years in yearly cycles for the three vaccination strategies. Occurrences of IMD were simulated for the period from 15 to 23 years of age for all three strategies, according to vaccine effectiveness (VE) and vaccination uptake in each strategy (). The age period 15–23 years of age was chosen to take into account the potential protection of the vaccine for all 5 years after vaccination at either 15 or 18 years of age. Modeling was based partly on ideas and templates provided by the Decision Analysis in R for Technologies in Health group.Citation20,Citation21 All individuals were assumed to be at risk of getting IMD, and all IMD cases were assumed admitted to hospital in the acute phase. Hospitalized patients would either recover, suffer permanent sequelae or die (). Individuals started in the model in the health state susceptible and were at risk of the event IMD from either of the meningococcal strains A, C, W, or Y. Individuals who acquired IMD, and thus were hospitalized in the acute phase could from there move to the health state sequelae, or they could recover. All individuals were at risk of dying, although hospitalized patients or those with sequelae were at an increased risk of death.
Figure 1. Illustration of time at risk of IMD, MCV4 vaccination uptake and duration of protection from MCV4 in all cohorts studied
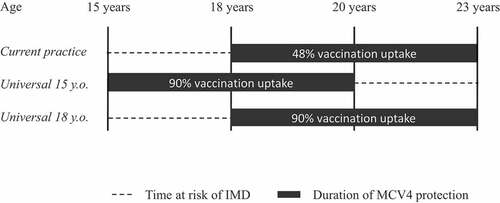
Figure 2. Markov model simulating the impact of vaccinating teenagers with MCV4
Input parameters () were based on sources from Norway where available, or from high-income countries with similar epidemiology or economy. Uncertain parameters in the model were incorporated as probability distributions and simulated 1,000 times using Monte Carlo simulation. Probability distributions were created based on 95% confidence intervals (CI) or standard errors where available. For parameters where no information about the magnitude of the uncertainty was available in published sources, probability distributions were based on assumed uncertainty around the average of each parameter. One-way sensitivity analyses were based on average of runs of the probabilistic model for each extreme value.
Table 2. Model input parameters
Health effects and costs were discounted according to Norwegian guidelines for single technology assessment (STA) of pharmaceuticals,Citation23 hence 4% over the first 40 years, 3% from 40 to 75 years and thereafter 2%. Due to the growing use of differential discounting of health and costs,Citation6 we performed a separate analysis without discounting health effects, as suggested in a Norwegian official report.Citation24
Epidemiology
The average Norwegian cohort of 15-year-olds has been fairly consistent at around 65,000 individuals in the last years.Citation25 The model only included IMD among age groups directly affected by the different vaccination strategies described above, and only included cases caused by MCV4-targeted serogroups. Data on IMD incidence were extracted from The Norwegian Surveillance System for Communicable Diseases (MSIS) between January 2008 and December 2017.Citation17 Cases of IMD are mandatorily reported to MSIS and administration of vaccines to the Norwegian Immunisation Registry SYSVAK.Citation19 The incidence of IMD in the study period might have been influenced by the increase in MCV4 uptake from zero to nearly 50% in some age groups between 2008 and 2017. We wanted to compare the cost-effectiveness of the different vaccination strategies if each strategy had been implemented before the recommendation of MCV4 to 16–19-year-olds in 2011/2012 induced a more widespread use of MCV4 among Norwegian teenagers. We therefore adjusted the observed cases of IMD registered in MSIS according to vaccination uptake in the cohorts registered in SYSVAK and the estimated vaccine effectiveness, in order to calculate a “baseline” incidence of IMD if MCV4 had not been used among teenagers in 2008–2017. In this way, we were able to analyze differences in cost-effectiveness between just a recommendation for the use of MCV4 among selected 16–19-year-olds with out-of-pocket payment (Current practice) to that of implementing MCV4 in the national immunization program to a full cohort of teenagers with the vaccine fully financed by the government (Universal 15 or Universal 18). With the adjusted incidences, we then calculated mean incidences for the full ten-year period (medium incidence), the first five-year period (high incidence) and the last five-year period (low incidence) to incorporate the effect of incidence variations in the model. The number of IMD cases per birth cohort in each year was relatively low. The number of cases was therefore combined for the age groups 15–17, 18–19, and 20–23-year-olds, respectively, in order to even out random fluctuations in IMD (). Probabilities of sequelae (amputation, hearing loss, seizures, and skin scarring) were based on a review by Olbrich et al.Citation2 The distributions of sequelae were based on medians, minimum and maximum values reported in the review.
Vaccine effectiveness and vaccination uptake
Assumed VE of MCV4 was extrapolated from a phase III immunogenicity study among 11–18-year-olds comparing two well-known types of MCV4.Citation12 We pooled the percentage of seroconversion for different serogroups into one estimate based on a weighted average of effects for the different serogroups, giving a VE of 73% (95%CI 71–76%) (). Duration of protection was assumed to be 5 years.Citation26,Citation27 Possible waning of vaccine protection was not included in the model. Herd effect of MCV4 has only been shown in one study.Citation28,Citation29 Since our study was explorative, we chose a static model not accounting for possible herd effect of MCV4.
The vaccine uptake in the universal strategies was assumed to be 90%, based on 88% uptake for the human papilloma virus vaccine and 93% for the diphtheria-tetanus-pertussis-polio booster among Norwegian 16-year-olds in 2018.Citation30 In sensitivity analysis, the uptake in a NIP setting was varied between 80 and 95%. With the current recommendation of out-of-pocket payment of MCV4 vaccination of 16–19-year-olds, vaccine uptake among 18–19-year-olds has gradually risen from 16% in 2012 to 48% in 2018, adapted from ref. 19.Citation19 Therefore, in the strategy, Current practice, the level of vaccine uptake was set to 48%.
Quality-adjusted life-year (QALY) weights
In order to comply with Norwegian guidelines for STA of pharmaceuticals,Citation23 data on health-related QoL were based on the EQ-5D standardized generic instrument,Citation31 and quality-adjusted life-year (QALY) weight among the healthy, general population was based on two Swedish studiesCitation32,Citation33 (). QALY weights for hospital stay and for having sequelae were based on a previous economic evaluation.Citation34 Patients with sequelae (amputation, skin scarring, hearing loss and seizures) were assumed to have sequelae for the rest of their lives. The event “hospital stay” was assumed to last for 10 days based on a publication by Stoof et al.Citation35
Costs
Costs were calculated in European euro (€) with an exchange rate of 9.60 Norwegian kroner (NOK) per € estimated from an average of exchange rates from 2018Citation36 (). In the strategy of Current practice, the price of MCV4 was based on the official retail price in Norway as given by the Norwegian Medicines Agency (NoMA).Citation37 Vaccines included in the Norwegian NIP are procured through national tenders. In the two universal strategies, a 50% price rebate was assumed as a base case estimate. This rebate was extensively varied in a one-way sensitivity analysis from 25 to 75%, based on experience with tenders on long-term contracts for vaccines already implemented in the Norwegian NIP.
Costs of hospital stay were based on annually published official estimates for 2018 of average costs provided by the Norwegian Directorate of Health.Citation38 These estimates are based on the diagnosis-related group (DRG) system, which has separate groups for patients presenting with meningitis and septicemia, the latter group divided into age groups above and below 18 years of age.
Time and resources used for school-based vaccination of adolescents were estimated using information provided by municipalities that are currently offering school-based meningococcal vaccination. Probability distributions were assumed to vary between minimum and maximum of values provided by the different municipalities.
Norwegian guidelines for the management of IMD cases recommend prophylaxis with one dose of ciprofloxacin and one dose of MCV4 to close contacts of IMD cases. Costs and time spent on contact tracing and prophylactic treatment were based on information provided by municipal doctors and nurses. Estimates of number of hours spent on contact tracing was assumed as a lower and upper end of a confidence interval. Salaries for nurses and doctors were based on average hourly wages as reported by Statistics Norway.Citation25 The estimate for the number of close contacts per IMD case was based on records from Norwegian IMD cases in the last decade. Estimates of lifetime costs for patients living with sequelae were based on a Canadian study from 2017.Citation39 This study was chosen due to strong and rigorous reporting of all relevant costs related to sequelae. Canadian dollars were converted into NOK and adjusted based on consumer price index before being converted into €.
According to Norwegian guidelines for STA of pharmaceuticals,Citation23 costs were gathered based on an extended healthcare perspective, implying that factors as productivity loss arising from the inability to work were not included. The Norwegian guidelines explicitly state that health-related costs borne by patients and relatives should be included in the analyses. Thus, costs related to vaccines paid out-of-pocket by 48% of the graduating students in the scenario Current practice were included in the analysis, similar to costs related to a potential vaccination program free of charge to all teenagers. Costs of adverse events monitoring after MCV4 vaccination were not included in the model, because such events were assumed either minor and/or of short duration.Citation40,Citation41
When the ratio between incremental costs divided by incremental effects (i.e., the incremental cost-effectiveness ratio (ICER)) is below a certain level (willingness-to-pay threshold), the intervention in question is regarded as cost-effective compared to the comparator. We assumed a maximum willingness-to-pay threshold for cost-effectiveness of €86,000 (NOK825,000) per QALY gained. The exact national threshold is uncertain, and our assumption was based on indications from two Norwegian official reports, proposing this maximum level for high priority diseases with high severity.Citation24,Citation42 There are yet no estimated threshold for Norway based on which health interventions are displaced when new health interventions are introduced. The best estimate so far is probably the estimate by Woods et al.,Citation43 which translates to approximately €35,000 per QALY gained for 2018.
Results
Comparison of the strategies Current practice vs Universal 15
Vaccinating a cohort of 15-year-olds with MCV4 as part of the Norwegian NIP with an estimated uptake of 90% (i.e., Universal 15) would result in 4.26 IMD-related hospitalizations during the 8-year-period from 15 to 23 years age, compared to 7.41 hospitalizations if vaccinating 48% of 18-year-olds (i.e. Current practice) (). In the same period, a change from Current practice to Universal 15, would lead to a reduction of 0.47 deaths and 0.20 sequelae in each birth cohort. This means that about every 2 years we would avoid a death, and every 5 years a sequelae due to IMD caused by serogroups A, C, W, or Y if MCV4 was included in the NIP for 15-year-olds.
Table 3. Costs and health gains of vaccination with one dose of MCV4. Estimates are displayed per birth cohort. Current practice refers to today’s practice of vaccinating 18-year-olds outside a NIP setting (48% uptake, retail vaccine price) and is therefore the base case strategy in the model. Universal 15 and Universal 18 refers to vaccination in a NIP setting (90% uptake, 50% rebate on vaccine price) of 15-year-olds and 18-year-olds, respectively. Estimates for the Universal 15 or Universal 18 strategies are the results of comparing these strategies individually with the base case strategy Current practice.
Expected discounted QALYs per individual would be 22.4 with Current practice. Changing to the Universal 15 strategy would give a 0.00015 increase in expected QALYs per person, translating to 9.67 QALYs gained per birth cohort (). Total costs would decrease with €30,000, assuming 50% rebate on the vaccine price in the NIP-setting. This would give both a slight decrease in costs and an increase in effects, resulting in Universal 15 being what is referred to as a “dominant” strategy.
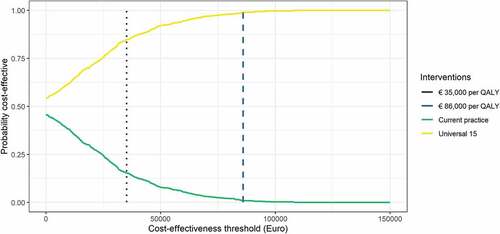
Our simulations indicated that there was a 100% chance of Universal 15 being more effective (i.e., giving more QALYs) than Current practice and a 54% chance of being less costly (). Overall, there was a 99% chance of Universal 15 being cost-effective compared to Current practice when assuming a willingness-to-pay threshold of €86,000/QALY for severe diseases (), although the uncertainty related to the input parameters indicates large variability around the estimated cost-effectiveness ratio (). Given the estimate of assumed displacement of other services, there was an 85% probability that introducing the MCV4 vaccine would give more health than the health displaced if the resources were taken from other health services ().
Figure 3. Scatterplot of simulations comparing the strategy Universal 15 with Current practice. Dotted red line represents the threshold for displaced health interventions of €35,000 per QALY and dashed blue line the willingness-to-pay threshold of €86,000 per QALY
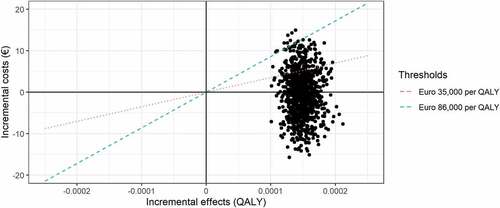
Figure 4. Cost-effectiveness acceptability curve comparing the strategy Universal 15 (yellow line) with Current practice (green line). Dotted purple line represents threshold for displaced health interventions of €35,000 per QALY and dashed blue line the willingness-to-pay threshold of €86,000 per QALY
Sensitivity and scenario analyses for the strategies Current practice vs Universal 15
Potential effects of variations in vaccine rebate, vaccination uptake, IMD incidence and discount rates on the ICER were explored comparing the Universal 15 strategy with Current practice in a tornado diagram ().
Figure 5. Tornado diagram of one-way sensitivity analysis on selected inputs with strategies Universal 15 compared to Current practice. Red indicates the lower boundaries and blue the upper boundaries for sensitivity analyses of input parameters in the model. Dotted line represents the threshold for displaced health interventions of €35,000 per QALY and dashed line the willingness-to-pay threshold of €86,000 per QALY. Black solid line represents input parameters used for base case cost-effectiveness calculations in the strategy Universal 15 (50% vaccine rebate, 90% vaccine uptake, medium IMD incidence and 2–4% discount rate)
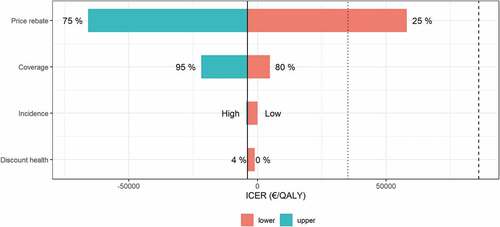
Not surprisingly, a 75% rebate on the current MCV4 vaccine price in Norway was even more cost-effective than the base case of 50% rebate. With only 25% rebate, the ICER would be €58,000 per QALY gained, which is still below the willingness-to-pay threshold.
Changing vaccination uptake to 80% or 95% resulted in incremental increases in QALYs of 8.54 and 10.2, respectively, and differences in costs of -€186,000 and €49,000. Assuming a willingness-to-pay threshold of €86,000/QALY for severe diseases, this resulted in both 80% and 95% uptake being cost-effective, and 80% uptake was also cost-saving.
Varying the IMD incidence from medium (i.e., the whole 10-year period) in the base case to low (i.e., the last 5-year period) indicated a gain of 6.32 QALYs, while a change from medium to high incidence (i.e., the first 5-year period) gave a gain of 12.5 QALYs. Furthermore, a high incidence was cost-saving compared to the base case incidence, while the low incidence gave a small increase of €400 per cohort, giving an ICER of €63 per QALY gained.
When performing the analysis without discounting health effects, differences in QALYs increased to 26.6 per birth cohort when comparing Universal 15 to Current practice. Although incremental effect is considerably higher without discounting QALYs, the costs are the same, indicating that Universal 15 still produces more QALYs and reduces costs compared to Current practice.
We did also perform an analysis of the expected value of perfect information for all parameters (data not shown) based on methods developed by Strong and colleagues.Citation44 Due to the high probability of cost-effectiveness and since only the vaccine rebate was informing this probability, the graph was not informative.
Comparison of the strategies Current practice vs Universal 18
We also looked into potentially vaccinating the 18-year-olds instead of the 15-year-olds with MCV4 in a NIP-setting (Universal 18), with 90% uptake and 50% rebate on the vaccine price. Compared to Current practice, the Universal 18 strategy lead to an annual reduction of 3.2 hospitalizations, 0.14 sequelae, and 0.38 deaths per cohort. Lifetime QALYs gained was 8.32 and costs were reduced by €20,000, giving an ICER below zero. The intervention had a 98% probability of being cost-effective compared to Current practice, when assuming a willingness-to-pay threshold of €86,000/QALY for severe diseases ().
Figure 6. Scatterplot of simulations for comparing the strategies Universal 15 and Current practice (black dots) versus comparing Universal 18 and Current practice (gray dots). Dotted red line represents the threshold for displaced health interventions of €35,000 per QALY and dashed blue line the willingness-to-pay threshold of €86,000 per QALY
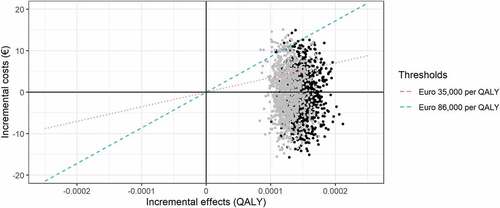
The Universal 18 strategy averted fewer IMD cases and the probability of cost-effectiveness was lower compared to the Universal 15 strategy (). Since the Universal 18 strategy was both more costly and less effective, the Universal 18 strategy was “dominated” by the Universal 15 strategy.
Discussion
Main findings
Vaccinating the 15-year-olds with one dose of MCV4 in a NIP-setting (Universal 15) is 99% likely to be cost-effective compared to Current practice of vaccinating 18-year-olds with out-of-pocket payment, when assuming a willingness-to-pay threshold of €86,000/QALY for severe diseases in Norway. Changing from the strategy Current practice to Universal 15 will prevent three hospitalizations annually, one sequelae every 5 years and one death every 2 years among the 15–23-year-olds. In addition, the intervention will be cost-saving, with a reduction in incremental costs of €30,000. Vaccine price and incidence of IMD have the greatest impact on the probability of cost-effectiveness of implementing MCV4 for the 15-year-olds in the NIP.
Vaccination of the 18-year-olds as part of the NIP (Universal 18) was also found to be cost-effective compared to Current practice. However, as the incidence rate in 15–17-year-olds was more than double that of the 20–23-year-olds, the Universal 18 strategy was more costly and gave lower health gains than the Universal 15 strategy. We have previously shown that the meningococcal carriage rate in Norwegian teenagers was higher in upper secondary school students (15–19-year-olds) than in lower secondary school students (12–15-year-olds).Citation45 Since duration of protection from MCV4 is estimated to be at least 5 years,Citation26,Citation27 and since the incidence of IMD among the 15–17-year-olds is higher than among the 20–23-year-olds, it would be advantageous to vaccinate the 15-year-olds.
There is no official explicit threshold for cost-effectiveness in Norway. We have therefore compared our results in the likely lowest and highest thresholds that would be relevant for implementing a meningococcal vaccine in the Norwegian NIP. Assuming the levels of meningococcal disease observed during the past years, universal vaccination of the 15-year-olds would be cost-effective, regardless of whether the lowest or highest threshold for cost-effectiveness are used.
Comparison with other countries
Cost-effectiveness of MCV4 vaccination of adolescents in a NIP-setting has been evaluated in a few published studies.Citation39,Citation46–51 To our knowledge, our study is the first using a healthcare perspective and where the study population has not been primed with meningococcal vaccine in infancy. An Australian study using a healthcare perspective showed that introducing a booster dose of MCV4 to the 15–19-year-olds in addition to MCV4 at 12 months age would lead to an ICER above the current willingness-to-pay threshold and only a 35% probability of being cost-effective.Citation47 In a US study, MCV4-vaccination of 11-year-olds without a catch-up would probably exceed the willingness-to-pay thresholds adapted for other vaccines implemented in the NIP.Citation49 When assessing the same scenario, but with addition of a catch-up program, the intervention would be cost-effective from a societal perspective if accounting for herd immunity.Citation48 Similarly, a Dutch study evaluating primary vaccination with MCV4 at 14 months and a booster dose at 12 years of age showed cost-effectiveness when accounting for herd immunity.Citation46 In Canada, where MCVC is provided at 12 months and MCVC or MCV4 in adolescence, switching from MCVC to MCV4 among the 12-year-olds would be cost-effective when assuming herd immunity and a high IMD incidence or declining vaccine prices.Citation50
Some of the aforementioned studies were highly dependent on high IMD incidence and low vaccine price for a positive result, and the majority of the studies would not reach cost-effectiveness without accounting for herd effects.Citation39,Citation46–51 Teenagers and young adults have higher rates of meningococcal carriage and play an important role in transmission of the bacterium.Citation52 Even though herd effects have been established for meningococcal conjugate vaccines against serogroup A (MCVA)Citation53 and C (MCVC),Citation54 there is only one study to our knowledge that has indicated possible herd effects for MCV4.Citation28 Adding herd effects would have made our results even more cost-effective, but without changing the conclusion. The willingness-to-pay threshold for diseases with high severity such as IMD is much higher in Norway compared to many other European countries, as well as in Canada and Australia.Citation39,Citation46,Citation47,Citation50 In the UK for example, the threshold was for many years assumed to be between £20,000/QALY and £30,000/QALY, and later reduced to £12,936/QALY.Citation55 In some cases with severe diseases, however, a threshold of £50,000 has been assumed. Similarly, the threshold in Norway has been estimated to about €35,000 per QALY,Citation43 although the government has decided to use thresholds in the range between €29,000/QALY and €86,000/QALY, depending on severity.Citation24,Citation42 Applying a lower threshold in our calculations would imply a lower probability of cost-effectiveness for MCV4 vaccination of Norwegian teenagers, but would not change the conclusion that the vaccination is both increasing health and reducing costs. As the epidemiology, vaccination programs, healthcare systems, insurance policies, and willingness-to-pay thresholds are country-specific, comparison of cost-effectiveness of vaccine interventions between different countries are challenging.
Decisions regarding implementation of a meningococcal vaccine in the NIP
Cost-effectiveness analyses tend to disfavor interventions on rare and severe diseases such as IMD. Generating adequate data on the magnitude of long-term or late-onset sequelae and their impact on for example future productivity loss or the need for special education and social welfare, requires systematic and long-term follow-up of a significant number of patients.Citation6 More subtle sequelae such as psychiatric, psychosocial, or cognitive conditions may be difficult to capture fully and are probably underreported.Citation56 Indirect effects on the QoL of relatives or caregivers of IMD patients can be significant,Citation57 but have not commonly been included in cost-effectiveness analyses.
With high developmental costs of new and technologically advanced vaccine platforms, some new vaccines have become expensive. If the decisions on whether to implement vaccines in a NIP were solely based on the intervention being cost-effective, few of the more costly vaccines would have a chance of achieving cost-effectiveness, maybe affecting the incentive for further vaccine development against rare diseases like IMD. The challenge could partly be overcome by accepting higher willingness-to-pay thresholds for more severe conditions such as recommended in Norwegian guidelines for STA of pharmaceuticals.Citation23 Another measure could be to adjust for the challenges of capturing the impact of long-term sequelae by adding a QALY adjustment factor, such as done by the Joint Committee on Vaccination and Immunisation in the UK.Citation6 Incorporating both a healthcare and a more broad societal perspective in the models might also contribute to a more balanced calculation.Citation6
Fortunately, health technology assessments for publicly financed meningococcal vaccination lean on more than economic evaluations. Other factors include societal preference and acceptability, equity in healthCitation6,Citation7, and feasibility of the intervention. Unexpected affliction of previously young and healthy individuals induces anxiety in the population and high media uptake. Prevention of severe diseases and especially those affecting young people have a high priority both to the public and other stakeholders.Citation7 Observations of a high vaccine uptake among the 18-year-olds with out-of-pocket payment in NorwayCitation19 indicate that the acceptability of MCV4 vaccination is high. Introducing MCV4 in the NIP would promote equity in access to health and probably increase the vaccine uptake among Norwegian teenagers, as already observed in regions sponsoring MCV4. Including MCV4 in the NIP for the 15-year-olds would easily be feasible since the framework for vaccination is already established. From a political and public health perspective, a universal vaccination program against IMD might also mitigate the unforeseen costs related to outbreak responsesCitation10,Citation11 and the need for rapid changes in immunization practice.Citation8,Citation9
Strengths and limitations
Cost-effectiveness studies usually apply a societal perspective, sometimes combined with a health-care perspective. Experiences from some countries indicate that including a wide range of societal costs in the analysis may be pivotal for the outcome.Citation6,Citation7 By using a healthcare perspective, our model did not capture the costs and burdens of IMD fully, which might have underestimated the benefits of vaccination. However, adhering to the Norwegian government’s recommendation of using a healthcare perspective makes our calculations more applicable for the Norwegian settings.
When exploring uncertainty of single parameters, the recommended choice of modeling strategy is still to use a probabilistic model, as for instance pointed out by Claxton and colleagues.Citation58 In essence, there are two ways to do this; the simple way shown in our tornado diagram () or an analysis of expected value of perfect information for parameters. We did also perform such an analysis based on methods developed by Strong and colleagues,Citation44 but due to high probability of the intervention being cost-effective (99%) and only one parameter informing this probability (the vaccine rebate), the graph was not informative. Instead, we included vaccine price, incidence of IMD and vaccination uptake in a tornado diagram as we believe these would be the most important parameters for Norwegian policy makers in the discussion regarding implementation of MCV4 into the NIP for teenagers. In addition, a discount rate was included since there is an ongoing discussion in Norway whether to discount health effects.Citation24
For all input data, we aimed at the best possible level of detail that would also be feasible in the model. In the case of sequelae after meningococcal disease, we found good sources for occurrence of different specific sequelae. For costs, however, our best available estimate was the estimate of lifetime loss due to different sequelae combined.Citation39 Although these approaches to data for sequelae were clearly different, we concluded that neither introducing more detail without data on one side nor reducing detail on the other side would make the model closer to a real life setting.
Country-specific data on mortality rate, frequency, quality-of-life impact and costs of acute and long-term sequelae from IMD were unfortunately not available from Norway. Using data from other countries with different epidemiology and healthcare systems might have over- or underestimated our results.
Vaccination uptake of MCV4 among Norwegian 18–19-year-olds is currently high (48%) and might have contributed to the observed drop in IMD incidence among Norwegian teenagers since 2010. We therefore attempted to wash out the effect of this MCV4 protection financed by out-of-pocket payments, to estimate a baseline incidence to assess a naïve population in all strategies. If the lower incidence of IMD was caused by natural fluctuation or other unknown factors instead of a vaccine effect, our incidence estimates might have been too high and might imply an overestimation of cost-effectiveness.
We did not account for waning of vaccine effectiveness during the eight-year study period, which might have overestimated vaccine protection. Cost-effectiveness studies from other countries evaluating implementation of adolescent MCV4 vaccination have assumed a VE similar to that for MCVC.Citation39,Citation46–51 The VE used in our model was based on assumed protection from serum bactericidal antibodies as a correlate of protection which, compared to other similar studies, might have underestimated the cost-effectiveness. However, more recent evaluations have indicated a lower VE for MCV4Citation59,Citation60 compared to MCVC.
As mentioned, the present analysis was intended as an exploratory analysis to find out whether meningococcal vaccination in Norway could be cost-effective. We therefore constructed a simpler Markov model instead of a more complex dynamic model. Since the intervention proved to be cost-effective in a Markov model, adding indirect effects would probably not change the conclusion of this study as such effects typically would avert even more IMD cases.
Conclusions
IMD is a rare, but highly severe disease striking young and previously healthy individuals unexpectedly. The benefits of meningococcal vaccination are high and risks of adverse events low. In a NIP-setting in Norway, vaccinating the 15-year-olds with one dose of MCV4 would be more cost-effective than vaccinating the 18-year-olds, but vaccination at either age has a high probability of being cost-effective, assuming a high vaccination uptake and a rebate on the vaccine price in a national tender. Implementing MCV4 in the NIP free of charge would contribute to better equity in health and comply with both public and political desire. Future health economic evaluations should strive to obtain Norwegian data on the frequency, impact, and costs of short- and long-term sequelae in survivors of IMD. Furthermore, exploring the costs from a societal perspective and using a more complex and dynamic model to assess the impact of herd effects should be considered, especially if assessing cost-effectiveness of the more expensive meningococcal B vaccines.
Authors contributions
SVW, LMN, DAC, GT, and TW initiated and designed the study. SVW drafted the manuscript. TW performed economic calculations. All authors contributed to the interpretation of the data, to writing and revising the manuscript, and to approval of the final manuscript. All authors attest they meet the ICMJE criteria for authorship.
Acknowledgments
We are grateful for helpful information on meningococcal disease from Petter Brandtzæg, and for information on current practice regarding contact tracing and meningococcal vaccination from Einar Sagberg, Wenche Hovde, Sissel Ourom, Line Sødal, Siv Kristin Larsen, Hilde Stølevik and Ellen M. Sæther.
Disclosure statement
The authors declare that there are no conflicts of interest.
References
- Wang B, Santoreneos R, Giles L, Haji Ali Afzali H, Marshall H. Case fatality rates of invasive meningococcal disease by serogroup and age: a systematic review and meta-analysis. Vaccine. 2019;37(21):2768–82. doi:10.1016/j.vaccine.2019.04.020.
- Olbrich KJ, Muller D, Schumacher S, Beck E, Meszaros K, Koerber F. Systematic review of invasive meningococcal disease: sequelae and quality of life impact on patients and their caregivers. Infect Dis Ther. 2018;7(4):421–38. doi:10.1007/s40121-018-0213-2.
- Caugant DA, Brynildsrud OB. Neisseria meningitidis: using genomics to understand diversity, evolution and pathogenesis. Nat Rev Microbiol. 2020;18(2):84–96. doi:10.1038/s41579-019-0282-6.
- Pelton SI. The global evolution of meningococcal epidemiology following the introduction of meningococcal vaccines. J Adolesc Health. 2016;59(2):S3–S11. doi:10.1016/j.jadohealth.2016.04.012.
- European Centre for Disease Prevention and Control. Invasive meningococcal disease. In: ECDC. Annual epidemiological report for 2017. Stockholm: ECDC; 2019 [accessed 2019 Oct 16]. https://www.ecdc.europa.eu/sites/default/files/documents/AER_for_2017-invasive-meningococcal-disease.pdf.
- Christensen H, Al-Janabi H, Levy P, Postma MJ, Bloom DE, Landa P, Damm O, Salisbury DM, Diez-Domingo J, Towse AK, et al. Economic evaluation of meningococcal vaccines: considerations for the future. Eur J Health Econ. 2020;21(2):297–309. doi:10.1007/s10198-019-01129-z.
- Stawasz A, Huang L, Kirby P, Bloom D. Health technology assessment for vaccines against rare, severe infections: properly accounting for serogroup B meningococcal vaccination’s full social and economic benefits. Front Public Health. 2020;8:261. doi:10.3389/fpubh.2020.00261.
- Knol MJ, Ruijs WL, Antonise-Kamp L, De Melker HE, Van Der Ende A. Implementation of menACWY vaccination because of ongoing increase in serogroup W invasive meningococcal disease, the Netherlands, 2018. Euro Surveill. 2018;23(16):18–00158. doi:10.2807/1560-7917.ES.2018.23.16.18-00158.
- Public Health England. Meningococcal ACWY immunisation programme for adolescents information for healthcare professionals. London (UK): Crown; 2020 [accessed 2020 Oct 16]. https://www.gov.uk/government/publications/menacwy-programme-information-for-healthcare-professionals.
- Clark SA, Lucidarme J, Angel G, Lekshmi A, Morales-Aza B, Willerton L, Campbell H, Gray SJ, Ladhani SN, Wade M, et al. Outbreak strain characterisation and pharyngeal carriage detection following a protracted group B meningococcal outbreak in adolescents in South-West England. Sci Rep. 2019;9(1):9990. doi:10.1038/s41598-019-46483-3.
- Grogan J, Roos K. Serogroup B meningococcus outbreaks, prevalence, and the case for standard vaccination. Curr Infect Dis Rep. 2017;19(9):30. doi:10.1007/s11908-017-0587-4.
- Jackson LA, Baxter R, Reisinger K, Karsten A, Shah J, Bedell L, Dull PM. Phase III comparison of an investigational quadrivalent meningococcal conjugate vaccine with the licensed meningococcal ACWY conjugate vaccine in adolescents. Clin Infect Dis. 2009;49(1):e1–10. doi:10.1086/599117.
- Baxter R, Baine Y, Ensor K, Bianco V, Friedland LR, Miller JM. Immunogenicity and safety of an investigational quadrivalent meningococcal ACWY tetanus toxoid conjugate vaccine in healthy adolescents and young adults 10 to 25 years of age. Pediatr Infect Dis J. 2011;30(3):e41–48. doi:10.1097/INF.0b013e3182054ab9.
- Santolaya ME, O’ryan ML, Valenzuela MT, Prado V, Vergara R, Munoz A, Toneatto D, Grana G, Wang H, Clemens R, et al. Immunogenicity and tolerability of a multicomponent meningococcal serogroup B (4CMenB) vaccine in healthy adolescents in Chile: a phase 2b/3 randomised, observer-blind, placebo-controlled study. Lancet. 2012;379(9816):617–24. doi:10.1016/s0140-6736(11)61713-3.
- Ostergaard L, Vesikari T, Absalon J, Beeslaar J, Ward BJ, Senders S, Eiden JJ, Jansen KU, Anderson AS, York LJ, et al. A bivalent meningococcal B vaccine in adolescents and young adults. N Engl J Med. 2017;377(24):2349–62. doi:10.1056/NEJMoa1614474.
- European Centre for Disease Prevention and Control. Vaccine schedules in all countries of the European Union. [accessed 2019 Oct 15]. https://vaccine-schedule.ecdc.europa.eu/.
- Norwegian Institute of Public Health. Norwegian surveillance system for communicable diseases. Oslo (Norway): Norwegian Institute of Public Health; [accessed 2019 Dec 16]. http://msis.no/.
- Taylor K. Norwegian teens celebrate a bizarre, month-long holiday full of drinking, sex, and wild dares — here’s what it’s like. Business Insider. 2018 Apr 18 [cited 2019 Dec 16]. https://www.businessinsider.com/what-the-norwegian-teen-holiday-russefeiring-is-like-2017-6?r=US&IR=T.
- Norwegian Immunisation Registry SYSVAK. Oslo (Norway): Norwegian Institute of Public Health; 2019 [accessed 2019 Apr 10]. https://www.fhi.no/en/hn/health-registries/norwegian-immunisation-registry-sysvak/.
- Alarid-Escudero F, Krijkamp EM, Enns EA, Myriam Hunink MG, Pechlivanoglou P, Jalal H. Cohort state-transition models in R: a tutorial. arXiv:200107824. 2020 Jan 22 [cited 2020 Feb 24]. https://ui.adsabs.harvard.edu/abs/2020arXiv200107824A.
- Krijkamp EM, Alarid-Escudero F, Enns EA, Pechlivanoglou P, Hunink MGM, Yang A, Jalal HJ. A multidimensional array representation of state-transition model dynamics. Medical Decision Making. 2020;40(2):242–48. doi:10.1177/0272989x19893973.
- Norwegian Ministry of Finance. Oslo (Norway); [accessed 2019 Oct 9]. https://www.regjeringen.no/en/dep/fin/id216/
- Norwegian Medicines Agency. Guidelines for the submission of documentation for single technology assessment (STA) of pharmaceuticals. Oslo (Norway): Norwegian Medicines Agency; 2018 [accessed 2019 Oct 10]. https://legemiddelverket.no/Documents/English/Public%20funding%20and%20pricing/Documentation%20for%20STA/Guidelines_april_2018.pdf.
- Norheim O, Allgott B, Gjul G, Kjellevold A, Moen A, Sjøli S, Aschim B, Gundersen T, Kvinnsland S, Ø M, et al. Norwegian [Transparent and fair – priorities in health services]. Oslo (Norway): Norwegian Government; 2014. https://www.regjeringen.no/no/dokumenter/NOU-2014-12/id2076730/?ch=1.
- Statistics Norway. Oslo (Norway); [accessed 2019 Oct 9]. https://www.ssb.no/en/.
- Jacobson RM, Jackson LA, Reisinger K, Izu A, Odrljin T, Dull PM. Antibody persistence and response to a booster dose of a quadrivalent conjugate vaccine for meningococcal disease in adolescents. Pediatr Infect Dis J. 2013;32(4):e170–177. doi:10.1097/INF.0b013e318279ac38.
- Baxter R, Baine Y, Kolhe D, Baccarini CI, Miller JM, Van Der Wielen M. Five-year antibody persistence and booster response to a single dose of meningococcal A, C, W and Y tetanus toxoid conjugate vaccine in adolescents and young adults: an open, randomized trial. Pediatr Infect Dis J. 2015;34(11):1236–43. doi:10.1097/inf.0000000000000866.
- Read RC, Baxter D, Chadwick DR, Faust SN, Finn A, Gordon SB, Heath PT, Lewis DJ, Pollard AJ, Turner DP, et al. Effect of a quadrivalent meningococcal ACWY glycoconjugate or a serogroup B meningococcal vaccine on meningococcal carriage: an observer-blind, phase 3 randomised clinical trial. Lancet. 2014;384(9960):2123–31. doi:10.1016/s0140-6736(14)60842-4.
- Balmer P, Burman C, Serra L, York LJ. Impact of meningococcal vaccination on carriage and disease transmission: a review of the literature. Hum Vaccin Immunother. 2018;14(5):1118–30. doi:10.1080/21645515.2018.1454570.
- Norwegian: [Statistics for childhood vaccination]. Oslo (Norway): Norwegian Institute of Public Health; 2018 [accessed 2019 Oct 14]. https://www.fhi.no/hn/helseregistre-og-registre/sysvak/barnevaksinasjon—statistikk/.
- EQ-5D (EuroQo 5 dimensions). Rotterdam (The Netherlands): EuroQol Group; 2020 [accessed 2019 Oct 9]. https://euroqol.org/.
- Sun S, Irestig R, Burstrom B, Beijer U, Burstrom K. Health-related quality of life (EQ-5D) among homeless persons compared to a general population sample in Stockholm county, 2006. Scand J Public Health. 2012;40(2):115–25. doi:10.1177/1403494811435493.
- Burstrom K, Johannesson M, Diderichsen F. Swedish population health-related quality of life results using the EQ-5D. Qual Life Res. 2001;10(7):621–35. doi:10.1023/a:1013171831202.
- Carroll AE, Downs SM. Improving decision analyses: parent preferences (utility values) for pediatric health outcomes. J Pediatr. 2009;155(1):21–25. doi:10.1016/j.jpeds.2009.01.040.
- Stoof SP, Rodenburg GD, Knol MJ, Rumke LW, Bovenkerk S, Berbers GA, Spanjaard L, Van Der Ende A, Sanders EA. Disease burden of invasive meningococcal disease in the Netherlands between June 1999 and June 2011: a subjective role for serogroup and clonal complex. Clin Infect Dis. 2015;61(8):1281–92. doi:10.1093/cid/civ506.
- Exchange rates. Oslo (Norway): Norges Bank; 2018 [accessed 2019 Oct]. https://www.norges-bank.no/en/topics/Statistics/exchange_rates/.
- Norwegian Medicines Agency. Oslo (Norway); [accessed 2019 Oct 14]. https://legemiddelverket.no/english.
- Norwegian: [ISF_regulations_2018 – DRGlist somatic]. Norwegian Directorate of Health; 2018 [accessed 2019 Oct 14]. https://www.helsedirektoratet.no/tema/finansiering/innsatsstyrt-finansiering-og-drg-systemet/innsatsstyrt-finansiering-isf/ISF_Regelverk_2018%20%E2%80%93%20DRGliste%20Somatikk%20(Vedlegg%20A1).xlsx?download=true.
- De Wals P, Zhou Z. Cost-effectiveness comparison of monovalent C versus quadrivalent ACWY meningococcal conjugate vaccination in Canada. Pediatr Infect Dis J. 2017;36(7):e203–e207. doi:10.1097/inf.0000000000001512.
- Summary of Product Characteristics – Nimenrix. European Medicines Agency; [accessed 2019 Oct 28]. https://www.ema.europa.eu/en/documents/product-information/nimenrix-epar-product-information_en.pdf.
- Summary of Product Characteristics – Menveo. European Medicines Agency; [accessed 2019 Oct 28]. https://www.ema.europa.eu/en/documents/product-information/menveo-epar-product-information_en.pdf.
- Norwegian Ministry of Health and Care Services. Principles for priority setting in health care - Summary of a white paper on priority setting in the norwegian health care sector. Oslo (Norway): Norwegian Government; 2016. https://www.regjeringen.no/contentassets/439a420e01914a18b21f351143ccc6af/en-gb/pdfs/stm201520160034000engpdfs.pdf.
- Woods B, Revill P, Sculpher M, Claxton K. Country-level cost-effectiveness thresholds: initial estimates and the need for further research. Value Health. 2016;19(8):929–35. doi:10.1016/j.jval.2016.02.017.
- Strong M, Oakley JE, Brennan A. Estimating multiparameter partial expected value of perfect information from a probabilistic sensitivity analysis sample: a nonparametric regression approach. Med Decis Making. 2014;34(3):311–26. doi:10.1177/0272989x13505910.
- Watle SV, Caugant DA, Tunheim G, Bekkevold T, Laake I, Brynildsrud OB, Næss LM. Meningococcal carriage in Norwegian teenagers: strain characterisation and assessment of risk factors. Epidemiol Infect. 2020;148:e80. doi:10.1017/s0950268820000734.
- Hepkema H, Pouwels KB, Van Der Ende A, Westra TA, Postma MJ. Meningococcal serogroup A, C, W(1)(3)(5) and Y conjugated vaccine: a cost-effectiveness analysis in the Netherlands. PLoS One. 2013;8(5):e65036. doi:10.1371/journal.pone.0065036.
- Si S, Zomer E, Fletcher S, Lee J, Liew D. Cost-effectiveness of meningococcal polysaccharide serogroups A, C, W-135 and Y conjugate vaccine in Australian adolescents. Vaccine. 2019;37(35):5009–15. doi:10.1016/j.vaccine.2019.07.008.
- Ortega-Sanchez IR, Meltzer MI, Shepard C, Zell E, Messonnier ML, Bilukha O, Zhang X, Stephens DS, Messonnier NE. Economics of an adolescent meningococcal conjugate vaccination catch-up campaign in the United States. Clin Infect Dis. 2008;46(1):1–13. doi:10.1086/524041.
- Shepard CW, Ortega-Sanchez IR, Scott RD, Rosenstein NE. Cost-effectiveness of conjugate meningococcal vaccination strategies in the United States. Pediatrics. 2005;115(5):1220–32. doi:10.1542/peds.2004-2514.
- Delea TE, Weycker D, Atwood M, Neame D, Alvarez FP, Forget E, Langley JM, Chit A. Cost-effectiveness of alternate strategies for childhood immunization against meningococcal disease with monovalent and quadrivalent conjugate vaccines in Canada. PLoS One. 2017;12(5):e0175721. doi:10.1371/journal.pone.0175721.
- De Wals P, Coudeville L, Trottier P, Chevat C, Erickson LJ, Nguyen VH. Vaccinating adolescents against meningococcal disease in Canada: a cost-effectiveness analysis. Vaccine. 2007;25(29):5433–40. doi:10.1016/j.vaccine.2007.04.071.
- Vetter V, Baxter R, Denizer G, Safadi MA, Silfverdal SA, Vyse A, Borrow R. Routinely vaccinating adolescents against meningococcus: targeting transmission & disease. Expert Rev Vaccines. 2016;15(5):641–58. doi:10.1586/14760584.2016.1130628.
- Kristiansen PA, Jorgensen HJ, Caugant DA. Serogroup A meningococcal conjugate vaccines in Africa. Expert Rev Vaccines. 2015;14(11):1441–58. doi:10.1586/14760584.2015.1084232.
- Trotter CL, Maiden MC. Meningococcal vaccines and herd immunity: lessons learned from serogroup C conjugate vaccination programs. Expert Rev Vaccines. 2009;8(7):851–61. doi:10.1586/erv.09.48.
- Thokala P, Ochalek J, Leech AA, Tong T. Cost-effectiveness thresholds: the past, the present and the future. Pharmacoeconomics. 2018;36(5):509–22. doi:10.1007/s40273-017-0606-1.
- Martinon-Torres F. Deciphering the burden of meningococcal disease: conventional and under-recognized elements. J Adolesc Health. 2016;59(2 Suppl):S12–20. doi:10.1016/j.jadohealth.2016.03.041.
- Al-Janabi H, Van Exel J, Brouwer W, Trotter C, Glennie L, Hannigan L, Coast J. Measuring health spillovers for economic evaluation: a case study in meningitis. Health Econ. 2016;25(12):1529–44. doi:10.1002/hec.3259.
- Claxton K, Sculpher M, Mccabe C, Briggs A, Akehurst R, Buxton M, Brazier J, O’hagan T. Probabilistic sensitivity analysis for NICE technology assessment: not an optional extra. Health Econ. 2005;14(4):339–47. doi:10.1002/hec.985.
- Im JH, Woo H, Ha BM, Lee JS, Chung MH, Jung J. Effectiveness of a single dose of the quadrivalent meningococcal conjugate vaccine, MenACWY-CRM, in the Korean Armed Forces. Vaccine. 2019;38(4):730–32. doi:10.1016/j.vaccine.2019.11.015.
- Cohn AC, Macneil JR, Harrison LH, Lynfield R, Reingold A, Schaffner W, Zell ER, Plikaytis B, Wang X, Messonnier NE, et al. Effectiveness and duration of protection of one dose of a meningococcal conjugate vaccine. Pediatrics. 2017;139(2):e20162193. doi:10.1542/peds.2016-2193.
