ABSTRACT
Pneumococcal disease can be serious and debilitating in older adults. Pneumococcal conjugate vaccines (PCVs), such as the 13-valent PCV (PCV13), reduce pneumococcal disease rates caused by vaccine serotypes. Development of PCVs offering additional coverage against serotypes not contained in PCV13 can reduce disease burden further. The complementary 7-valent PCV (cPCV7) contains seven non-PCV13 serotypes (8, 10A, 11A, 12F, 15B, 22F, 33F) and can expand coverage by supplementing direct or indirect protection from existing PCVs. This phase 1/2, randomized, active-controlled, observer-blinded study evaluated cPCV7 safety and immunogenicity in healthy adults 50–85 years of age. Stage 1 randomized 66 healthy adults (50–64 years) naive to pneumococcal vaccines to receive cPCV7 or licensed tetanus, diphtheria, and acellular pertussis vaccine; Stage 2 randomized 445 healthy adults (65–85 years) previously vaccinated with PCV13 to receive cPCV7 or 23-valent polysaccharide vaccine. Local reactions and systemic events up to 14 days and adverse events (AEs) through 1 month after vaccination were assessed. Immunogenicity was evaluated by serotype-specific opsonophagocytic activity (OPA) assays before and 1 month after vaccination (and after 12 months in Stage 2). Rates of local reactions, systemic events, and AEs were generally similar after receipt of cPCV7 or control. Robust OPA responses were observed for all seven serotypes 1 month after cPCV7; titers declined yet remained above baseline 12 months after vaccination. Overall, this study found that in adults ≥50 years of age, cPCV7 was safe, well tolerated, and elicited functional immune responses to vaccine serotypes. ClinicalTrials.gov: NCT03313050
Introduction
Streptococcus pneumoniae can cause serious invasive diseases (e.g., bacteremic pneumonia, meningitis) as well as noninvasive diseases (e.g., acute otitis media, sinusitis, nonbacteremic pneumonia).Citation1–3 Pneumococcal disease is a global public health concern: in 2016, there were 197 million cases and 1.1 million deaths from pneumococcal disease globally.Citation4
Although pneumococcal disease poses a serious risk for all individuals, older adults remain particularly susceptible to pneumococcal infection.Citation1–3,Citation5 In the United States, an estimated 503,000 cases of nonbacteremic pneumococcal pneumonia and 30,000 cases of invasive pneumococcal disease (IPD) occur annually among adults ≥50 years of age, resulting in 25,000 deaths each year for this population.Citation6 Serious pneumococcal disease is predominantly caused by a limited subset of the >95 known S pneumoniae serotypes.Citation2,Citation3,Citation7
To protect against pneumococcal disease, vaccines have been developed and are recommended for use in numerous countries.Citation2 While the 23-valent pneumococcal polysaccharide vaccine (PPSV23) has been available since the early 1980s,Citation2,Citation8,Citation9 unconjugated polysaccharide vaccines like PPSV23 elicit a T-cell independent response and do not induce immunological memory.Citation10 Additionally, several PPSV23 studies have not shown effectiveness in preventing nonbacteremic pneumococcal pneumonia in older adults.Citation10–12 In contrast, pneumococcal conjugate vaccines (PCVs), such as 13-valent PCV (PCV13), induce T-cell-dependent immune responses, resulting in more robust and long-lasting immune responsesCitation10,Citation13 with demonstrated efficacy and real-world effectiveness against nonbacteremic pneumococcal pneumonia.Citation14,Citation15 PCV13 targets 13 pneumococcal serotypes (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F), of which 12 are also targeted by PPSV23.Citation2,Citation9
Widespread use of PCVs in children and adults has resulted in substantial reductions of the disease burden associated with vaccine serotypes;Citation16–19 however, after the introduction of PCV13, pneumococcal disease burden due to nonvaccine serotypes has persisted and increased in some settings, including among older adults.Citation20 As such, expanding serotype coverage beyond that provided by current PCVs would be expected to further reduce the worldwide burden of pneumococcal disease.Citation18,Citation20–23
To augment protection provided by PCV13, the complementary 7-valent PCV (cPCV7) was developed to target seven additional serotypes (8, 10A, 11A, 12F, 15B, 22F, and 33F) and extend coverage beyond PCV13. These seven serotypes were selected based on prevalence, geographic distribution, and association with antibiotic resistance and greater disease severity.Citation17,Citation21–34 Of note, in Germany in 2017 and 2018, these 7 additional serotypes accounted for approximately 30% of all cases of IPD in adults 60 years of age and older.Citation33 Moreover, in US adults of any age, it has been estimated that these serotypes account for approximately 9990 cases of IPD and 44,000 cases of inpatient pneumonia annually.Citation35 A strategy of using a complementary PCV such as cPCV7 that extends coverage but does not contain the serotypes already covered by available PCVs could be implemented in cases where protection against PCV13 serotypes is provided either indirectly from herd protection from a coexisting pediatric PCV13 program, which may have certain limitations, or directly with PCV13 given separately or concomitantly with cPCV7. Thus, supplementing PCV13 serotype coverage with cPCV7 could potentially address an unmet need to expand protection in older adults against pneumococcal disease caused by these seven serotypes.
This two-stage, phase 1/2, first-in-human study was conducted to describe the safety and immunogenicity of cPCV7 in adults 50 to 64 years of age naive to pneumococcal vaccines and in adults 65 to 85 years of age previously vaccinated with PCV13.
Methods
Study design and participants
This two-stage, phase 1/2, randomized, active-controlled, observer-blinded study conducted between October 12, 2017, and May 24, 2019, at 16 study sites in the United States evaluated the safety and immunogenicity of cPCV7 in healthy adults 50 to 85 years of age (NCT03313050). The purpose of Stage 1 (phase 1 portion of the study) was to provide first-in-human safety and immunogenicity data for cPCV7 in 50- to 64-year-old adults. Stage 2 functioned as the phase 2 part of the study and provided additional safety and immunogenicity data for cPCV7, with PPSV23 serving as the control group in adults 65 to 85 years of age.
For Stage 1, eligible participants were 50 to 64 years of age and healthy or had chronic, nonserious, stable disease not requiring a significant change in therapy in the 12 weeks preceding enrollment or not worsening or requiring hospitalization within 6 months before receipt of the investigational product. Key exclusion criteria for Stage 1 included history of clinically significant or major disease or other acute or chronic medical or psychiatric condition that may have increased the risk associated with participation or with investigational product administration or that may have interfered with result interpretation and, in the investigator’s judgment, would have made participation in the study inappropriate, or who had a known or suspected immunodeficiency or had received immunosuppressive therapy. For Stage 2, eligible participants were 65 to 85 years of age, were either healthy or had stable preexisting disease (as described for Stage 1) and had received PCV13 at least 2 months before investigational product administration. Key exclusion criteria were otherwise identical to Stage 1.
Participants were randomized in a 1:1 ratio to receive either cPCV7 or licensed tetanus, diphtheria, and acellular pertussis (Tdap) vaccine (Stage 1) or to receive either cPCV7 or PPSV23 (Stage 2). Participant allocation to the vaccine group was performed using an interactive response technology system (interactive Web-based response) that provided site personnel with the participant randomization number. Study staff administering vaccines were unblinded, but all other study personnel (i.e., principle investigator, study coordinator, and any other site staff) and study participants were blinded. For Stage 1, sponsor core study team members remained blinded until completion of the analysis of all available safety data 1 month after vaccination. Similarly, for Stage 2, sponsor blinding remained until completion of the analysis of all available safety and immunogenicity data 1 month after vaccination. Investigator site personnel remained blinded until after the primary clinical study report was complete.
A single 0.5-mL dose of the investigational cPCV7 (Stage 1 and 2) or licensed Tdap vaccine (Stage 1; Adacel®, Sanofi Pasteur, Ltd) was administered intramuscularly into the deltoid muscle. cPCV7 contains saccharides from seven pneumococcal serotypes individually conjugated to the nontoxic variant of diphtheria toxin cross-reactive material 197 (CRM197). A single cPCV7 dose contains 2.2 µg of each saccharide and 0.125 mg aluminum as aluminum phosphate. Tdap is a sterile isotonic suspension of tetanus and diphtheria toxoids and pertussis antigens adsorbed on aluminum phosphate. A single dose (0.5 mL) of PPSV23 (Stage 2; Pneumovax ®, Merck & Co., Inc.) consists of 25 µg of each purified capsular polysaccharides from 23 serotypes (1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F, and 33F) in an isotonic saline solution containing 0.25% phenol as a preservative.
The study was conducted in compliance with the ethical principles originating in or derived from the Declaration of Helsinki and in compliance with all International Council for Harmonization Good Clinical Practice Guidelines. The protocol was approved by the institutional review board or independent ethics committee for each participating study site. All participants provided written informed consent before performance of any study-specific activity.
Assessments
Safety assessments included local reactions, systemic events, adverse events (AEs), serious AEs (SAEs), and newly diagnosed chronic medical conditions (NDCMCs). Prompted local reactions and systemic events were recorded in an electronic diary for 14 days after vaccination. Local reactions (redness, swelling, pain at the injection site) and systemic events (fatigue, headache, muscle pain, joint pain) were graded as mild (grade 1), moderate (grade 2), severe (grade 3), or grade 4 based on increasing severity levels. Grading was based on size or description of the affected area for redness and swelling and on degree to which the event interfered with activity for pain and all systemic events. Maximum daily temperature was recorded in the electronic diary; fever was collected as a systemic event and grouped into 1 of 4 categories: 38.0–38.4°C, 38.5–38.9°C, 39.0–40.0°C, and >40°C. Data on AEs were collected through 1 month after vaccination and on SAEs and NDCMCs, through a telephone follow-up 6 months after vaccination in both stages, and also at a visit 12 months after vaccination for Stage 2 only.
For both Stages 1 and 2, blood samples for immunogenicity assessments were collected before vaccination and 1 month after vaccination. Stage 2 included an additional blood sample collection at 12 months after vaccination.
Endpoints
Primary endpoints included the percentage of participants reporting prompted local reactions or systemic events occurring within 14 days after vaccination, the percentage of participants reporting AEs occurring within 1 month after vaccination, and the percentage of participants reporting SAEs or NDCMCs occurring within 6 months after vaccination (Stages 1 and 2) and within 12 months after vaccination (Stage 2 only).
Secondary endpoints included cPCV7-type opsonophagocytic activity (OPA) geometric mean titers (GMTs) at 1 month after vaccination and cPCV7-type OPA geometric mean fold rises (GMFRs) from before vaccination to 1 month after vaccination.
Exploratory endpoints (Stage 2 only) included percentages of participants with fourfold rises in OPA titers from before to 1 month after vaccination, immunoglobulin G (IgG) geometric mean concentrations (GMCs) at 1 month after vaccination, and IgG GMFRs from before to 1 month after vaccination, and OPA GMTs at 12 months after vaccination and OPA GMFRs from before vaccination to 12 months after vaccination.
Analyses
The study sample size was not based on any formal statistical hypothesis testing. The study planned to enroll approximately 33 participants per group in Stage 1 and 220 per group in Stage 2 to provide a sample size sufficient to detect infrequent local reactions, systemic events, and AEs.
The safety population used for the analysis of safety endpoints included any participant who received a dose of cPCV7 or Tdap (Stage 1) or of cPCV7 or PPSV23 (Stage 2). Descriptive summaries of the safety endpoints, the percentages of participants with the indicated endpoint, and the associated 95% CIs were calculated using the Clopper-Pearson method.
The evaluable immunogenicity population at 1 month after vaccination (in each of Stages 1 and 2) or the evaluable immunogenicity population at 12 months after vaccination (Stage 2 only) was used to assess immunogenicity endpoints and included all eligible participants who received the assigned vaccine as randomized, had a blood collection within 27–49 days after vaccination (or 350–385 days after vaccination for the analysis at 12 months), had OPA titers for ≥1 serotype after vaccination, and had no other major protocol deviations.
Descriptive summaries of geometric means and corresponding 95% CIs were calculated for OPA titers and IgG concentrations at each time point by vaccine group within Stage 1 or Stage 2, with each serotype analyzed separately. GMTs or GMCs and corresponding exact 2-sided 95% CIs were obtained by log-transforming OPA titers or IgG concentrations, respectively, then calculating the 95% CI based on the Student’s t distribution and exponentiating the mean and limits. Serotype-specific GMFRs were summarized by each vaccine group for the fold change in OPA titer or IgG concentration from before vaccination to 1 month after vaccination (Stages 1 and 2) and from before vaccination to 12 months after vaccination (Stage 2 only). GMFRs were calculated by first dividing the later time point assay result by the earlier assay result and log transforming the ratios, then applying the same geometric mean and associated 95% CI calculations as described above for GMTs and GMCs. OPA titers or IgG concentrations below the lower limit of quantitation (LLOQ) were set to 0.5 × LLOQ for analyses; missing assay results were not imputed.
Results
Stage 1
Participants
In Stage 1, 66 participants were randomized to receive either cPCV7 (n = 34) or Tdap (n = 32). All participants received vaccination and completed the 6-month follow-up visit. All 66 participants were included in the safety population, and 65 participants were included in the evaluable immunogenicity population for 1 month after vaccination (1 participant with a blood sample drawn outside the protocol specified time window was excluded). Demographic characteristics were similar between vaccine groups; mean (SD) age at vaccination was 58.1 (4.46) years, and the majority of participants were White, non-Hispanic, and female (Supplementary Table 1).
Safety
Local reactions were reported by 22 (64.7%) participants in the cPCV7 group and 25 (78.1%) participants in the Tdap group (Supplementary Figure 1). All local reactions were mild or moderate in severity; pain at the injection site was the most common local reaction in both vaccine groups. Systemic events were reported by 23 (67.6%) participants in the cPCV7 group and 22 (68.8%) participants in the Tdap group (Supplementary Figure 1). The most frequently reported systemic event was muscle pain. All systemic events were mild or moderate in severity, and fever (temperature ≥38.0°C) was not reported in either group.
A total of 3 (8.8%) participants in the cPCV7 group and 3 (9.4%) participants in the Tdap group reported any AE; all were considered unrelated to vaccination (Supplementary Table 2). Two participants (both in the cPCV7 group) reported 1 SAE each (intestinal obstruction, cholecystitis) within 1 month after vaccination, neither of which was considered related to vaccination. NDCMCs were reported by 2 participants in the Tdap group (1 event each of hypercholesterolemia, lactose intolerance, and hypertension) and were not considered related to the vaccine. No safety-related withdrawals were reported.
Immunogenicity
Robust increases in serotype-specific OPA GMTs were observed 1 month after vaccination in the cPCV7 group for all seven vaccine serotypes (GMT range: 1401–9558) compared to before vaccination (Supplementary Table 3). The serotype-specific OPA GMFRs from baseline to 1 month after vaccination for all seven serotypes ranged from 6.5-fold (serotype 11A) to 261.8-fold (serotype 12 F) in the cPCV7 group (Supplementary Table 4). As expected, no increases in the Tdap control group were observed.
Stage 2
Participants
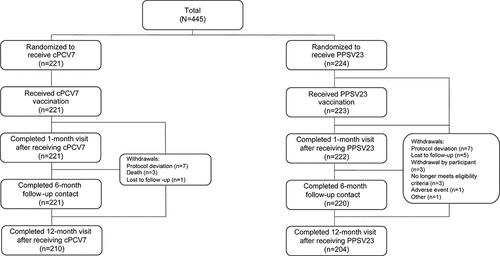
Of the 444 participants receiving either cPCV7 (n = 221) or PPSV23 (n = 223) in Stage 2, 414 participants completed the study through 12 months after vaccination (cPCV7: 210 participants, 95.0%; PPSV23: 204 participants, 91.1%; ). Reasons for withdrawal included protocol deviation (e.g., prohibited vaccine received; n = 14), lost to follow-up (n = 6), death (n = 3), withdrawal by participant (n = 3), no longer meeting the eligibility criteria (n = 3), AE (n = 1), and other (n = 1). All 444 participants were included in the safety population. The 1-month evaluable immunogenicity population included 436 participants (cPCV7: 219 participants; PPSV23: 217 participants), and the 12-month evaluable immunogenicity population included 407 participants (cPCV7: 207 participants; PPSV23: 200 participants). Demographic characteristics were similar between vaccine groups; mean (SD) age at vaccination was 68.9 (4.36) years, and the majority of study participants were White, non-Hispanic, and female (Supplementary Table 1). The mean interval between the prior dose of PCV13 and randomization was 15.4 months in both groups.
Safety
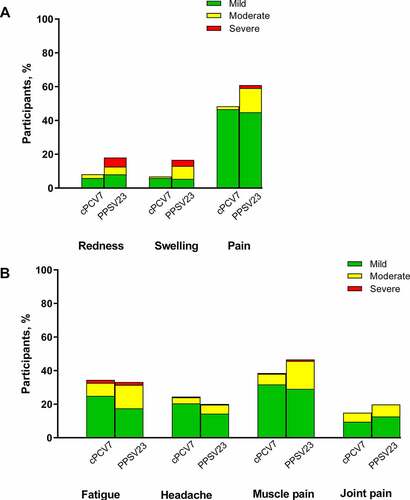
Local reactions were reported by 113 (51.5%) participants in the cPCV7 group and 141 (63.2%) participants in the PPSV23 group. A total of 129 (58.4%) and 139 (62.3%) participants in the cPCV7 and PPSV23 groups, respectively, reported a systemic event. Most local reactions and systemic events were mild or moderate in severity (). No severe local reactions were reported in the cPCV7 group; however, severe local reactions were reported in the PPSV23 group (redness, n = 12 [5.4%]; swelling, n = 8 [3.6%]; pain at the injection site, n = 4 [1.8%]). Fever was reported by one participant in the cPCV7 group (38.0–38.4°C) and 2 participants in the PPSVS23 group (38.5–38.9°C, 39.0–40.0°C).
Figure 2. Reactogenicity events including (A) local reactions and (B) systemic events occurring within 14 days after vaccination (Stage 2). Three reports of fever (1 and 2 participants in cPCV7 and PPSV23 groups, respectively) are not shown. Number of participants: cPCV7, n = 221; PPSV23, n = 223. cPCV7 = complementary 7-valent pneumococcal conjugate vaccine; PPSV23 = 23-valent pneumococcal polysaccharide vaccine
Adverse events occurred in 22 (10.0%) and 30 (13.5%) participants in the cPCV7 and PPSV23 groups, respectively, in the month after vaccination; no AEs were considered related to vaccination (). Severe AEs occurred in 2 participants in the cPCV7 group (one each of breast abscess and gastroenteritis) and in 1 participant in the PPSV23 group (thrombophlebitis) in the month after vaccination, but none of these AEs were assessed as related to vaccination. From vaccination through 6 months after vaccination, 5 participants in the cPCV7 group and 6 participants in the PPSV23 group reported ≥1 SAE; the only SAE considered by the investigator to be related to vaccination was polymyalgia rheumatica, reported by 1 participant in the PPSV23 group 44 days after vaccination. In the 12 months after vaccination, SAEs were reported by 9 and 8 participants in the cPCV7 and PPSV23 groups, respectively, and 15 participants in the cPCV7 group and 14 participants in the PPSV23 group reported ≥1 NDCMC. One participant in the PPSV23 group was withdrawn from the study (hernia hiatus repair). Three deaths occurred during the study in participants in the cPCV7 group (one each of myocardial infarction, arteriosclerosis, and acute respiratory distress syndrome); deaths occurred 259–272 days after vaccination and none were considered related to vaccination.
Table 1. Summary of AEs (Stage 2 Safety Population)
Immunogenicity
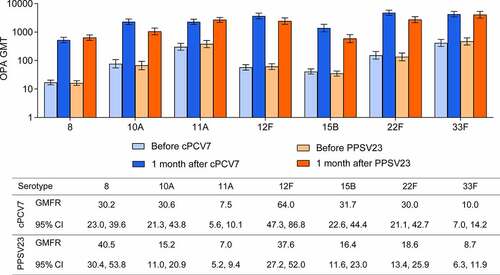
One month after vaccination, robust increases in serotype-specific OPA GMTs were observed for all seven cPCV7 serotypes in the cPCV7 group (), with GMTs 1 month after vaccination ranging from 528 (serotype 8) to 4775 (serotype 22F). Among PPSV23 recipients, OPA GMTs 1 month after vaccination ranged from 586 (serotype 15B) to 4043 (serotype 33F). OPA GMFRs from baseline to 1 month after vaccination for all seven serotypes ranged from 7.5-fold (serotype 11A) to 64.0-fold (serotype 12F) in the cPCV7 group and from 7.0-fold (serotype 11A) to 40.5-fold (serotype 8) in the PPSV23 group (). Across all seven serotypes, 54.0% (serotype 11A) to 87.0% (serotype 12F) of participants in the cPCV7 group achieved a ≥ fourfold rise in OPA titers from baseline at 1 month after vaccination. For the PPSV23 group, 52.5% (serotype 11A) to 85.8% (serotype 8) of participants achieved ≥fourfold rise in OPA titers 1 month after vaccination. The IgG responses to the seven vaccine serotypes showed a trend consistent with the OPA responses (Supplementary Figure 2).
Figure 3. Pneumococcal OPA GMTs before and 1 month after vaccination and GMFRs 1 month after vaccination for the cPCV7 serotypes (Stage 2). The LLOQs for each serotype were as follows: 8, 16; 10A, 14; 11A, 32; 12F, 51; 15B, 36; 22F, 28; 33F, 49. cPCV7 = complementary 7-valent pneumococcal conjugate vaccine; GMTs = geometric mean titers; GMFRs = geometric mean fold-rises; LLOQ = lower limit of quantitation; OPA = opsonophagocytic activity; PPSV23 = 23-valent pneumococcal polysaccharide vaccine
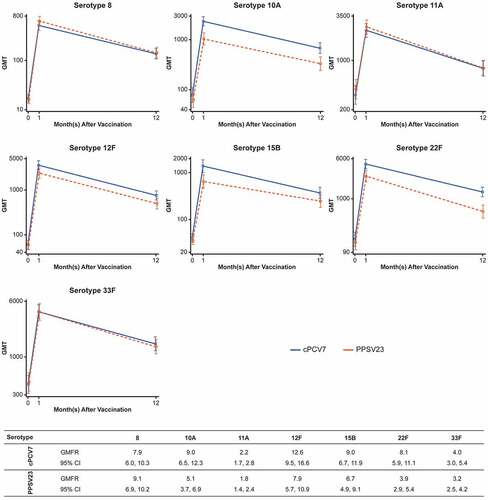
At 12 months after vaccination, pneumococcal OPA GMTs for the 7 serotypes declined from the levels observed at 1 month after vaccination but remained elevated above levels before vaccination for both cPCV7 and PPSV23 groups. Plots of the OPA GMTs for each of the vaccine serotypes at the three immunogenicity time points (before vaccination, 1 and 12 months after vaccination) are provided by vaccine group in . OPA GMFRs from before vaccination to 12 months after vaccination were from 2.2-fold (serotype 11A) to 12.6-fold (serotype 12F) among cPCV7 recipients and 1.8-fold (serotype 11A) to 9.1-fold (serotype 8) among PPSV23 recipients.
Figure 4. Pneumococcal OPA GMT antibody response curves and OPA GMFRs for the cPCV7 serotypes from baseline to 12 months after vaccination (Stage 2). Note that the y-axis scales for each graph differ. cPCV7 = complementary 7-valent pneumococcal conjugate vaccine; GMT = geometric mean titer; OPA = opsonophagocytic activity; PPSV23 = 23-valent pneumococcal polysaccharide vaccine
Discussion
While the worldwide introduction of PCVs has resulted in substantial reductions in pneumococcal disease among both children and adults, certain serotypes not included in these vaccines continue to cause disease.Citation16–19,Citation34 This 2-stage phase 1/2 study assessed the safety and immunogenicity of cPCV7, a novel PCV that targets 7 pneumococcal serotypes not included in PCV13; these serotypes were selected based on relative prevalence as a cause of IPD and nonbacteremic pneumonia, geographic distribution, antibiotic resistance, and disease severity characteristics.Citation17,Citation21–34
In this first-in-human study, cPCV7 was well tolerated and elicited functional immune responses in healthy adults 50 to 85 years of age. The two study stages allowed for an efficient transition to move from a phase 1 type evaluation that focused primarily on describing initial safety and immunogenicity to a phase 2 evaluation with a larger and older population with a licensed pneumococcal vaccine as a control to further assess safety and immunogenicity. Because the participants in the control group in Stage 2 were to receive PPSV23, the study enrolled only those who had received the recommended dose of PCV13 at least 2 months previously as part of their routine care, and excluded those who had prior vaccination with PPSV23. This was to ensure that the study population would have received PCV13 before a dose of PPSV23 (if randomized to the control group), consistent with the sequence recommended by the Advisory Committee on Immunization Practices based on diminished responses when PPSV23 is given initially.Citation22 Although all Stage 2 participants had received prior PCV13 before enrollment, they were naive to vaccines containing the polysaccharides for the serotypes in cPCV7.
cPCV7 was well tolerated in Stage 1, with all local and systemic reactions being mild to moderate in severity. In Stage 2, local reactions and systemic events experienced by cPCV7 and PPSV23 recipients were generally mild or moderate in severity. However, cPCV7 had a lower rate of moderate and severe local reactions than PPSV23. The severe reactions observed in participants receiving PPSV23 were typical of PPSV23 in those with prior pneumococcal vaccination.Citation36,Citation37 Frequencies of AEs were similar for cPCV7 and PPSV23, and no new safety concerns were identified.
Robust immune responses to all vaccine serotypes were seen 1 month after vaccination with cPCV7 in Stages 1 and 2. In Stage 2, observed OPA GMTs at 1 month after cPCV7 were similar to or higher than the PPSV23 group. OPA GMTs to the vaccine serotypes had declined by 12 months after cPCV7 but remained above baseline levels. Although PPSV23 was used as the benchmark for the seven vaccine serotypes in Stage 2 of this study, it is important to note that the absolute OPA GMTs alone do not completely reflect the important advantages of PCVs in terms of quality of immune responses, including eliciting memory responses and ability to protect against noninvasive disease.Citation10,Citation14
Taken together, these results show that cPCV7 is immunogenic in older adults. Thus, if developed further, cPCV7 could have the potential to supplement the protection provided by PCV13. Expanding serotype coverage of PCVs would be expected to reduce the substantial burden of pneumococcal disease due to these additional serotypes.Citation1–3,Citation5 It should be noted that an alternative approach to cPCV7, a 20-valent PCV (PCV20), is also in clinical development. PCV20 contains the components in PCV13 and the polysaccharide conjugates of cPCV7 in a single formulation and represents a more efficient approach to expanding vaccine coverage. PCV20 has shown that it is well tolerated and elicits functional antibodies in phase 1 and phase 2 studies, and findings from phase 3 clinical studies are forthcoming (NCT03828617, NCT03760146, NCT03835975).Citation38,Citation39
This study to evaluate cPCV7 was strengthened by its randomized 2-stage design. Potential limitations include that it was performed in a single country, thereby constraining the global generalizability of these results. The sample size was appropriate for a phase 1/2 study and allowed a descriptive assessment of safety and immunogenicity.
Conclusion
In this two-stage, phase 1/2, first-in-human study, cPCV7 was well tolerated and induced functional immune responses to the vaccine serotypes in adults 50 through 85 years of age.
Disclosure of potential conflicts of interest
Brandon Essink and James Peterson are investigators in Pfizer-sponsored studies. Kari Yacisin, Xia Xu, Himal Lal, Sarah Mirza, Daniel A. Scott, Ingrid L. Scully, Kathrin U. Jansen, William C. Gruber, and Wendy Watson are current or former employees of Pfizer and may hold stock or stock options.
Data sharing statement
Upon request, and subject to certain criteria, conditions, and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual de-identified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines, and medical devices (1) for indications that have been approved in the US and/or EU or (2) in programs that have been terminated (i.e., development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
Supplemental Material
Download MS Word (259 KB)Acknowledgments
Editorial/medical writing support was provided by Emily Stackpole, PhD, of ICON plc (North Wales, PA) and was funded by Pfizer Inc.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2021.1890511
Additional information
Funding
References
- Blasi F, Mantero M, Santus P, Tarsia P. Understanding the burden of pneumococcal disease in adults. Clin Microbiol Infect. 2012;18(5 suppl):7–14. doi:10.1111/j.1469-0691.2012.03937.x.
- World Health Organization. Pneumococcal vaccines WHO position paper–2012. Wkly Epidemiol Rec. 2012;87(14):129–44.
- Drijkoningen JJ, Rohde GG. Pneumococcal infection in adults: burden of disease. Clin Microbiol Infect. 2014;20(5 suppl):45–51. doi:10.1111/1469-0691.12461.
- Collaborators GBDLRI. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018;18(11):1191–210. doi:10.1016/S1473-3099(18)30310-4.
- Said MA, Johnson HL, Nonyane BA, Deloria-Knoll M, O’Brien KL, AGEDD Adult Pneumococcal Burden Study Team, Andreo F, Beovic B, Blanco S, Boersma WG, Estimating the burden of pneumococcal pneumonia among adults: a systematic review and meta-analysis of diagnostic techniques. PLoS One. 2013;8(4):e60273. doi:10.1371/journal.pone.0060273.
- Weycker D, Strutton D, Edelsberg J, Sato R, Jackson LA. Clinical and economic burden of pneumococcal disease in older US adults. Vaccine. 2010;28(31):4955–60. doi:10.1016/j.vaccine.2010.05.030.
- Brueggemann AB, Griffiths DT, Meats E, Peto T, Crook DW, Spratt BG. Clonal relationships between invasive and carriage Streptococcus pneumoniae and serotype- and clone-specific differences in invasive disease potential. J Infect Dis. 2003;187(9):1424–32. doi:10.1086/374624.
- Matanock A, Lee G, Gierke R, Kobayashi M, Leidner A, Pilishvili T. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68(46):1069–75. doi:10.15585/mmwr.mm6846a5.
- Menveo (Meningococcal Group A, C, W-135 and Y Conjugate Vaccine). Siena (Italy): Summary of Product Characteristics, GSK Vaccines S.r.l.; 2020.
- Clutterbuck EA, Lazarus R, Yu LM, Bowman J, Bateman EA, Diggle L, Angus B, Peto TE, Beverley PC, Mant D, et al. Pneumococcal conjugate and plain polysaccharide vaccines have divergent effects on antigen-specific B cells. J Infect Dis. 2012;205(9):1408–16. doi:10.1093/infdis/jis212.
- Jackson LA, Neuzil KM, Yu O, Benson P, Barlow WE, Adams AL, Hanson CA, Mahoney LD, Shay DK, Thompson WW, et al. Effectiveness of pneumococcal polysaccharide vaccine in older adults. N Engl J Med. 2003;348(18):1747–55. doi:10.1056/NEJMoa022678.
- Leventer-Roberts M, Feldman BS, Brufman I, Cohen-Stavi CJ, Hoshen M, Balicer RD. Effectiveness of 23-valent pneumococcal polysaccharide vaccine against invasive disease and hospital-treated pneumonia among people aged ≥65 years: a retrospective case-control study. Clin Infect Dis. 2015;60(10):1472–80. doi:10.1093/cid/civ096.
- Vadlamudi NK, Parhar K, Altre Malana KL, Kang A, Marra F. Immunogenicity and safety of the 13-valent pneumococcal conjugate vaccine compared to 23-valent pneumococcal polysaccharide in immunocompetent adults: a systematic review and meta-analysis. Vaccine. 2019;37(8):1021–29. doi:10.1016/j.vaccine.2019.01.014.
- Bonten MJ, Huijts SM, Bolkenbaas M, Webber C, Patterson S, Gault S, van Werkhoven CH, van Deursen AM, Sanders EA, Verheij TJ, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015;372(12):1114–25. doi:10.1056/NEJMoa1408544.
- McLaughlin JM, Jiang Q, Isturiz RE, Sings HL, Swerdlow DL, Gessner BD, Carrico RM, Peyrani P, Wiemken TL, Mattingly WA, et al. Effectiveness of 13-valent pneumococcal conjugate vaccine against hospitalization for community-acquired pneumonia in older US adults: a test-negative design. Clin Infect Dis. 2018;67(10):1498–506. doi:10.1093/cid/ciy312.
- Cohen R, Cohen JF, Chalumeau M, Levy C. Impact of pneumococcal conjugate vaccines for children in high- and non-high-income countries. Expert Rev Vaccines. 2017;16(6):625–40. doi:10.1080/14760584.2017.1320221.
- Moore MR, Link-Gelles R, Schaffner W, Lynfield R, Lexau C, Bennett NM, Petit S, Zansky SM, Harrison LH, Reingold A, et al. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance. Lancet Infect Dis. 2015;15(3):301–09. doi:10.1016/S1473-3099(14)71081-3.
- Vadlamudi NK, Chen A, Marra F. Impact of 13-pneumococcal conjugate vaccine among adults: a systematic review and meta-analysis. Clin Infect Dis. 2018. doi:10.1093/cid/ciy872.
- World Health Organization. Pneumococcal conjugate vaccines in infants and children under 5 years of age: WHO position paper - February 2019. [accessed 2019 Jun 28] https://apps.who.int/iris/bitstream/handle/10665/310968/WER9408.pdf?ua=1.
- Hausdorff WP, Hanage WP. Interim results of an ecological experiment - conjugate vaccination against the pneumococcus and serotype replacement. Hum Vaccin Immunother. 2016;12(2):358–74. doi:10.1080/21645515.2015.1118593.
- Metcalf BJ, Gertz RE Jr., Gladstone RA, Walker H, Sherwood LK, Jackson D, Li Z, Law C, Hawkins PA, Chochua S, et al. Strain features and distributions in pneumococci from children with invasive disease before and after 13-valent conjugate vaccine implementation in the USA. Clin Microbiol Infect. 2016;22(1):60 e69–60 e29. doi:10.1016/j.cmi.2015.08.027.
- Tomczyk S, Lynfield R, Schaffner W, Reingold A, Miller L, Petit S, Holtzman C, Zansky SM, Thomas A, Baumbach J, et al. Prevention of antibiotic-nonsusceptible invasive pneumococcal disease with the 13-valent pneumococcal conjugate vaccine. Clin Infect Dis. 2016;62(9):1119–25. doi:10.1093/cid/ciw067.
- Wantuch PL, Avci FY. Invasive pneumococcal disease in relation to vaccine type serotypes. Hum Vaccin Immunother. 2019;15(4):874–75. doi:10.1080/21645515.2018.1564444.
- Cohen R, Biscardi S, Levy C. The multifaceted impact of pneumococcal conjugate vaccine implementation in children in France between 2001 to 2014. Hum Vaccin Immunother. 2016;12(2):277–84. doi:10.1080/21645515.2015.1116654.
- Flasche S, Van Hoek AJ, Sheasby E, Waight P, Andrews N, Sheppard C, George R, Miller E. Effect of pneumococcal conjugate vaccination on serotype-specific carriage and invasive disease in England: a cross-sectional study. PLoS Med. 2011;8(4):e1001017. doi:10.1371/journal.pmed.1001017.
- Lepoutre A, Varon E, Georges S, Gutmann L, Levy-Bruhl D. Impact of infant pneumococcal vaccination on invasive pneumococcal diseases in France, 2001-2006. Euro Surveill. 2008;13:35. doi:10.2807/ese.13.35.18962-en.
- Harboe ZB, Thomsen RW, Riis A, Valentiner-Branth P, Christensen JJ, Lambertsen L, Krogfelt KA, Konradsen HB, Benfield TL. Pneumococcal serotypes and mortality following invasive pneumococcal disease: a population-based cohort study. PLoS Med. 2009;6(5):e1000081. doi:10.1371/journal.pmed.1000081.
- Wroe PC, Lee GM, Finkelstein JA, Pelton SI, Hanage WP, Lipsitch M, Stevenson AE, Rifas-Shiman SL, Kleinman K, Dutta-Linn MM, et al. Pneumococcal carriage and antibiotic resistance in young children before 13-valent conjugate vaccine. Pediatr Infect Dis J. 2012;31(3):249–54. doi:10.1097/INF.0b013e31824214ac.
- Balsells E, Guillot L, Nair H, Kyaw MH. Serotype distribution of Streptococcus pneumoniae causing invasive disease in children in the post-PCV era: a systematic review and meta-analysis. PLoS One. 2017;12(5):e0177113. doi:10.1371/journal.pone.0177113.
- Yahiaoui RY, Bootsma HJ, den Heijer CDJ, Pluister GN, John Paget W, Spreeuwenberg P, Trzcinski K, Stobberingh EE. Distribution of serotypes and patterns of antimicrobial resistance among commensal Streptococcus pneumoniae in nine European countries. BMC Infect Dis. 2018;18(1):440. doi:10.1186/s12879-018-3341-0.
- Cui YA, Patel H, O’Neil WM, Li S, Saddier P. Pneumococcal serotype distribution: a snapshot of recent data in pediatric and adult populations around the world. Hum Vaccin Immunother. 2017;13(6):1–13. doi:10.1080/21645515.2016.1277300.
- Kalina WV, Souza V, Wu K, Giardina P, McKeen A, Jiang Q, Tan C, French R, Ren Y, Belanger K, et al. Qualification and clinical validation of an immunodiagnostic assay for detecting 11 additional S. pneumoniae serotype-specific polysaccharides in human urine. Clin Infect Dis. 2020. Epub ahead of print. doi:10.1093/cid/ciaa158.
- van der Linden M, Imohl M, Perniciaro S. Limited indirect effects of an infant pneumococcal vaccination program in an aging population. PLoS One. 2019;14(8):e0220453. doi:10.1371/journal.pone.0220453.
- Zintgraff J, Fossati S, Pereira CS, Veliz O, Regueira M, Moscoloni MA, Irazu L, Lara C, Napoli D, Argentina Spn Working G. Distribution of PCV13 and PPSV23 Streptococcus pneumoniae serotypes in Argentinean adults with invasive disease, 2013-2017. Rev Argent Microbiol. 2020. doi:10.1016/j.ram.2019.11.004.
- Perdrizet J, Chilson E, Wasserman M, Farkouh RA, Sato R Current and future pneumococcal conjugate vaccine serotype-specific burden in the United States adult population. Paper presented at: ISPPD; 2020; Toronto, Canada.
- Greenberg RN, Gurtman A, Frenck RW, Strout C, Jansen KU, Trammel J, Scott DA, Emini EA, Gruber WC, Schmoele-Thoma B. Sequential administration of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naive adults 60-64 years of age. Vaccine. 2014;32(20):2364–74. doi:10.1016/j.vaccine.2014.02.002.
- Jackson LA, Gurtman A, Rice K, Pauksens K, Greenberg RN, Jones TR, Scott DA, Emini EA, Gruber WC, Schmoele-Thoma B. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine in adults 70 years of age and older previously vaccinated with 23-valent pneumococcal polysaccharide vaccine. Vaccine. 2013;31(35):3585–93. doi:10.1016/j.vaccine.2013.05.010.
- Thompson A, Lamberth E, Severs J, Scully I, Tarabar S, Ginis J, Jansen KU, Gruber WC, Scott DA, Watson W. Phase 1 trial of a 20-valent pneumococcal conjugate vaccine in healthy adults. Vaccine. 2019;37(42):6201–07. doi:10.1016/j.vaccine.2019.08.048.
- Hurley D, Griffin C, Young M, Scott DA, Pride MW, Scully IL, Ginis J, Severs J, Jansen KU, Gruber WC, et al. Safety, tolerability, and immunogenicity of a 20-valent pneumococcal conjugate vaccine (PCV20) in adults 60 to 64 years of age. Clin Infect Dis. 2020. doi:10.1093/cid/ciaa1045.