ABSTRACT
Skin cancers are among the most physically accessible malignancies, so local delivery of a medication into the tumor, so-called intratumoral therapy, is an appealing route of drug administration. Intratumoral therapies have the potential to increase local drug concentration and/or attract immune cells to the local tumor microenvironment, possibly with fewer systemic side effects. A wide array of intratumoral agents have been studied to date in patients with advanced melanoma, including chemotherapeutic drugs, immune modulating agents, and cancer-directed vaccines. In this review, we will summarize the key pre-clinical and clinical data supporting the use of intratumoral therapy for advanced unresectable and metastatic melanoma. First, we will discuss the history of intratumoral immunotherapy for the treatment of melanoma and the various agents studied to date. Second, we will explore how intratumoral therapies can constitute an in situ vaccine, potentially leading to disease control both locally and systemically. Finally, we will highlight opportunities in the field and key future directions.
Introduction
There have been dramatic improvements in the treatment of melanoma over the last two decades. Previously, cytotoxic chemotherapy was the mainstay of treatment, but more recently systemic immune therapy and targeted therapies have significantly improved the treatment options for patients with advanced melanoma. While some patients derive marked benefit from these therapies, there is still a substantial number of patients who do not respond, develop treatment resistance, or experience serious toxicities with these therapies. Therefore, novel treatment strategies are still needed.
An alternative emerging treatment strategy has been proposed is to administer drugs directly into the tumor, a drug delivery strategy termed “intratumoral” or “intralesional” therapy. Melanoma lesions are typically cutaneous and have a propensity for cutaneous/subcutaneous spread, providing a unique opportunity for direct injection of tumors. This route of drug administration has the potential to increase local drug concentrations, stimulate a local immune response, and decrease systemic toxicity. It is possible that intratumoral treatment can induce a systemic immune response, causing regression of both injected and non-injected lesions. Moreover, it is possible that the administration of intratumoral treatment can enhance systemic treatment response when administered in conjunction with other systemic therapies.
To better understand the potential impact of intratumoral therapy, it is important to discuss recent advances in our understanding of the tumor microenvironment (TME). The TME is defined as the local environment around a tumor, including the proliferating tumor cells, the tumor stroma, blood vessels, infiltrating inflammatory cells, and the surrounding tissues.Citation1 Innate immune cells such as dendritic cells as well as adaptive immune cells such as CD8+ and CD4 + T cells respond to pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) in the vicinity of the tumor and recruit other immune cells.Citation2–6 Tumor infiltrating lymphocytes (TILs) in the TME can also initiate a positive feedback loop, secreting cytokines such as Interferon-γand Interleukin-12 (IL-12) to help recruit effector T cells.Citation7 Indeed, the presence of TILs is associated with improved clinical response to immunotherapy.Citation8 Thus, the ability to directly inject drugs that impact the TME is an appealing strategy.
The TME can be classified into three broad categories based on human and preclinical mouse model data.Citation9 The TME most unresponsive to immune therapy is termed “immune excluded”; these tumors have various immune cells but no CD8+ cells and what few CD8+ cells exist are located at the periphery. These immune-excluded TMEs are seen in epithelial cancers such as colorectal carcinomaCitation10 and pancreatic adenocarcinoma but also in some instances of melanoma. The second category is the “immune-infiltrated” TME, where there are plentiful CD8+ cells expressing checkpoint molecules such as PD-1 and CTLA4.Citation11 This category is often sensitive to PD-1 therapy and comprises many melanomas as well as microsatellite instability (MSI) high colorectal cancers. The third subtype, which is a type of immune infiltrated TME, has histological evidence of tertiary lymphoid structures (TLSs), lymphoid aggregates whose cellular composition is similar to that in lymph nodes. TLSs are often, but not always, correlated with a positive prognosis.Citation12 Similar to lymph nodes, TLSs can contain a substantial diversity of lymphocytes, including naive and activated conventional T cells, regulatory T (Treg) cells, B cells and dendritic cells (DCs).Citation13 TLSs are generally present at the invasive tumor margin and in the stroma. They are thought to act as sites of lymphoid recruitment and immune activation that are typically formed in the setting of enhanced inflammation, such as after administration of an autologous tumor vaccine.Citation14
In this review, we review intratumoral strategies studied to date, including the use of intralesional chemotherapy, oncolytic viruses, immunotherapy, and combination strategies. Then, we discuss the possibility of in situ vaccination as a treatment strategy for the treatment and prevention of metastatic malignancy. Finally, we discuss challenges and opportunities in the field of intratumoral therapies for the treatment of melanoma.
Intratumoral strategies
Intralesional chemotherapy and electrochemotherapy
The accessibility of cutaneous metastatic and in-transit melanoma lesions led to the hypothesis that these malignancies could be treated using intralesional therapies. Metastatic melanoma was originally treated with cytotoxic chemotherapy, so naturally antineoplastic and cytotoxic drugs were the first to be tested as intratumoral therapies. One of the earliest agents studied was 10% Rose Bengal, also known as PV-10. In murine models, intratumoral administration of PV-10 caused an immune-recruiting response that leads to enzymatic cell death, although the precise mechanism of action was unclear.Citation15 In vivo trials in humans demonstrated that PV-10 has a dose-dependent response in both target and non-target (non-injected) lesions.Citation16,Citation17 However, subsequent work suggested that the response rate is short, with a median lesion-based response duration of 4.0 months,Citation17 so the clinical utility of this agent has been questioned.
Since many chemotherapy agents cannot adequately penetrate tumor cells after systemic therapy or direct injection, the use of electric pulses to increase the uptake of chemotherapy drugs, known as electrochemotherapy (ECT), was developed. In ECT, a drug such as cisplatin or bleomycin is administered systemically or intratumorally and then electric pulses are applied perilesionally to allow the drug to better permeate cell membranes given the transient increase in cell permeability caused by the modulated electric field.Citation18,Citation19 Intravenous bleomycin was first tested with ECT, and then subsequently the use of intratumoral bleomycin was described.Citation20,Citation21 Studies showed an ORR of approximately 90% with the use of intratumoral bleomycin administered with electroporation. There have also been studies evaluating ECT with cisplatin. In a meta-analysis of twelve studies that investigated the use of ECT in the treatment of melanoma, there was a 74% objective response rate in 502 patients.Citation22 Only six of the twelve studies had an intratumoral injection component and there was limited information about staging provided in the study. More recently, in a prospective cohort study using intratumoral or intravenous injection of bleomycin followed by application of electric pulses, the authors noted that among 151 patients with 394 treated lesions, 306 lesions (78%) showed an overall response, with 229 (58%) showing a complete response.Citation23 Factors significantly associated with complete response to ECT treatment were coverage of deep margins, previous irradiation of the treated area, and smaller tumor size (<3 cm). Additional studies are needed to evaluate the mechanism and clinical outcomes of this treatment strategy. However, given the advent of immunotherapy and its increased efficacy in the treatment of advanced melanoma compared to chemotherapy, it is likely that focusing intratumoral efforts on the study of immune modulating agents may yield more promising results.
Intralesional therapy with “oncolytic” viruses
The use of viruses has also been studied to try to increase tumor-targeting of local therapy. Many cancer cells lack cell cycle machinery such as hypophosphorylated-RB, functional p53, and interferon signaling which make them susceptible to viral infection. As a result, viruses can with some selectivity infect and destroy malignant cells with minimal effect on normal cells and are referred to as “oncolytic” viruses. Viruses can also be engineered to carry additional payloads such as cytokine or chemokine genes to increase recruitment of immune cells, such as dendritic cells (DC) or T cells, to the TME.
One of the most successful examples of using a viral vector in cancer therapy is the use of talimogene laherparepvec (T-VEC), which uses a modified herpes simplex virus (HSV). Specifically, the neurovirulence factor ICP34.5 is inactivated to prevent neuronal involvement and is replaced by the coding sequence for granulocyte-macrophage colony stimulating factor (GM-CSF).Citation24–26 Local oncolysis is thought to attract T cells, increasing tumoral inflammation and in turn inducing a more distant immune response. Indeed, some pre-clinical studies have demonstrated tumor infiltration by CD8 + T cells in both injected and non-injected metastases which supports this hypothesis.Citation27 In murine models, intratumoral inoculation of GM-CSF with inactivated HSV vectors resulted in greater tumor inhibition and improved mouse survival compared to HSV alone.Citation28 In humans, an initial phase II clinical trial enrolled 50 patients with stage IIIc-IV melanoma and treated them with intratumoral T-VEC every three weeks.Citation29 In this trial, most patients (74%) had received prior systemic therapies (which at that time were essentially cytotoxic chemotherapies or interferon). The objective response rate by bi-dimensional WHO measurement was 26%, with 8 out of 13 responding patients having complete responses and 12 responses lasting >6 months. The authors noted that both injected and non-injected lesions responded. However, overall survival was only 58% at 1 y and 52% at 2 y. Subsequently, a randomized open-label phase III trial (OPTiM) was conducted that randomized patients with unresectable stage IIIB-IV melanoma 2:1 to receive intratumoral T-VEC or subcutaneous recombinant GM-CSF.Citation30,Citation31 Durable responses were higher in the T-VEC arm than the subcutaneous recombinant GM-CSF arm (16.3 vs. 2.1%, p < .001), and objective response rate was also higher in the T-VEC arm (26.4 vs. 5.7%). Durable responses were more commonly seen in patients with stage III (33%) and IVM1a (16%) disease, and less common in patients with stage IVM1c disease (8%). Median overall survival was 23.2 months (95% CI 19.5–29.6) and 18.9 months (95% CI 16.0–23.7) in the T-VEC and GM-CSF arms, respectively (hazard ratio, 0.79; 95% CI, 0.62 to 1.00; p = .051). Thus, these T-VEC monotherapy studies suggest that this agent may stimulate distant responses at non-injected lesions, likely through immune activation, although few durable responses were seen in patients with stage IVM1c metastatic disease. This trial led to the FDA approval of T-VEC in unresectable cutaneous, subcutaneous, and nodal lesions in patients with melanoma that was recurrent after initial surgery.
Other viral vectors and tumor-targeting agents have also been described. A phase I trial used a recombinant canarypox viral vector system called ALVAC to compare the efficacy of GMCSF to interleukin-2 (IL-2).Citation32 In this small study, IL-2 lead to partial regression in three of eight tumors, whereas GM-CSF lead to only stable disease. The phase II CALM trial tested the safety and efficacy of coxsackievirus A21 (CVA21) in 57 patients with stage IIc and IV melanoma. The study met its primary endpoint of 38.6% immune-related progression-free survival (irPFS) at 6.0 months and a median irPFS of 4.2 months, and demonstrated a best overall response rate of 24% (9 out of 28 evaluable patients).Citation33
Given the promising activity of oncolytic viruses to augment the immune response, it was hypothesized that oncologic viruses and systemic immunotherapy may be an effective treatment strategy in combination. Preclinical studies suggested that combining oncolytic viral therapy and immune checkpoint blockade enhanced treatment response.Citation27 Results of a randomized phase II trial of T-VEC plus ipilimumab versus ipilimumab alone demonstrated a 39% ORR with the combination therapy versus an 18% ORR for ipilimumab alone (odds ratio 2.9; 95% CI 1.5 to 5.5, p = .002).Citation34 Responses were not limited to injected lesions; they observed decrease in visceral lesions in 52% of patients in the combination arm but only 23% of patients in the ipilimumab arm. A trial combining T-VEC followed by TVEC+PD-1 has also been conducted on 21 patients who were PD-1 naive.Citation35 This combination had a 61.9% objective response rate by irRC (immune response criteria). Interestingly, T-VEC injected lesions had increased PD-1/PD-L1 expression as well as increased CD4 and CD8 and Foxp3+ ve cells while these changes were not seen clearly in the small sample of non-injected lesions biopsied.Citation35 No changes in cross presenting cDC1 dendritic cells were seen. While circulating proliferating (Ki-67+ CD3+/CD8+) CD8+ cells were not induced by TVEC alone, these cells were increased following pembrolizumab administration by week 8. Based on these and other data, a large phase III clinical trial of pembrolizumab ± T-VEC is ongoing (NCT02263508), with study completion estimated in 2023. This trial and others are actively investigating the safety and efficacy of combining oncolytic viruses and systemic immunotherapy, so additional data is forthcoming.
The concept of using neoadjuvant treatment prior to tumor resection is less commonly utilized in melanoma as compared to breast and gastrointestinal cancers, but it also has the ability to constitute a vaccine. Several studies have investigated the use of intratumoral therapy using oncolytic viruses in the neoadjuvant setting. For example, one study compared neoadjuvant intratumoral T-VEC to immediate surgical resection.Citation36 Per the interim analysis of this study, 11 subjects progressed on the T-VEC arm compared to 17 patients in the control arm with an overall response rate of 14.7% in the T-VEC arm. Interestingly, of the patients who responded to T-VEC, 21% achieved a complete pathologic response with no melanoma cells detected at the time of surgery. Similarly, intratumoral CMP-001 with IV nivolumab showed promising interim safety and efficacy data,Citation37 as previously discussed. Further work is needed to address whether there is a role for neoadjuvant intratumoral treatment for patients with resectable melanoma.
Of note, a theoretical concern with the use of oncologic viruses is that the virus may be able to mutate and regain pathogenicity. However, this has not been a significant clinical issue in over two decades of clinical trials with various oncolytic viruses.Citation38 Instead, side effects are generally mild and arise from an anti-inflammatory response, causing fatigue, mild flu-like symptoms and/or arthralgias,Citation1 and/or local irritation or lesion ulceration.Citation32,Citation39
Intralesional immunotherapy: modulation of TME with cytokines
The number of TILs in the TME is correlated with clinical response to immunotherapy in patients with melanoma.Citation8 Therefore, a key question is whether intratumoral injections can alter the TME, thus increasing the number and quality of TILs and improving clinical outcomes.
Initial attempts to modulate TILs included injection of intratumoral cytokines. A phase I dose-escalation study used a plasmid vector to produce IL-12 that was administered in conjunction with electroporation, demonstrating safety and tolerability of this approach and a durable increase in both IL-12 and interferon-γwith minimal toxicity.Citation40 More recently, a phase II prospective open-label clinical trial of electroporated plasmid IL-12 in patients with advanced melanoma demonstrated an objective overall response rate of 35.7% (by RECIST criteria) with a complete response rate of 17.9%.Citation41 Interestingly, the transcriptomic and immunohistochemistry analysis showed that immune activation and co-stimulatory transcripts were up-regulated, increasing adaptive immune resistance which could have limited the response duration. Pre-clinical studies suggest that the combination of intratumoral IL-12 and anti-PD1 therapy may be more efficacious given the development of adaptive resistance in patients on monotherapy pIL-12 EP (Tavo)Citation42 and the frequent responses seen in patients who progressed on pIL-12 EP when treated with PD-1 therapy appear to corroborate this data.Citation42 Interestingly, an analysis of 29 patients treated in this trial showed emergent responses to shared melanoma tumor antigens.Citation43 Samples from 25 patients were evaluated by ELISpot assay to assess for antigen-specific T-cell responses to shared melanoma antigens. Systemic interferon-γ responses to gp100 were significantly higher at 6 months after treatment compared with baseline (p = .0313). In the same analysis population, interferon-γ responses to NY-ESO-1, MAGE-A3, or Melan-A/MART-1 did not significantly change after treatment showing variability in emergent responses to these tumor-associated antigens.Citation43 One patient had a very high preexisting T-cell response to MAGE-A3 and this patient had a complete clinical response with treatment. At 90 d post-treatment, interferon-γ responses to MAGE-A3 were significantly lower for responders compared with non-responders (p = .0236), but were not significantly different pretreatment or at 39, 180, or 360 d after treatment. Interferon-γ responses to gp100, NY-ESO-1, and Melan-A/MART-1 were not significantly different between responders and non-responders pre- or post-treatment. At the same time, “exhausted” CD8 + T cells in circulation decreased during treatment.
Plasmid IL-12 EP treatment was also explored in a phase II trial of the rare but increasing skin malignancy, Merkel Cell Cancer (MCC).Citation44 This cancer is characterized by the presence in cancer cells of the Merkel cell polyoma virus (MCPyV) and its large T antigen. The T antigen drives carcinogenesis and patients can mount immune responses to the T antigen. In the trial by Bhatia et al., patients with metastatic MCC had an objective response rate of 25% (3/12 patients). Using a library of MCPyV-specific HLA class-I tetramers they tracked MCPyV-specific T-cell responses, both locally in the treated lesions and in peripheral blood. The frequency of MCPyV-specific tetramer+ TIL significantly increased (≥1.5-fold change in at least one MCPyV-specific T-cell population) in 3 of 5 patients following treatment, and decreased in 1 patient with 1 patient not having any detectable MCPyV-specific T cells in pre- or post-treatment specimens. Interestingly, all three patients with increased frequency of MCPyV-specific TIL-experienced clinical responses.
Given the adaptive immune resistance seen in melanoma patients treated with pIL-12 EP, Algazi et al. conducted a phase II trial of pembrolizumab plus pIl-12 EP in patients with immunologically cold melanoma.Citation45 Currently, immune-excluded tumors are thought to lack CD8 + T cells and consequently are unresponsive to immune checkpoint blockade.Citation46 Some hallmarks of these “cold” tumors are the lack of exhausted T cellsCitation11 and reduced PD-L1 expressionCitation47 as well as reduced interferon-γ transcriptional profile.Citation48 In this trial, patients were eligible only if they had tumors with an exhausted T cell frequency <25%, which in previous studies was associated with an objective response of ≤10%. The objective response rate (by RECIST) in this trial was 41% including complete response in 36% of patients. The combination of pIL-12 EP and pembrolizumab drove an increased density of CD8+ TIL as well as a significant increase in the number of unique T cell clones in the TME. In addition, significant increases in intratumoral PD-L1 expression by immunohistochemistry (p = .016) and transcriptomic analysis (p = .026), as well as increases in systemic proliferating PD-1 + T cells (p = .012) were noted. Along with these changes, the combination also increased adaptive PD-L1 expression in both responding and nonresponding patients. In quantitative spatial analysis, nonresponding patients had more Tregs in close proximity to CD8 + T cells than responding patients (p = .018). This balance of effector to suppressive immune subsets could also be seen in the positive ratio of CD8 + T cells to M2 macrophages (PD-L1+/CD163+) in responding patients (p = .011). This trial along with the previous two pIL-12 trials highlights the complex role played by the TME in inducing and maturing T cell responses and sometimes contradictory immune changes visualized in the immune microenvironment.
While these trials demonstrate the role of intratumoral IL-12 in altering the TME, very interesting experimental data also show the role of IL-12 in mediating immune checkpoint response. In a pivotal study, Garris et al. used an intravital imaging system responsive to IL-12 and interferon-γ to examine in a mouse tumor model the changes in the TME produced by PD-1 blockade.Citation49 They demonstrated intratumoral DCs (predominantly cross presenting DC1s) produced IL-12 in response to PD-1 blockade. If the IL-12 was neutralized, mice with MC38 tumors failed to reject them in response to PD-1 blockade. These results augment previous findings that tumor-infiltrating DCs can foster T cell immunity.Citation4 The authors thus proposed a DC1 licensed T cell killing model where DC are not just involved in priming T cells but also provide cytokine support to the T cells within the TME. Specifically, they examined samples from patients treated with pIL-12 in the clinical trials above and showed that pIL-12 enhanced expression of core cytolytic genes within tumors. These genes, namely CD2, CD3E, CD247, GZMA, GZMH, GZMK, NKG7, and PRF1, are associated with immunoediting and antitumor immune responses.
Intratumoral administration of interleukin-2 (IL-2) has also been studied with promising results. Specifically, intratumoral IL-2 induced complete responses in more than 60% of patients with melanoma, with two year overall survival rates of 95% for patients with stage IIIB disease, 66% for stage IV M1a disease, but only 9.1% for those with visceral metastases (stage IV M1b and M1c).Citation50 Several studies have also evaluated the safety and efficacy of combining intratumoral IL-2 with other agents. For example, a phase I study demonstrated that the combination of intratumoral IL-2 and ipilimumab was safe and tolerable, with promising clinical results including an abscopal effect in 89% of treated patients.Citation51 It should be noted that cytokine administration directly in the tumor results in only transient changes.
The administration of intratumoral interferons (INFs) has also been investigated for the treatment of melanoma. For example, an early study by Fierlbeck et al. demonstrated that intralesional injection of recombinant interferon beta resulted in a dose-dependent regression of melanoma lesions.Citation52 Subsequent studies have evaluated the combination of interferon with other agents, such as tumor necrosis factor (TNF), IL-2, and radiotherapy.Citation53–55
Intralesional immunotherapy: innate immune agonists
Another powerful approach to modulating the TME and changing it from immune-excluded to immune-infiltrated is the use of TLR and STING agonists to attract and stimulate DC. CMP-001 is a differentiated TLR-9 agonist that activates local tumor-associated plasmacytoid dendritic cells (pDCs) leading to type I interferon secretion and tumor antigen presentation to T cells and systemic antitumor T cell responses. Intratumoral CMP-001 has been studied in combination with systemic PD-1 therapy, and two ongoing clinical trials recently reported promising interim analyses. First, in a phase 1b Checkmate study (NCT02680184), patients with PD-1 refractory melanoma were randomized to receive the combination of intratumoral CMP-001 and IV pembrolizumab versus intratumoral CMP-001 alone. According to results presented during the 2019 Society for Immunotherapy of Cancer (SITC) Annual Meeting, CMP-001 was well tolerated in both arms.Citation56 The patients who received the combination therapy had an objective response rate (ORR) of 25% in patients. The median duration of response of 16.9+ months for the 28 RECIST v1.1 responders in this study. Second, in a phase II study of patients with stage III PD-1 naïve melanoma (NCT03618641), patients received neoadjuvant intratumoral CMP-001 and IV nivolumab. According to interim analysis also presented at the 2019 SITC Annual Meeting, this treatment combination was well tolerated and did not result in any surgical delays.Citation37 A major pathologic response rate (MPR) of 71% (15/21) was reported in 21 evaluable patients to date. Of the 15 responding patients, 13 patients had a pathological complete response at the time of surgery. Translational studies demonstrated that the combination therapy augmented peripheral blood and intra-tumoral tumor-specific CD8 + T cell immune responses. Similarly, intratumoral CMP-001 with IV nivolumab showed promising interim safety and efficacy data,Citation37 as previously discussed.
There have also been studies evaluating the safety and efficacy of combining intratumoral injection of the TLR-9 agonist IMO-2125 (tilsotolimod) with ipilimumab. In a phase I/II study, intratumoral IMO-2125 plus ipilimumab resulted in immune response in both injected and uninjected tumors in patients who had progressed on prior anti-PD1 therapy.Citation57 Specifically, they found that intratumoral tilsotolimod 8 mg plus ipilimumab was well tolerated with an objective response rate of 38%. A randomized phase III trial evaluating the efficacy of intratumoral tilsotolimod plus ipilimumab in patients with unresectable or metastatic melanoma who have progressed on prior anti-PD1 therapy is ongoing (NCT03445533).
Intralesional immunotherapy: checkpoint inhibitors
Checkpoint inhibitors have dramatically improved the treatment landscape for patients with advanced melanoma with a relatively low toxicity profile compared to systemic chemotherapy and other agents. Therefore, there has been interest in determining whether intratumoral injection of these agents may result in improved clinical response with decreased systemic side effects. Interim analysis from a pilot study by Samoylenko et al. recently demonstrated intralesional pembrolizumab or nivolumab resulted in an overall response in four out of the seven patients.Citation58 Additional studies are ongoing to study the safety and efficacy of intralesional CPI therapy in melanoma and other cutaneous malignancies, such as the use of intertumoral ipilimumab in combination with systemic nivolumab in patients with metastatic melanoma (NCT02857569) and the use of intratumoral nivolumab in patients with Kaposi’s sarcoma (NCT03316274).
In-situ vaccination using intralesional therapies
The immune system has the ability to detect and destroy cancer cells, so the concept of creating a cancer vaccine is an exciting prospect. A key problem in creating an effective cancer vaccine is the selection of antigen and the combination of adjuvant and antigen. Conventional vaccine approaches using cancer neoantigen(s) have been described, but this remains a technically and financially challenging strategy and the effector T cells generated are specific to the injected antigen(s). An alternative strategy is in situ vaccination, which relies on immunoenhancing agents that are injected locally into a tumor, thereby activating a local T cell response that then triggers a systemic immune response against diverse tumor antigens. The idea of using in-situ vaccination would be to use the tumor itself as the vaccine, which we will discuss here (also see ).
Figure 1. Conventional vaccination vs. in situ vaccination.
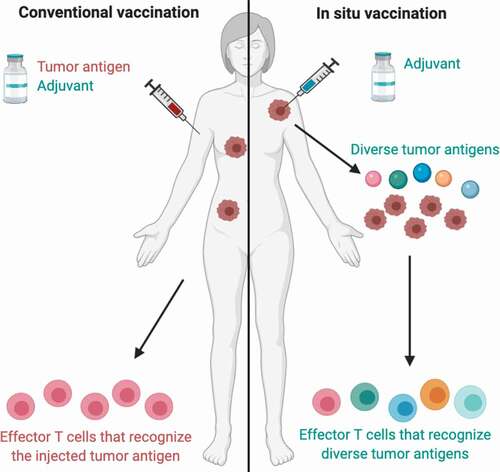
In a landmark study published in 2018, Sagiv-Barfi et al. described how low doses of CpG injected into a tumor induce the expression of OX40 on CD4+ T cells in the microenvironment in mouse or human tumors, triggering a systemic immune response.Citation59 They found that the combination of a TLR ligand and an anti-OX40 antibody could cure multiple types of cancer and prevent spontaneous genetically driven cancers, creating enthusiasm for the concept of in situ vaccination to treat and prevent cancer.
Several preclinical models of in situ cancer vaccines have demonstrated promising findings. Stimulator of interferon genes (STING) is required for the spontaneous generation of tumor-specific T cell responses.Citation60 Therefore, it was hypothesized that direct engagement of STING through intratumoral administration of specific agonists would result in effective anti-tumor therapy. In a murine model, intratumoral STING agonists induced profound regression of established tumors in mice and generated substantial systemic immune responses capable of rejecting distant metastases and providing long-lived immunologic memory.Citation61 In a dose escalation model, increased doses of intratumoral STING agonists resulted in increased frequency of cytokine-positive monocytes and increased diversity of responding cell types in the injected tumor.Citation62
In has been hypothesized that STING-based therapies may be particularly effective in combination with checkpoint inhibitors. In a subset of melanoma cell lines, it was demonstrated that intact activation of STING signaling enhanced both tumor antigenicity and susceptibility to lysis by human melanoma tumor-infiltrating lymphocytes through the augmentation of MHC class I expression.Citation63 Conversely, defects in the STING signaling pathway protected the melanoma cells from increased immune recognition by TILs and limited their sensitivity to TIL lysis. Based on these findings, it is possible that defects in STING signaling may mediate tumor immune evasion as well as resistance to TIL-based immunotherapies, so modulating this pathway in combination with checkpoint inhibitors may help overcome resistance. Further studies are needed in humans to assess the safety and efficacy of intratumoral STING agonists as monotherapy and in combination with other immunotherapies.
Aside from STING adjuvants, there has also been interest in studying whether toll-like receptor (TLR) adjuvants may have applications as in situ cancer vaccines. The interim results of a phase Ib/II clinical trial (NCT02521870) evaluating the safety and efficacy of the intratumoral TLR agonist SD-101 in combination with systemic pembrolizumab were recently presented at the American Society of Clinical Oncology (ASCO) Annual Meeting in 2019.Citation64 The authors observed an overall response rate of 70% and 6-month progression-free survival rate of 76% in patients naïve to anti-PD-1 treatment. The clinical trial was terminated prematurely due to a strategic restructuring within the company and plans to discontinue production of SD-101, but nonetheless this offers encouraging preliminary data for similar TLR agonists.
Furthermore, since STING and TLR signaling are non-redundant, one hypothesis is that TLR and STING adjuvants would further enhance DC activation in combination. Using the multidimensional synergy of combinations (MuSyc), a novel synergy algorithm, Taylor et al. recently demonstrated that the combination of R848 (TLR7-8) plus STING agonists provided a synergistic efficacious and potent response on bone marrow dendritic cells.Citation65 Additional studies are needed to better understand how the use of cancer vaccines can help prevent and treatment melanoma in the future, and we anticipate that this will be an active area of future research.
Future directions: challenges and opportunities
Challenges
One major limitation of the use of intratumoral therapy is in cases of advanced metastatic disease with visceral metastases. It is technically risky to inject visceral lesions, and the repeated administrations done with cutaneous lesions would not be feasible at these sites. Moreover, the tumor microenvironment is likely quite different in different organs, so the efficacy of intratumoral drug administration will likely be different. For example, studies have demonstrated that liver metastases in patients with melanoma have lower TIL counts and these patients have worse response to anti-PD1 therapy.Citation66
Another challenge in this field is how to deliver effective doses of the medication to each patient. The ideal dose and schedule for intratumoral therapy has not yet been established and is likely to vary significantly depending on the size of the tumor, injected agent, and combination therapy utilized. Systemic therapy is often dosed by patient weight, whereas intratumoral therapy can be dosed according to the size of the lesion or a ratio of tumor size to patient weight. It is unclear what dosing approach is most safe and efficacious.
Opportunities
The main advantage of intratumoral therapy is the opportunity to deliver medications directly into the TME. Most studies to date have suggested that intratumoral drug administration is generally safe and tolerable, with minimal side effects in most cases.
Perhaps the most exciting application of intratumoral therapy is the opportunity for in situ vaccine development using intratumoral agents. By injecting immune-stimulating agents into the TME, it is possible to induce both a local and systemic anti-cancer immune response. As depicted in , a significant advantage of this strategy compared to conventional vaccination approaches is that it is not necessary to choose tumor antigens or neoantigens in advance, but rather the injected agents can stimulate an immune response with the antigens already present in the TME with the potential for a more specific and comprehensive immune response. Thus, in situ vaccination can potentially generate a systemic immune response against the metastatic cells already present and prevent the development of new metastatic sites. Further studies are needed to evaluate the efficacy of in situ vaccination strategies and to optimize the immune response, but this remains a promising avenue for the treatment and prevention of metastatic melanoma.
Conclusion
Intratumoral therapies are a safe and tolerable drug administration strategy for patients with advanced or metastatic melanoma. In studies to date, the administration of intratumoral therapies alone generally do not exceed the response rate of systemic standard of care treatments, but the use of intratumoral therapies in combination with systemic therapies is a promising treatment strategy that warrants additional study. In particular, the possibility of in situ vaccination using intratumoral drug administration remains an exciting future direction, and further studies are needed to better understand and optimize this effect.
Disclosure of potential conflicts of interest
L.H. has no declarations.
A.D. Research funding from Merck, BMS, Roche, Incyte, OncoSec, Checkmate, Novartis.
Acknowledgments
Figure created with BioRender (BioRender.com).
References
- Whiteside T. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27(45):5904–9. doi:https://doi.org/10.1038/onc.2008.271.
- Engelhardt JJ, Boldajipour B, Beemiller P, Pandurangi P, Sorensen C, Werb Z, Egeblad M, Krummel M. Marginating dendritic cells of the tumor microenvironment cross-present tumor antigens and stably engage tumor-specific T cells. Cancer Cell. 2012;21(3):402–17. doi:https://doi.org/10.1016/j.ccr.2012.01.008.
- Spranger S, Sivan A, Corrales L, Gajewski TF. Tumor and host factors controlling antitumor immunity and efficacy of cancer immunotherapy. Adv Immunol. 2016;130:75–93.
- Broz ML, Binnewies M, Boldajipour B, Nelson A, Pollack J, Erle D, Barczak A, Rosenblum M, Daud A, Barber D, et al. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell. 2014;26(5):638–52. doi:https://doi.org/10.1016/j.ccell.2014.09.007.
- Spranger S, Dai D, Horton B, Gajewski TF. Tumor-residing Batf3 dendritic cells are required for effector T cell trafficking and adoptive T cell therapy. Cancer Cell. 2017;31(5):711–723.e4. doi:https://doi.org/10.1016/j.ccell.2017.04.003.
- Barry KC, Hsu J, Broz ML, Cueto FJ, Binnewies M, Combes AJ, Nelson AE, Loo K, Kumar R, Rosenblum MD, et al. A natural killer-dendritic cell axis defines checkpoint therapy-responsive tumor microenvironments. Nat Med. 2018;24(8):1178–91. doi:https://doi.org/10.1038/s41591-018-0085-8.
- Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol. 2006;6(10):715–27. doi:https://doi.org/10.1038/nri1936.
- Daud AI, Loo K, Pauli ML, et al. Tumor immune profiling predicts response to anti-PD-1 therapy in human melanoma. J Clin Invest. 2016;126(9):3447–52.
- Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, Coussens LM, Gabrilovich DI, Ostrand-Rosenberg S, Hedrick CC, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24(5):541–50. doi:https://doi.org/10.1038/s41591-018-0014-x.
- Mlecnik B, Bindea G, Angell HK, Maby P, Angelova M, Tougeron D, Church SE, Lafontaine L, Fischer M, Fredriksen T, et al. Integrative analyses of colorectal cancer show immunoscore is a stronger predictor of patient survival than microsatellite instability. Immunity. 2016;44(3):698–711. doi:https://doi.org/10.1016/j.immuni.2016.02.025.
- Daud AI, Loo K, Pauli ML, Sanchez-Rodriguez R, Sandoval PM, Taravati K, Tsai K, Nosrati A, Nardo L, Alvarado MD, et al. Tumor immune profiling predicts response to anti-PD-1 therapy in human melanoma. J Clin Invest. Published online 2016 Aug 15;126(9):3447–52. doi:https://doi.org/10.1172/JCI87324.
- Helmink BA, Reddy SM, Gao J, Zhang S, Basar R, Thakur R, Yizhak K, Sade-Feldman M, Blando J, Han G, et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature. 2020;577(7791):549–55. doi:https://doi.org/10.1038/s41586-019-1922-8.
- Neyt K, Perros F, GeurtsvanKessel CH, Hammad H, Lambrecht BN. Tertiary lymphoid organs in infection and autoimmunity. Trends Immunol. 2012;33(6):297–305. doi:https://doi.org/10.1016/j.it.2012.04.006.
- Coppola D, Mulé JJ. Ectopic lymph nodes within human solid tumors. J Clin Oncol. 2008;26(27):4369–70. doi:https://doi.org/10.1200/JCO.2008.17.6149.
- Liu H, Weber A, Morse J, Kodumudi K, Scott E, Mullinax J, Sarnaik AA, Pilon-Thomas S. T cell mediated immunity after combination therapy with intralesional PV-10 and blockade of the PD-1/PD-L1 pathway in a murine melanoma model. PLoS One. 2018;13(4):e0196033. doi:https://doi.org/10.1371/journal.pone.0196033.
- Thompson JF, Hersey P, Wachter E. Chemoablation of metastatic melanoma using intralesional Rose Bengal. Melanoma Res. 2008;18(6):405–11. doi:https://doi.org/10.1097/CMR.0b013e32831328c7.
- Thompson JF, Agarwala SS, Smithers BM, Ross MI, Scoggins CR, Coventry BJ, Neuhaus SJ, Minor DR, Singer JM, Wachter EA, et al. Phase 2 study of intralesional PV-10 in refractory metastatic melanoma. Ann Surg Oncol. 2015;22(7):2135–42. doi:https://doi.org/10.1245/s10434-014-4169-5.
- Campana L, Valpione S, Mocellin S, Sundararajan R, Granziera E, Sartore L, Chiarion-Sileni V, Rossi CR. Electrochemotherapy for disseminated superficial metastases from malignant melanoma. Br J Surg. Published online 2012;99(6):821–30. doi:https://doi.org/10.1002/bjs.8749.
- Belehradek M, Domenge C, Luboinski B, Orlowski S, Belehradek J, Mir LM. Electrochemotherapy, a new antitumor treatment. First clinical phase I-II trial. Cancer. 1993;72(12):3694–700. doi:https://doi.org/10.1002/1097-0142(19931215)72:12<3694::AID-CNCR2820721222>3.0.CO;2-2.
- Glass LF, Pepine ML, Fenske NA, Jaroszeski M, Reintgen DS, Heller R. Bleomycin-mediated electrochemotherapy of metastatic melanoma. Arch Dermatol. 1996;132(11):1353–57. doi:https://doi.org/10.1001/archderm.1996.03890350095015.
- Rudolf Z, Stabuc B, Cemazar M, Miklavcic D, Vodovnik L, Sersa G. Electrochemotherapy with bleomycin. The first clinical experience in malignant melanoma patients. Radiol Oncol. 1995;29:229–35.
- Serša G, Štabuc B, Čemažar M, Miklavčič D, Rudolf Z. Electrochemotherapy with cisplatin: clinical experience in malignant melanoma patients. Clin Cancer Res. 2000;6:863–67.
- Kunte C, Letulé V, Gehl J, Dahlstroem K, Curatolo P, Rotunno R, Muir T, Occhini A, Bertino G, Powell B, et al. Electrochemotherapy in the treatment of metastatic malignant melanoma: a prospective cohort study by inspect. Br J Dermatol. 2017;176(6):1475–85. doi:https://doi.org/10.1111/bjd.15340.
- Liu BL, Robinson M, Han Z-Q, Branston RH, English C, Reay P, McGrath Y, Thomas SK, Thornton M, Bullock P, et al. ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties. Gene Ther. 2003;10(4):292–303. doi:https://doi.org/10.1038/sj.gt.3301885.
- Johnson DB, Puzanov I, Kelley MC. Talimogene laherparepvec (T-VEC) for the treatment of advanced melanoma. Immunotherapy. 2015;7(6):611–19. doi:https://doi.org/10.2217/imt.15.35.
- Kohlhapp FJ, Kaufman HL. Molecular pathways: mechanism of action for talimogene laherparepvec, a new oncolytic virus immunotherapy. Clin Cancer Res. 2016;22(5):1048–54. doi:https://doi.org/10.1158/1078-0432.CCR-15-2667.
- Zamarin D, Holmgaard RB, Subudhi SK, Park JS, Mansour M, Palese P, Merghoub T, Wolchok JD, Allison JP. Localized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy. Sci Transl Med. 2014;6(226):226ra32. doi:https://doi.org/10.1126/scitranslmed.3008095.
- Toda M, Martuza RL, Rabkin SD. Tumor growth inhibition by intratumoral inoculation of defective herpes simplex virus vectors expressing granulocyte–macrophage colony-stimulating factor. Molecular Therapy. 2000;2(4):324–29. doi:https://doi.org/10.1006/mthe.2000.0130.
- Senzer NN, Kaufman HL, Amatruda T, Nemunaitis M, Reid T, Daniels G, Gonzalez R, Glaspy J, Whitman E, Harrington K, et al. Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. J Clin Oncol. 2009;27(34):5763–71. doi:https://doi.org/10.1200/JCO.2009.24.3675.
- Andtbacka RHI, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, Delman KA, Spitler LE, Puzanov I, Agarwala SS, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. 2015;33(25):2780–88. doi:https://doi.org/10.1200/JCO.2014.58.3377.
- Andtbacka RHI, Collichio F, Harrington KJ, Middleton MR, Downey G, Ohrling K, Kaufman HL. Final analyses of OPTiM: a randomized phase III trial of talimogene laherparepvec versus granulocyte-macrophage colony-stimulating factor in unresectable stage III–IV melanoma. J Immunother Cancer. 2019;7(1):7. doi:https://doi.org/10.1186/s40425-019-0623-z.
- Hofbauer GFL, Baur T, Bonnet M-C, Tartour E, Burg G, Berinstein NL, Dummer R. Clinical phase I intratumoral administration of two recombinant ALVAC canarypox viruses expressing human granulocyte-macrophage colony-stimulating factor or interleukin-2: the transgene determines the composition of the inflammatory infiltrate. Melanoma Res. 2008;18(2):104–11. doi:https://doi.org/10.1097/CMR.0b013e3282f702cf.
- Andtbacka R, Curti B, Kaufman H, Daniels GA, Nemunaitis JJ, Spitler LE, Hallmeyer S, Lutzky J, Schultz S, Whitman ED, et al. CALM study: a phase II study of an intratumorally delivered oncolytic immunotherapeutic agent, coxsackievirus A21, in patients with stage IIIc and stage IV malignant melanoma. J Clin Oncol. 2014;32(15_suppl):3031–3031. doi:https://doi.org/10.1200/jco.2014.32.15_suppl.3031.
- Chesney J, Puzanov I, Collichio F, Singh P, Milhem MM, Glaspy J, Hamid O, Ross M, Friedlander P, Garbe C, et al. Randomized, open-label phase II study evaluating the efficacy and safety of talimogene laherparepvec in combination with ipilimumab versus ipilimumab alone in patients with advanced, unresectable melanoma. J Clin Oncol. 2018;36(17):1658–67. doi:https://doi.org/10.1200/JCO.2017.73.7379.
- Ribas A, Dummer R, Puzanov I, VanderWalde A, Andtbacka RHI, Michielin O, Olszanski AJ, Malvehy J, Cebon J, Fernandez E, et al. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell. 2017;170(6):1109–1119.e10. doi:https://doi.org/10.1016/j.cell.2017.08.027.
- Andtbacka R, Dummer R, Gyorki D, Berger AC, Conry RM, Demidov LV, Chan E, Treichel S, Faries MB, Ross MI, et al. Interim analysis of a randomized, open-label phase 2 study of talimogene laherparepvec (T-VEC) neoadjuvant treatment (neotx) plus surgery (surgx) vs surgx for resectable stage IIIB-IVM1a melanoma (MEL). J Clin Oncol. 2018;36(15_suppl):9508–9508. doi:https://doi.org/10.1200/JCO.2018.36.15_suppl.9508.
- Davar D, Karunamurthy A, Hartman D. Phase II trial of neoadjuvant nivolumab (nivo) and intra-tumoral (IT) CMP-001 in high risk resectable melanoma (MEL): preliminary results. Presented at the: 2019 Annual Meeting of the Society for Immunotherapy of Cancer; 2019 Nov 6–10; National Harbor (MD).
- Liu T-C, Galanis E, Kirn D. Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress. Nat Clin Pract Oncol. 2007;4(2):101–17. doi:https://doi.org/10.1038/ncponc0736.
- Sun J, Gastman BR, McCahon L, Buchbinder EI, Puzanov I, Nanni M, Lewis JM, Carvajal RD, Singh-Kandah S, Desai AM, et al. Observational study of talimogene laherparepvec use in the anti-PD-1 era for melanoma in the US (COSMUS-2). Melanoma Manag. 2020;7(2):MMT41. doi:https://doi.org/10.2217/mmt-2020-0005.
- Daud A, DeConti R, Andrews S, Urbas P, Riker AI, Sondak VK, Munster PN, Sullivan DM, Ugen KE, Messina JL, et al. Phase I trial of interleukin-12 plasmid electroporation in patients with metastatic melanoma. J clin oncol. 2008;26(36):5896–903. doi:https://doi.org/10.1200/JCO.2007.15.6794.
- Algazi A, Bhatia S, Agarwala S, Molina M, Lewis K, Faries M, Fong L, Levine LP, Franco M, Oglesby A, et al. Intratumoral delivery of tavokinogene telseplasmid yields systemic immune responses in metastatic melanoma patients. Ann Oncol. 2020;31(4):532–40.
- Algazi A, Bhatia S, Agarwala S, Molina M, Lewis K, Faries M, Fong L, Levine LP, Franco M, Oglesby A et al. Intratumoral delivery of tavokinogene telseplasmid yields systemic immune responses in metastatic melanoma patients. Ann Oncol. 2020 Feb 1;31(4):532–40. doi:https://doi.org/10.1016/j.annonc.2019.12.008.
- Greaney SK, Algazi AP, Tsai KK, Takamura KT, Chen L, Twitty CG, Zhang L, Paciorek A, Pierce RH, Le MH, et al. Intratumoral plasmid IL12 electroporation therapy in patients with advanced melanoma induces systemic and intratumoral T-cell responses. Cancer Immunol Res. 2020;8(2):246–54. doi:https://doi.org/10.1158/2326-6066.CIR-19-0359.
- Bhatia S, Longino NV, Miller NJ, Kulikauskas R, Iyer JG, Ibrani D, Blom A, Byrd DR, Parvathaneni U, Twitty C, et al. Intratumoral delivery of plasmid interleukin-12 via electroporation leads to regression of injected and non-injected tumors in merkel cell carcinoma. Clin Cancer Res. 2020 Feb 1;26(3):598–607. doi:https://doi.org/10.1158/1078-0432.CCR-19-0972.
- Algazi AP, Twitty CG, Tsai KK, Le M, Pierce R, Browning E, Hermiz R, Canton DA, Bannavong D, Oglesby A, et al. Phase II trial of IL-12 plasmid transfection and PD-1 blockade in immunologically quiescent melanoma. Clin Cancer Res. Published online 2020 May 6;26(12):2827–37. doi:https://doi.org/10.1158/1078-0432.CCR-19-2217.
- Gajewski TF, Corrales L, Williams J, Horton B, Sivan A, Spranger S. Cancer immunotherapy targets based on understanding the T cell-inflamed versus non-T cell-inflamed tumor microenvironment. Adv Exp Med Biol. 2017;1036:19–31.
- Daud AI, Wolchok JD, Robert C, Hwu W-J, Weber JS, Ribas A, Hodi FS, Joshua AM, Kefford R, Hersey P, et al. Programmed death-ligand 1 expression and response to the anti-programmed death 1 antibody pembrolizumab in melanoma. J Clin Oncol. 2016;34(34):4102–09. doi:https://doi.org/10.1200/JCO.2016.67.2477.
- Ayers M, Lunceford J, Nebozhyn M, Murphy E, Loboda A, Kaufman DR, Albright A, Cheng JD, Kang SP, Shankaran V, et al. IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest. Published online 2017 June 26;127(8):2930–40. doi:https://doi.org/10.1172/JCI91190.
- Garris CS, Arlauckas SP, Kohler RH, Trefny MP, Garren S, Piot C, Engblom C, Pfirschke C, Siwicki M, Gungabeesoon J, et al. Successful anti-PD-1 cancer immunotherapy requires T cell-dendritic cell crosstalk involving the cytokines IFN-γ and IL-12. Immunity. 2018;49(6):1148–1161.e7. doi:https://doi.org/10.1016/j.immuni.2018.09.024.
- Weide B, Eigentler TK, Pflugfelder A, Leiter U, Meier F, Bauer J, Schmidt D, Radny P, Pföhler C, Garbe C, et al. Survival after intratumoral interleukin-2 treatment of 72 melanoma patients and response upon the first chemotherapy during follow-up. Cancer Immunol Immunother. 2011;60(4):487–93. doi:https://doi.org/10.1007/s00262-010-0957-3.
- Ray A, Williams MA, Meek SM, Bowen RC, Grossmann KF, Andtbacka RHI, Bowles TL, Hyngstrom JR, Leachman SA, Grossman D, et al. A phase I study of intratumoral ipilimumab and interleukin-2 in patients with advanced melanoma. Oncotarget. 2016;7(39):64390–99. doi:https://doi.org/10.18632/oncotarget.10453.
- Fierlbeck G, d’Hoedt B, Stroebel W, Stutte H, Bogenschütz O, Rassner G. Intralesional therapy of melanoma metastases with recombinant interferon-beta. Hautarzt. 1992;43:16–21.
- Retsas S, Leslie M, Bottomley D. Intralesional tumour necrosis factor combined with interferon gamma in metastatic melanoma. BMJ. 1989;298(6683):1290–91. doi:https://doi.org/10.1136/bmj.298.6683.1290.
- Sparano JA, Fisher RI, Sunderland M, Margolin K, Ernest ML, Sznol M, Atkins MB, Dutcher JP, Micetich KC, Weiss GR, et al. Randomized phase III trial of treatment with high-dose interleukin-2 either alone or in combination with interferon alfa-2a in patients with advanced melanoma. J Clin Oncol. 1993;11(10):1969–77. doi:https://doi.org/10.1200/JCO.1993.11.10.1969.
- Paul E, Muller I, Renner H, Bodeker R-H, Cochran AJ. Treatment of locoregional metastases of malignant melanomas with radiotherapy and intralesional interferon injection. Melanoma Res. 2003;13(6):611–17. doi:https://doi.org/10.1097/00008390-200312000-00011.
- Kirkwood J, Milhem M, Zakharia Y. Durable responses in anti-PD-1 refractory melanoma following intratumoral injection of toll-like receptor 9 (TLR9) agonist CMP-001, in combination with pembrolizumab. Presented at the: 2019 Annual Meeting of the Society for Immunotherapy of Cancer; 2019 Nov 6–10; National Harbor (MD).
- Diab A, Haymaker C, Bernatchez C, Andtbacka RHI, Shaheen M, Johnson D, Markowitz J, Puzanov I, Murthy R, Johnson DH, et al. Intratumoral (IT) injection of the TLR9 agonist tilsotolimod (IMO-2125) in combination with ipilimumab (ipi) triggers durable responses in PD-1 inhibitor refractory metastatic melanoma (rMM): results from a multicenter, phase I/II study. Ann Oncol. 2018;29:viii442. doi:https://doi.org/10.1093/annonc/mdy289.001.
- Samoylenko I, Korotkova OV, Zabotina T, Demidov LV. Intralesional anti-PD1 treatment in patients with metastatic melanoma: the pilot study. JCO. 2018;36(5_suppl):188–188. doi:https://doi.org/10.1200/JCO.2018.36.5_suppl.188.
- Sagiv-Barfi I, Czerwinski DK, Levy S, Alam IS, Mayer AT, Gambhir SS, Levy R. Eradication of spontaneous malignancy by local immunotherapy. Sci Transl Med. 2018;10:426. doi:https://doi.org/10.1126/scitranslmed.aan4488.
- Corrales L, McWhirter SM, Dubensky TW, Gajewski TF. The host STING pathway at the interface of cancer and immunity. J Clin Invest. 2016;126(7):2404–11. doi:https://doi.org/10.1172/JCI86892.
- Corrales L, Glickman LH, McWhirter SM, Kanne DB, Sivick KE, Katibah GE, Woo SR, Lemmens E, Banda T, Leong JJ, et al. Direct activation of STING in the tumor microenvironment leads to potent and systemic tumor regression and immunity. Cell Rep. 2015;11:1018–30.
- Sivick KE, Desbien AL, Glickman LH, Reiner GL, Corrales L, Surh NH, Hudson TE, Vu UT, Francica BJ, Banda T, et al. Magnitude of therapeutic STING activation determines CD8+ T cell-mediated anti-tumor immunity. Cell Rep. 2018;25(11):3074–3085.e5. doi:https://doi.org/10.1016/j.celrep.2018.11.047.
- Falahat R, Perez-Villarroel P, Mailloux AW, Zhu G, Pilon-Thomas S, Barber GN, Mulé JJ, et al. STING signaling in melanoma cells shapes antigenicity and can promote antitumor T-cell activity. Cancer Immunol Res. 2019;7(11):1837–48.
- Ribas A, Milhem M, Hoimes C, Amin A. Phase 1b/2, open label, multicenter, study of the combination of SD-101 and pembrolizumab in patients with advanced melanoma who are naïve to anti-PD-1 therapy. Presented at the: ASCO Annual Meeting; 2019; Chicago, IL.
- Taylor DR, Young K, Korrer MJ. The synergy of TLR and STING in cancer immunity. J Immunol. 2020;204:91.30–91.30.
- Tumeh PC, Hellmann MD, Hamid O, Tsai KK, Loo KL, Gubens MA, Rosenblum M, Harview CL, Taube JM, Handley N, et al. Liver metastasis and treatment outcome with anti-PD-1 monoclonal antibody in patients with melanoma and NSCLC. Cancer Immunol Res. 2017;5(5):417–24. doi:https://doi.org/10.1158/2326-6066.CIR-16-0325.
