ABSTRACT
In most countries worldwide, pneumococcal conjugate vaccines have been included in the infant immunization program, resulting in a significant reduction in the burden of pneumococcal disease in children and adults. Shifting serotype distribution due to the indirect effect of infant vaccination with the 13-valent pneumococcal conjugate vaccine (PCV13) may continue to increase the gap between 23-valent pneumococcal polysaccharide vaccine (PPSV23) and PCV13 serotype coverage for older adults in the coming years. This clinical study (V110-029; NCT02225587) evaluated the safety and immunogenicity of sequential administration of PCV13 followed approximately 8 weeks later, or approximately 26 weeks later, by PPSV23 in healthy adults ≥50 years of age. Both dosing intervals were generally well tolerated as measured by the nature, frequency, and intensity of reported adverse events (AEs) in both vaccination groups. Serotype-specific opsonophagocytic activity (OPA) geometric mean titers (GMTs) measured 30 days following receipt of PPSV23 in either group and at Week 30 were generally comparable between the 2 groups for 6 serotypes unique to PPSV23 and 12 serotypes shared between PCV13 and PPSV23, regardless of the interval between receipt of PCV13 and PPSV23. In addition, administration of PPSV23 given either 8 weeks or 26 weeks following PCV13 did not negatively impact immune responses induced by PCV13. Furthermore, administration of PPSV23 given either 8 weeks or 26 weeks after PCV13 elicited serotype-specific OPA GMTs to serotypes unique to PPSV23, which could provide earlier protection against pneumococcal disease caused by these serotypes in comparison with the current Advisory Committee on Immunization Practices recommended interval of at least 12 months.
Introduction
Despite the availability of several pneumococcal vaccines for adult and infant immunization, pneumococcal disease remains a high health priority worldwide. Polyvalent polysaccharide vaccines (PPSVs) were first developed and licensed based on the demonstrated efficacy of 6-valent and 13-valent vaccines against pneumococcal pneumonia in adults.Citation1,Citation2 Subsequently, both 14-valent and 23-valent vaccines were licensed based on comparable safety and immunogenicity profiles to lower valency vaccines for shared serotypes. However, PPSVs were shown to be ineffective in children <2 years of age due to the immaturity of their immune system and pneumococcal conjugate vaccines (PCVs) were subsequently developed.Citation3,Citation4 A PCV containing the 7 leading serotypes causing invasive pneumococcal disease (IPD) in infants was licensed in 2000 followed by 10-valent and 13-valent PCVs.Citation5–7 Widespread use of PCVs in infants resulted in a significant reduction of pneumococcal disease caused by serotypes included in these PCVs in both children targeted by the immunization programs and indirectly in unvaccinated older children and adults (indirect protection).Citation7–9 The success of PCVs among young children led to renewed interest in their use in older adults. A large clinical study demonstrated the efficacy of PCV13 (PREVNAR™ 13: pneumococcal 13-valent conjugate vaccine [diphtheria CRM197 protein]; Wyeth Pharmaceuticals Inc., a subsidiary of Pfizer, Inc., Philadelphia, PA, USA)Citation10 against vaccine-type (VT) community-acquired pneumonia (CAP) in adults 65 years of age,Citation11 which led to the inclusion of PCV13 in the adult pneumococcal vaccination program by the Advisory Committee for Immunization Practices (ACIP) of the United States Centers for Disease Control and Prevention (US-CDC) and other countries.Citation12
For people living with immunocompromising conditions such as HIV infection and transplant recipients, PCV was added into the immunization practice due to the lower immunogenicity of PPSV23 (PNEUMOVAX™ 23: pneumococcal vaccine polyvalent; Merck & Co., Inc.;, Kenilworth, NJ, USA) in comparison to PCVs in this populationCitation13 and limited duration of protective antibody levels associated with the T-independent nature of pneumococcal polysaccharide vaccines. The rationale for sequential administration of PCV and PPSV23 in immunocompromised individuals was based on the high burden of pneumococcal disease and the need to provide broader protection against disease caused by serotypes unique to PPSV23 in that population, as well as the positive benefit-risk ratio of an additional dose of PPSV23 following receipt of PCV13.Citation14–20 Pneumococcal vaccination strategies for immunocompetent adults ≥65 years of age vary between countries and include PCV13-PPSV23, PPSV23 only, or PCV13 only.Citation21,Citation22 Although a 2-month interval between PCV and PPSV23 has been widely accepted for immunocompromised individuals, the recommended dosing interval for the two vaccines in immunocompetent adults ≥65 years has been the subject of many scientific debates and differs between countries. Previous studies evaluating sequential administrations of PCV7 or PCV13 followed by PPSV23 at intervals of 2, 6, 12 months or 3–4 years have shown an increase in serotype-specific antibodies following receipt of PPSV23.Citation23–27 A study involving a small number of volunteers (approximately 29 per group) compared the safety and immunogenicity of sequential administration of PCV7 and PPSV23 given either 2 months or 6 months apart. Study results showed that more individuals vaccinated using the shorter interval reported injection site redness and swelling than those vaccinated using the longer interval; most of these reactions were transient and mild-to-moderate in intensity and no differences were observed between the two vaccination groups regarding the frequencies and severity of systemic adverse events (AEs) or serotype-specific antibodies measured after PPSV23.Citation23 Although some studies have suggested that a longer interval of at least 12 months between PCV and PPSV23 could induce numerically higher antibody titers for some shared vaccine serotypes,Citation25,Citation26 a longer interval could leave individuals unprotected against pneumococcal disease caused by serotypes unique to PPSV23.
The present study (V110–029; NCT02225587) evaluated the safety, tolerability, and immunogenicity of sequential administration of PCV13 followed either 2 months or 6 months later by PPSV23 in pneumococcal vaccine-naïve adults 50 years of age in order to determine the benefit-risk (immunological benefit versus safety) of a shorter interval in the administration of the two vaccines in a larger population than that evaluated in previous studies.
Materials and methods
Participants and study design
This phase 3 randomized, double-blind trial was conducted between September 2014 and July 2015 at 20 clinical sites in the United States to compare the safety, tolerability, and immunogenicity profiles of a sequential administration of PCV13 followed by PPSV23, given either 2 months or 6 months later in pneumococcal vaccine-naïve adults ≥50 years of age. Key eligibility criteria were no previous pneumococcal vaccination, stable underlying medical condition, and absence of protocol-defined known or suspected immunocompromising conditions (e.g. HIV infection, generalized malignancy). The protocol was approved by the central ethical review committee and conducted in conformance with applicable country or local requirements. Written informed consent was obtained from each subject prior to the performance of any study procedure.
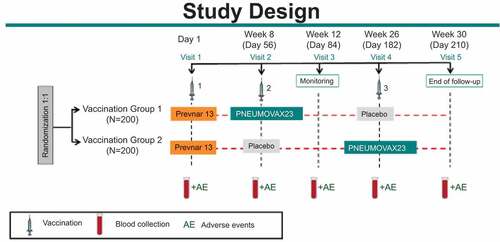
A total of 400 community-dwelling adults were randomly assigned to 2 different intervention groups: Group #1 received PCV13 on Day 1, PPSV23 at Week 8 (Month 2), and Placebo (saline solution) at Week 26 (Month 6). Group #2 received PCV13 on Day 1, Placebo at Week 8 (Month 2), and PPSV23 at Week 26 (Month 6) []. All subjects were followed for AEs for 14 days post each injection. Solicited injection-site AEs included redness, swelling, and pain/tenderness; and solicited systemic AEs included muscle pain (myalgia), joint pain (arthralgia), headache, and fatigue. SAEs were collected through 30 days after the last injection (Placebo in Group #1 and PPSV23 in Group #2) and/or completion of the subject’s participation in the study. The daily oral temperature was collected for the first 5 days postvaccination.
Blood samples were collected prior to the first vaccination on Day 1, at Week 8, Week 12, Week 26, and Week 30. The functional opsonophagocytic activity was assessed using a multiplex OPA (MOPA-4) assay (Laboratory of Prof. David Goldblatt, Institute of Child Health, London, UK).Citation28 Serotype-specific OPA GMTs were evaluated in all subjects from both vaccination groups with sufficient volume of serum on Day 1 and Week 12. In each vaccination group, OPA GMTs were also measured at Week 30 in the first 50% of the subjects who had sufficient serum after testing at Day 1 and Week 12 were completed. Furthermore, OPA GMTs were also measured at Week 8 and Week 26 in the first 25% of the subjects who had sufficient serum after testing at Day 1, Week 12, and Week 30 were completed. Serotype-specific OPA responses to all 13 serotypes included in PCV13 (serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F) and 2 serotypes unique to PPSV23 (22F, 33F) were evaluated using a validated MOPA-4 assay and qualified MOPA-4 was used to evaluate OPA responses to 4 other serotypes unique to PPSV23 (8, 9N, 11A, 17F).
Vaccines
PPSV23 (PNEUMOVAX™23; Merck & Co., Inc., Kenilworth, NJ, USA) is a 23-valent pneumococcal polysaccharide vaccine indicated for the prevention of pneumococcal disease caused by the 23 serotypes of Streptococcus pneumoniae contained in the vaccine (1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19F, 19A, 20, 22F, 23F, and 33F) for persons ≥50 years of age and persons ≥2 years of age who are at increased risk for pneumococcal disease. Each 0.5 mL dose of PPSV23 contains 25 µg of each capsular polysaccharide. PCV13 (Prevnar 13™; Wyeth Pharmaceuticals Inc., a subsidiary of Pfizer, Inc., Philadelphia, PA) is a 13-valent pneumococcal conjugate vaccine indicated for the prevention of invasive pneumococcal disease and pneumococcal pneumonia caused by the 13 serotypes contained in the vaccine (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F) in adults 18 years of age and older. PCV13 is also indicated for the prevention of invasive pneumococcal disease in children 6 weeks of age through 17 years of age and prevention of acute otitis media in children 6 weeks through 5 years of age. Each 0.5 mL dose of PCV13 contains 2.2 µg of each capsular polysaccharide (except serotype 6B at 4.4 µg), 34 µg of CRM197 used a carrier protein, and formulated with 125 µg of aluminum phosphate adjuvant.
Statistical methods
There were no safety hypotheses in this study. Proportions of subjects reporting AEs following vaccination were compared between Group #1 and Group #2. The analysis of safety results followed a tiered approach. For Tier 1 safety endpoints, point estimates, risk differences with 95% CIs and corresponding p values are provided. Tier 2 parameters were assessed via point estimates and risk differences with 95% CIs; only point estimates by the group are provided for Tier 3 safety parameters. Tier 1 safety endpoints included solicited injection-site AEs (redness, swelling, and pain/tenderness) during Day 1 to Day 5 postvaccination, and solicited systemic AEs (muscle pain, joint pain, headache, and tiredness) during Day 1 to Day 14 postvaccination. Temperatures collected from Day 1 through Day 5 were treated as Tier 2 events. Statistical significance of observed differences between vaccination groups was assessed for solicited AEs using the methodology of Miettinen.Citation29
Study primary immunogenicity hypotheses were based on serotypes evaluated using validated MOPA-4 assay, aimed at demonstrating that Group #1 was superior to Group #2 based on OPA GMTs measured at Week 12 for 2 serotypes unique to PPSV23 (serotypes 22F and 33F) and Group #1 was noninferior to Group #2 based on serotype-specific OPA GMTs measured for the 12 shared serotypes between PCV13 and PPSV23 at Week 12. Superiority was declared if the lower bound of the two-sided 95% CI of the OPA GMT ratio (Group #1/Group #2) was >2.0 for both 22F and 33F. Non-inferiority was declared if lower bound of two-sided 95% CI of the OPA GMT ratios (Group #1/Group #2) for each shared serotype was >0.5 (twofold noninferiority margin). GMT ratio estimation, 95% CI, and hypothesis test (one-sided p value) for noninferiority comparisons were calculated using constrained longitudinal data analysis method and response vector consisted of natural log-transformed prevaccination and postvaccination antibody titers.Citation30 Additional secondary objectives included OPA GMTs at Weeks 8, 26, and 30 as well as summaries of geometric mean fold-rises, the proportion of subjects with ≥4-fold rise from baseline to Week 12 and Week 30 and in each intervention group, and reverse cumulative distribution curves (RCDCs) at the different timepoints up to Week 30 for the 12 shared serotypes between PCV13 and PPSV23 as well as the 6 serotypes unique to PPSV23. A sample size of ~200 adults per vaccination group provided at least 90% power to demonstrate that Group #1 leads to a higher GMT to 2 serotypes unique to PPSV23 (serotypes 22F and 33F) at 12 weeks after the first dose as compared to Group#2 and 88.6% power to demonstrate non-inferiority between the 2 vaccination groups for the 12 shared serotypes between PCV13 and PPSV23 at 1-sided, 2.5% alpha level. Since the declaration of non-inferiority between the 2 vaccination groups required demonstration of noninferiority for all 12 serotypes, no multiplicity adjustments were required. There was no formal hypothesis testing for the GMTs difference between the 2 dosing regimens (2-month versus 6-month intervals between PCV13 and PPSV23) based on the comparison of OPA GMTs measured at Week 12 for Group #1 and Week 30 for Group #2.
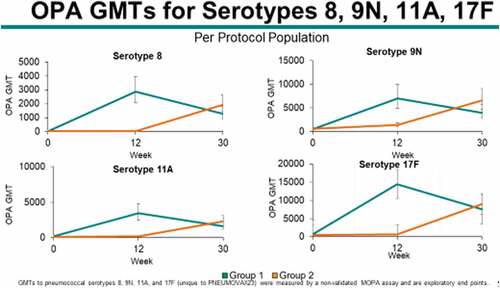
A post-hoc analysis was conducted for additional 4 serotypes unique to PPSV23 (8, 9N, 11A, and 17F) to evaluate the superiority of OPA GMTs at Week 12 in Group 1 compared to Group 2 and noninferiority of serotype 6A (unique to PCV13) in both groups at Week 12.
Results
Study population
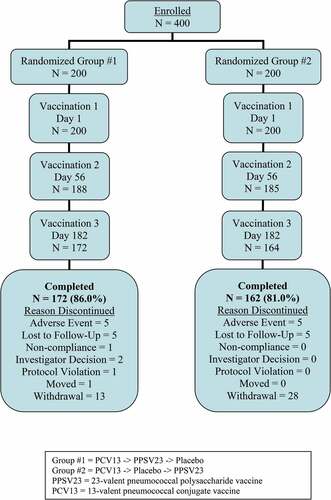
A total of 400 participants were enrolled in the study and were equally distributed across the 2 intervention groups with respect to age strata, gender, and race/ethnicity . Most study participants in both vaccination groups received both study vaccines (PCV13 and PPSV23) and completed the study (334/400; 83.5%). A total of 66 (16.5%) participants prematurely discontinued from the study primarily due to withdrawal by subject (41 subjects, 10.3%), an AE (10 participants, 2.5%), or lost to follow-up (10 participants, 2.5%) . The proportions of participants who discontinued study intervention or withdrew from the study were generally comparable between Group #1 and Group #2.
Table 1. Participant demographics
Safety
Overall, the proportions of subjects reporting injection-site and/or systemic AEs from Days 1 to 14 following any vaccination were generally comparable between Group #1 and Group #2. Most frequently reported AEs were those solicited in the electronic vaccination report card (eVRC) . Rates of subjects reporting at least one injection site AE during Days 1–5 following any vaccination were comparable between the two vaccination groups although a numerically higher number of subjects reported injection site pain, swelling, and erythema in individuals given the shorter (Group #1) than longer (Group #2) dosing interval between PCV13 and PPSV23. Rates of systemic AEs reported during Days 1 to 14 following any vaccination were generally comparable between the 2 vaccination groups and most frequently reported AEs included muscle pain, fatigue, headache, and joint pain. The majority of AEs were transient (lasting less than 3 days on average) and mild (Grade 1) to moderate (Grade 2) in intensity Supplement Tables S1 and S2. Body temperature measurements were taken at approximately the same time each day and very few study participants (2/385; 0.5%) reported an elevated temperature (≥100.4°F [≥38.0°C] oral or equivalent) during Days 1–5 after any vaccination.
Table 2. Summary of adverse events (AEs) occurring on Days 1–14 after any vaccination (all subjects as treated)
A total of 24 serious AEs (SAEs) were reported by 20 out of 400 study subjects and no death was reported during the protocol-specified safety follow-up period. None of these SAEs was assessed to be related to the study vaccine . A total of 10 subjects (5 in each group) were discontinued from the study due to an AE. One subject in Group #2 was discontinued from the study due to an SAE of death that occurred 95 days after receipt of PPSV23; the final study visit had to occur 30 days after receipt of PPSV23 in Group #2 but the study volunteer did not visit the study site at the scheduled date nor respond to several attempts from the site personnel; the SAE of death due to brain injury (secondary to trauma/fall) was determined by the investigator to be unrelated to study vaccine .
Analyses of AEs reported after each vaccination showed comparable rates of injection site and systemic AEs following receipt of PCV13 or Placebo between the two vaccination groups but higher rates of injection site and systemic AEs in Group #1 than Group #2 during Days 1–14 following receipt of PPSV23 . Although rates of reported systemic AEs following PPSV23 were generally comparable between the two vaccination groups, the shorter interval (2 months in Group #1) compared to the longer interval (6 months in Group #2) between PCV13 and PPSV23 was associated with significantly higher rates of injection site pain (79.3% versus 64.0%), injection site swelling (50.5% versus 29.3%) and injection site erythema (41.5% versus 29.3%) .
Table 3. Listing of most frequently reported adverse events (>5% in either group)
Immunogenicity
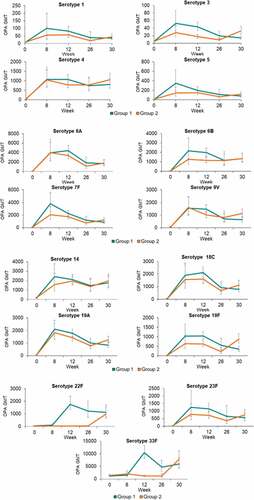
Opsonophagocytic activity (OPA) geometric mean titers (GMTs) to all 19 serotypes tested in the study (6 serotypes unique to PPSV23, 1 serotype unique to PCV13, and 12 shared serotypes between PCV13 and PPSV23) were generally comparable between the 2 vaccination groups prior to any vaccination (Baseline or Day 1) and 8 weeks after both groups had only received PCV13 (Week 8) . At Week 12, corresponding to 4 weeks after study volunteers in Group #1 had received PPSV23 but those in Group #2 received Placebo, OPA GMTs to 6 pneumococcal serotypes unique to PPSV23 (serotypes 8, 9 N, 11A, 17 F, 22 F and 33 F) in Group #1 were superior to OPA GMTs in Group #2 . Additionally, at Week 12, OPA GMTs to 12 shared pneumococcal serotypes contained in both PPSV23 and PCV13 (serotypes 1, 3, 4, 5, 6B, 7 F, 9 V, 14, 18 C, 19A, 19 F, and 23 F) in Group #1 were noninferior to OPA GMTs in Group #2 . By Week 26, OPA GMTs were slightly higher in Group #1 than Group #2 for most serotypes tested at that timepoint but antibody titers were again comparable between the 2 vaccine groups by Week 30, approximately 4 weeks after subjects in Group #2 were given PPSV23 and those in Group #1 received Placebo.
Table 4. Summary of OPA GMTs measured
Table 5. Analysis of postvaccination OPA GMTs to pneumococcal serotypes at week 12 (per protocol population)
In order to assess the possible benefit of an earlier administration of PPSV23 following PCV13, OPA GMTs measured 4 weeks after receipt of PPSV23 (Week 12 in Group #1 and Week 30 in Group #2) were compared between the 2 vaccination groups. OPA GMTs to the 6 serotypes unique to PPSV23 and to 12 serotypes in common between PCV13 and PPSV23 were generally comparable between the 2 vaccination groups Table 6 and .
Discussion
Pneumococcal vaccination policy for adults varies between countries and has been driven by recent changes in the burden of pneumococcal disease, serotype distribution following widespread use of PCVs in infants in many countries worldwide, and economic constraints.Citation15,Citation22,Citation31–34 In our study, sequential administration of PCV13 followed by PPSV23 given at either 2-month or 6-month intervals was generally well tolerated as measured by the nature, frequency, and intensity of reported AEs in the two vaccination groups. Although a shorter interval (2 months) was associated with higher frequency of injection-site AEs (pain, swelling, and erythema) after PPSV23, the observed difference was not clinically significant as most AEs were transient and mild to moderate in intensity. Serotype-specific OPA GMTs measured following receipt of PPSV23 were generally comparable between the 2 groups, regardless of the interval between receipt of PCV13 and PPSV23 for the 6 serotypes unique to PPSV23 and the 12 shared serotypes between PCV13 and PPSV23. In addition, administration of PPSV23 given either 8 weeks or 26 weeks following PCV13 did not hinder the immune responses induced by PCV13 as OPA GMTs increased for most shared serotypes at 4 weeks following receipt of PPSV23. Our safety and immunogenicity findings are consistent with previous studies evaluating the safety and immunogenicity of sequential administration of PCV and PPSV23 using intervals of 2 or 6 months between the two vaccines.Citation23,Citation24
Furthermore, administration of PPSV23 given either 8 weeks or 26 weeks after PCV13 elicited serotype-specific OPA GMTs to the serotypes unique to PPSV23, which could provide earlier protection against pneumococcal disease caused by these serotypes in comparison with the current Advisory Committee on Immunization Practices recommended interval of at least 12 months for adults ≥65 years of age requiring a shared clinical decision. It has been hypothesized that a longer interval (≥12 months) between PCV13 and PPSV23 in adults could yield higher levels of antipneumococcal antibodies than shorter intervals of 2 or 6 monthsCitation25–27,Citation35 but no head-to-head study has thus far compared antibody titers between short (≤6 months) and long (≥12 months) intervals of sequential PCV-PPSV23 in older adults.Citation34 Previous head-to-head studies have shown that PCVs generally induced higher OPA GMTs than PPSV23 for most shared serotypes.Citation26,Citation36–39 Limited data from studies comparing antibody levels following sequential regimens of pneumococcal vaccines in adults (PCV13/PCV13, PCV13/PPSV23 or PPSV23/PCV13) given 1 year apart suggested that antibody levels following the second vaccine were lower than those measured 30 days after the first dose with PCV13 but slightly higher than those measured after a first dose with PPSV23.Citation25 The clinical significance of the observed differences in antibody titers between PCVs and PPSV23 as well as those observed between various dosing regimens is unclear.
In the absence of an accepted antibody threshold value that can be correlated with protection against pneumococcal disease in adults and lack of clinical data comparing levels of vaccine-induced antibodies following short and long intervals in the sequential administration of PCV and PPSV23, our study showed that a shorter interval of 2 months induces OPA GMTs comparable to those of a longer interval, which could provide earlier protection against pneumococcal disease in older adults. Previous studies have shown that a substantial proportion of adults ≥ 18 years of age who do not have a high-risk, immunocompromising condition have at least one medical condition associated with increased risk for pneumococcal disease (at-risk condition includes asthma, chronic obstructive pulmonary disease, heart failure, liver disease, alcoholism, cigarette smoking). Furthermore, the percentage of subjects with two or three concurrent at-risk conditions (risk stacking) was also high, especially in older adults ≥65 years of age.Citation40,Citation41 In addition, mortality from pneumococcal disease in those with six stacked conditions was estimated to be twice higher than in those with two conditions,Citation41 highlighting the value of broad and early protection against pneumococcal disease in older adults.
The dosing interval between PCV and PPSV23 has been the subject of many debates as reflected by the many changes in pneumococcal vaccination policy for adults ≥65 years of age in the United States between 2012 and 2019.Citation21,Citation42 The major challenge has been the difficulty to distinguish the indirect effect of infant immunization and the direct contribution of PCV13 in adults, especially for vaccine-type non-bacteremic pneumococcal pneumonia.Citation32,Citation42,Citation43 Given the difference in the burden of residual pneumococcal disease and distribution of serotypes between children and adults, novel strategies are needed in order to better address the transmission dynamic of serotype replacement and minimize the selection of antibiotic-resistant strains of S. pneumoniae.Citation44
Our study has several limitations. Although it involved a larger number of subjects than previous studies evaluating safety and immunogenicity of shorter PCV-PPSV23 interval, the sample size was not sufficient to reliably assess the proportion of individuals with one or more underlying medical conditions associated with increased risk for pneumococcal disease, and thus supports the value of shorter interval and early protection against disease caused by serotypes unique to PPSV23 in that population. In addition, vaccine-induced immune responses were not measured for 5 of the 11 serotypes unique to PPSV23. However, several studies demonstrated that PPSV23 induces serotype-specific antibodies to all serotypes included in the vaccine and therefore these missing data should not raise concerns about the value of PPSV23 for the protection against disease caused by these serotypes.Citation39,Citation45 Our study did not evaluate the clinical efficacy of the sequential administration of PCV13-PPSV23 and the question remains unresolved. We measured immune responses up to 30 days after receipt of all study vaccines and did not provide information about long-term persistence (≥1 year) of vaccine-induced immune responses between subjects given PCV13-PPSV23 with 2-month compared to 6-month intervals.
Overall, our study showed that both 2-month and 6-month dosing intervals of PCV13-PPSV23 were generally well tolerated and induced comparable levels of serotype-specific OPA GMTs at Week 30, after volunteers in both groups had received PCV13 on Day 1 and PPSV23 either 8 weeks or 26 weeks later. Receipt of PPSV23 approximately 2 months after PCV13 was associated with earlier and higher levels of OPA GMTs for the serotypes unique to PPSV23 than in subjects given PPSV23 approximately 6 months after PCV13, which could possibly provide early protection against pneumococcal disease and is an approach already taken for immunocompromised populations.
Disclosure of potential conflicts of interest
No author was paid for their work on this manuscript.
Conflicts of Interest
CP Andrews, J Ervin, and JT Peterson were investigators for the sponsor supported by research grants.
UK Buchwald, GM Tamms, D Krupa, P Ajiboye, L Roalfe, AL Krick, TM Sterling, M Wang, J Martin, JE Stek, MA Kohn, T Folaranmi, C Abeygunawardana, J Hartzel, and LK Musey are employees of the sponsors and may hold stock and/or stock options in Merck & Co., Inc., Kenilworth, NJ, USA.
Study identification: V110-029
CLINICALTRIALS.GOV identifier: NCT02225587
EudraCT #: 2013-003027-11
Supplemental Material
Download MS Word (42.2 KB)Acknowledgments
Karyn Davis of Merck & Co., Inc., Kenilworth, NJ, USA assisted the authors with various administrative activities related to the submission of the manuscript and provided editorial assistance.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website
Additional information
Funding
Notes on contributors
CP Andrews, J Ervin, and JT Peterson: enrollment of participants and/or data collection, review of the manuscript.
UK Buchwald, GM Tamms, D Krupa, P Ajiboye, L Roalfe, AL Krick, TM Sterling, M Wang, J Martin, JE Stek, MA Kohn, and T Folaranmi: analysis and interpretation of data, and preparation of manuscript.
C Abeygunawardana, J Hartzel, and LK Musey: study concept and design, analysis and interpretation of data, and preparation of the manuscript.
References
- Austrian R, Douglas RM, Schiffman G, Coetzee AM, Koornhof HJ, Hayden-Smith S, Reid RD. Prevention of pneumococcal pneumonia by vaccination. Trans Assoc Am Physicians. 1976;89:184–94.
- Smit P, Oberholzer D, Hayden-Smith S, Koornhof HJ, Hilleman MR. Protective efficacy of pneumococcal polysaccharide vaccines. JAMA. 1977;238(24):2613–16. doi:10.1001/jama.1977.03280250039019.
- Cadoz M. Potential and limitations of polysaccharide vaccines in infancy. Vaccine. 1998;16(14–15):1391–95. doi:10.1016/S0264-410X(98)00097-8.
- Westerink MA, Schroeder HW Jr, Nahm MH. Immune Responses to pneumococcal vaccines in children and adults: rationale for age-specific vaccination. Aging Dis. 2012;3:51–67.
- Nuorti JP, Whitney CG. Centers for Disease Control and Prevention (CDC). Prevention of pneumococcal disease among infants and children - use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine - recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2010;59:1–18.
- Principi N, Esposito S. Development of pneumococcal vaccines over the last 10 years. Expert Opin Biol Ther. 2018;18(1):7–17. doi:10.1080/14712598.2018.1384462.
- Pilishvili T, Lexau C, Farley MM, Hadler J, Harrison LH, Bennett NM, Reingold A, Thomas A, Schaffner W, Craig AS, et al. Active bacterial core surveillance/emerging infections program network. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201(1):32–41. doi:10.1086/648593.
- Angoulvant F, Levy C, Grimprel E, Varon E, Lorrot M, Biscardi S, Minodier P, Dommergues MA, Hees L, Gillet Y, et al. Early impact of 13-valent pneumococcal conjugate vaccine on community-acquired pneumonia in children. Clin Infect Dis. 2014;58(7):918–24. doi:10.1093/cid/ciu006.
- Waight PA, Andrews NJ, Ladhani SN, Sheppard CL, Slack MP, Miller E. Effect of the 13-valent pneumococcal conjugate vaccine on invasive pneumococcal disease in England and Wales 4 years after its introduction: an observational cohort study. Lancet Infect Dis. 2015;15(5):535–43. doi:10.1016/S1473-3099(15)70044-7.
- Prevnar 13 [package insert]. Philadelphia (PA, USA): Wyeth Pharmaceuticals Inc. Accessed 2020 Mar 23. https://www.fda.gov/media/107657/download
- Bonten MJ, Huijts SM, Bolkenbaas M, Webber C, Patterson S, Gault S, van Werkhoven CH, van Deursen AM, Sanders EA, Verheij TJ, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015;372(12):1114–25. doi:10.1056/NEJMoa1408544.
- Tomczyk S, Bennett NM, Stoecker C, Gierke R, Moore MR, Whitney CG, Hadler S, Pilishvili T. Centers for disease control and prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2014;63:822–25.
- Pneumovax 23 [package insert]. Kenilworth (NJ, USA): Merck & Co., Inc. Accessed 2020 Mar 23. https://www.fda.gov/media/80547/download
- Schenkein JG, Park S, Nahm MH. Pneumococcal vaccination in older adults induces antibodies with low opsonic capacity and reduced antibody potency. Vaccine. 2008;26(43):5521–26. doi:10.1016/j.vaccine.2008.07.071.
- Musher DM. How effective is vaccination in preventing pneumococcal disease? Infect Dis Clin North Am. 2013;27(1):229–41. doi:10.1016/j.idc.2012.11.011.
- Smith KJ, Nowalk MP, Raymund M, Zimmerman RK. Cost-effectiveness of pneumococcal conjugate vaccination in immunocompromised adults. Vaccine. 2013;31(37):3950–56. doi:10.1016/j.vaccine.2013.06.037.
- Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2012;61(40):816–19.
- Crum-Cianflone NF, Sullivan E. Vaccinations for the HIV-Infected adult: a review of the current recommendations, part I. Infect Dis Ther. 2017;6(3):303–31. doi:10.1007/s40121-017-0166-x.
- Crum-Cianflone NF, Sullivan E. Vaccinations for the HIV-Infected adult: a review of the current recommendations, part II. Infect Dis Ther. 2017;6(3):333–61. doi:10.1007/s40121-017-0165-y.
- Lopez A, Mariette X, Bachelez H, Belot A, Bonnotte B, Hachulla E, Lahfa M, Lortholary O, Loulergue P, Paul S, et al. Vaccination recommendations for the adult immunosuppressed patient: a systematic review and comprehensive field synopsis. J Autoimmun. 2017;80:10–27. doi:10.1016/j.jaut.2017.03.011.
- Matanock A, Lee G, Gierke R, Kobayashi M, Leidner A, Pilishvili T. Use of 13-Valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: updated recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2019;68(46):1069–75. doi:10.15585/mmwr.mm6846a5.
- Bonnave C, Mertens D, Peetermans W, Cobbaert K, Ghesquiere B, Deschodt M, Flamaing J. Adult vaccination for pneumococcal disease: a comparison of the national guidelines in Europe. Eur J Clin Microbiol Infect Dis. 2019;38(4):785–91. doi:10.1007/s10096-019-03485-3.
- Miernyk KM, Butler JC, Bulkow LR, Singleton RJ, Hennessy TW, Dentinger CM, Peters HV, Knutsen B, Hickel J, Parkinson AJ. Immunogenicity and reactogenicity of pneumococcal polysaccharide and conjugate vaccines in Alaska native adults 55–70 years of age. Clin Infect Dis. 2009;49(2):241–48. doi:10.1086/599824.
- Goldblatt D, Southern J, Andrews N, Ashton L, Burbidge P, Woodgate S, Pebody R, Miller E. The immunogenicity of 7-valent pneumococcal conjugate vaccine versus 23-valent polysaccharide vaccine in adults aged 50–80 years. Clin Infect Dis. 2009;49(9):1318–25. doi:10.1086/606046.
- Greenberg RN, Gurtman A, Frenck RW, Strout C, Jansen KU, Trammel J, Scott DA, Emini EA, Gruber WC, Schmoele-Thoma B. Sequential administration of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naïve adults 60–64 years of age. Vaccine. 2014;32(20):2364–74. doi:10.1016/j.vaccine.2014.02.002.
- Jackson LA, Gurtman A, van Cleeff M, Frenck RW, Treanor J, Jansen KU, Scott DA, Emini EA, Gruber WC, Schmoele-Thoma B. Influence of initial vaccination with 13-valent pneumococcal conjugate vaccine or 23-valent pneumococcal polysaccharide vaccine on anti-pneumococcal responses following subsequent pneumococcal vaccination in adults 50 years and older. Vaccine. 2013;31(35):3594–602. doi:10.1016/j.vaccine.2013.04.084.
- Juergens C, de Villiers PJ, Moodley K, Jayawardene D, Jansen KU, Scott DA, Emini EA, Gruber WC, Schmoele-Thoma B. Safety and immunogenicity of 13-valent pneumococcal conjugate vaccine formulations with and without aluminum phosphate and comparison of the formulation of choice with 23-valent pneumococcal polysaccharide vaccine in elderly adults: a randomized open-label trial. Hum Vaccin Immunother. 2014;10(5):1343–53. doi:10.4161/hv.27998.
- Burton RL, Nahm MH. Development and validation of a fourfold multiplexed opsonization assay (MOPA4) for pneumococcal antibodies. Clin Vaccine Immunol. 2006;13:1004–09. doi:10.1128/CVI.00112-06.
- Miettinen O, Nurminen M. Comparative analysis of two rates. Stat Med. 1985;4:213–26. doi:10.1002/sim.4780040211.
- Liang K-Y, Zeger SL. Longitudinal data analysis of continuous and discrete responses for pre-post designs. Sankhya Indian J Stat. 2000;62:134–48.
- Musher DM, Rodriguez-Barradas MB. Why the recent ACIP recommendations regarding conjugate pneumococcal vaccine in adults may be irrelevant. Hum Vaccin Immunother. 2016;12(2):331–35. doi:10.1080/21645515.2015.1098794.
- Weinberger DM, Shapiro ED. Prevention of pneumococcal infections in adults using conjugate vaccines: no easy answers. Clin Infect Dis. 2019 Jun 18;69(1):50–51. doi:10.1093/cid/ciy873.
- Djennad A, Ramsay ME, Pebody R, Fry NK, Sheppard C, Ladhani SN, Andrews NJ. Effectiveness of 23-valent polysaccharide pneumococcal vaccine and changes in invasive pneumococcal disease incidence from 2000 to 2017 in those aged 65 and over in England and Wales. EClinicalMedicine. 2019;6:42–50. doi:10.1016/j.eclinm.2018.12.007.
- Marra F, Vadlamudi NK. Efficacy and safety of the pneumococcal conjugate-13 valent vaccine in adults. Aging Dis. 2019;10(2):404–18. doi:10.14336/AD.2018.0512.
- Kobayashi M, Bennett NM, Gierke R, Almendares O, Moore MR, Whitney CG, Pilishvili T. Intervals between PCV13 and PPSV23 vaccines: recommendations of the advisory committee on immunization practices (ACIP). MMWR Morb Mortal Wkly Rep. 2015;64(34):944–47. doi:10.15585/mmwr.mm6434a4.
- de Roux A, Schmöle-Thoma B, Siber GR, Hackell JG, Kuhnke A, Ahlers N, Baker SA, Razmpour A, Emini EA, Fernsten PD, et al. Comparison of pneumococcal conjugate polysaccharide and free polysaccharide vaccines in elderly adults: conjugate vaccine elicits improved antibacterial immune responses and immunological memory. Clin Infect Dis. 2008;46(7):1015–23. doi:10.1086/529142.
- Shiramoto M, Hanada R, Juergens C, Shoji Y, Yoshida M, Ballan B, Cooper D, Gruber WC, Scott DA, Schmoele-Thoma B. Immunogenicity and safety of the 13-valent pneumococcal conjugate vaccine compared to the 23-valent pneumococcal polysaccharide vaccine in elderly Japanese adults. Hum Vaccin Immunother. 2015;11(9):2198–206. doi:10.1080/21645515.2015.1030550.
- Ermlich SJ, Andrews CP, Folkerth S, Rupp R, Greenberg D, McFetridge RD, Hartzel J, Marchese RD, Stek JE, Abeygunawardana C, et al. Safety and immunogenicity of 15-valent pneumococcal conjugate vaccine in pneumococcal vaccine-naïve adults ≥50 years of age. Vaccine. 2018;36(45):6875–82. doi:10.1016/j.vaccine.2018.03.012.
- Hurley D, Griffin C, Young M Jr, Scott DA, Pride MW, Scully IL, Ginis J, Severs J, Jansen KU, Gruber WC, et al. Safety, tolerability, and immunogenicity of a 20-valent pneumococcal conjugate vaccine (PCV20) in adults 60 to 64 years of age. Clin Infect Dis. 2020; in press. doi:10.1093/cid/ciaa1045.
- Pelton SI, Shea KM, Weycker D, Farkouh RA, Strutton DR, Edelsberg J. Rethinking risk for pneumococcal disease in adults: the role of risk stacking. Open Forum Infect Dis. 2015;2(1):ofy020. doi:10.1093/ofid/ofv020.
- Morton JB, Morrill HJ, LaPlante KL, Caffrey AR. Risk stacking of pneumococcal vaccination indications increases mortality in unvaccinated adults with Streptococcus pneumoniae infections. Vaccine. 2017;35(13):1692–97. doi:10.1016/j.vaccine.2017.02.026.
- Ahmed SS, Pondo T, Xing W, McGee L, Farley M, Schaffner W, Thomas A, Reingold A, Harrison L, Lynfield R, et al. Early Impact of 13-valent pneumococcal conjugate vaccine use on invasive pneumococcal disease among adults with and without underlying medical conditions-United States. Clin Infect Dis. 2020;70(12):2484–92. doi:10.1093/cid/ciz739.
- McLaughlin JM, Swerdlow DL, Isturiz RE, Jodar L. Decision-making for PCV in adults. Hum Vaccin Immunother. 2019;15(3):584–93. doi:10.1080/21645515.2018.1538611.
- Colijn C, Corander J, Croucher NJ. Designing ecologically optimized pneumococcal vaccines using population genomics. Nat Microbiol. 2020 Mar 5;3:473–85. doi:10.1038/s41564-019-0651-y.
- Robbins JB, Austrian R, Lee CJ, Rastogi SC, Schiffman G, Henrichsen J, Makela PH, Broome CV, Facklam RR, Tiesjema RH, et al. Considerations for formulating the second-generation pneumococcal capsular polysaccharide vaccine with emphasis on the cross-reactive types within the groups. J Infect Dis. 1983;148(6):1136–59. doi:10.1093/infdis/148.6.1136.