ABSTRACT
A national immunization program using two doses of live attenuated varicella vaccine was introduced for children aged one to two years in Japan in October 2014. Varicella cases declined after 2014, and immunological status against varicella among vaccinated children changed in post-vaccination era. A retrospective observational study of anti-varicella antibody seroprevalence, varicella vaccination status, and history of varicella among 528 students in the first grade of elementary school was conducted. The percentage of students who received at least a single dose of varicella vaccination increased from 67% (187 of 279 students) in 2007–2008 to 91% (226 of 249 students) in 2017. Students with a history of varicella decreased from 114 of 279 (41%) in 2007–2008 to 48 of 249 (19%, P < .01) in 2017. Among them, the rate of breakthrough varicella after a single dose of vaccine in students with a history of varicella significantly increased from 38% (43 of 114 students) in 2007–2008 to 58% (28 of 48 students) in 2017 (P < .05). The antibody-positive rate significantly decreased from 50% among subjects without varicella zoster who received a single dose (95%CI: 41–58%) in 2007–2008 to 29% (95%CI: 21–38%) in 2017 (P < .01). The antibody-positive rate among students without varicella history who received two doses of vaccine was only 43% (95%CI: 32–55%) in 2017. The number of varicella infections and antibody-positive rate among students without history of varicella who received varicella vaccination decreased after the introduction of a national immunization program.
Introduction
Varicella is a common disease among children, causing characteristic skin lesions with accompanying fever. Recovery usually occurs within a week. Encephalitis, pneumonia, and disseminated complications of varicella can occur among infants and immunocompromised patients. The varicella zoster virus (VZV) is the varicella pathogen and the live attenuated VZV vaccine Oka strain is used to prevent VZV infection worldwide .Citation1 The conventional vaccination of children aged 12–18 months with a single dose of varicella vaccine was introduced in the USA in 1996, and for children aged 11–24 months in several European countries from the 2000s.Citation2–4 Varicella incidence decreased approximately 90% in the USA by 2005.Citation5 On the other hand, varicella among vaccinated children increased after introduction of a national immunization program (NIP) in the USA.Citation5,Citation6 Breakthrough varicella (BV) is defined as a wild-type varicella virus infection among vaccinated subjects that occurs more than 42 days after vaccination. BV basically causes milder symptoms than varicella in unvaccinated individuals. The effectiveness of a single dose of varicella vaccine against varicella was almost 80%, and a second dose of varicella vaccine given to children aged 4–6 years was introduced in the USA in 2006 .Citation3,Citation5–7 The decrease in varicella incidence from 2005 to 2006 to 2013–2014 was 84.6% in the USA.Citation8 Several European countries introduced a second dose of varicella vaccine later in the 2000s, and a marked reduction in the incidence of varicella was reported in these countries .Citation3,Citation9 The effectiveness of two doses of varicella vaccine against varicella was reported to be 92% .Citation7
Voluntary vaccination with the varicella vaccine was started from 1987 in Japan. Coverage of varicella vaccination was estimated at less than <40% and yearly outbreaks of varicella continued until to the 2010s in Japan.Citation10,Citation11 The Japanese government introduced a NIP with two doses of varicella vaccine manufactured by Biken in October 2014. The first dose was recommended for children aged 12-<36 months with the second dose given at 3–6 months interval after the first dose by the Japanese Pediatric Society, similar to the immunization policy in Germany .Citation3 Children aged 36-<60 months without varicella or varicella vaccine history were temporarily given a single dose of vaccine from Oct 2014 to Mar 2015 in Japan. Before the introduction of the NIP, 175,030 to 238,645 cases of varicella per year were reported in surveillance conducted by approximately 3,000 pediatric sentinel clinics and hospitals in Japan from 2010 to 2013.Citation12 The number of varicella cases decreased to 77,614 in 2015 and 65,383 in 2016 .Citation12
Varicella outbreaks occurred even in countries that adopted NIP with two doses of varicella vaccine. In 2013–2014, 55% of varicella patients received at least a single dose of varicella vaccine in the USA .Citation8 In cases of varicella among vaccinated patients, 39% of them had received a single dose of varicella vaccine, and 56% of them had received two doses.Citation8 A total of 29 outbreaks of varicella were reported during 2012–2015 in several jurisdictions of the USA.Citation13 In large outbreaks (more than 8 cases per an outbreak), 55% of varicella patients were unvaccinated, and 16% and 18% of patients were vaccinated with a single or two doses of vaccine .Citation13 In small outbreaks (less than 7 cases per an outbreak), 33% of varicella patients were unvaccinated, and 17% and 39% of patients were vaccinated with one or two doses of vaccine.Citation13 In those outbreaks, 55% of the patients were 5–9 years old, and 26% were 10–14 years old.Citation13 The immunity induced by a single dose of varicella vaccine decreased with the time after vaccination; however, the duration of immunity induced by two-dose vaccination is still unclear .Citation14
Japan was the first country to introduce a NIP with two doses of varicella vaccine from the outset. The epidemiology of varicella in Japan has changed since 2014. The present study aimed to clarify the changes in susceptibility to varicella among Japanese children after the introduction of the NIP.
Materials and methods
Subjects
We conducted a retrospective observational study on elementary school students in the capital area of Japan to investigate the immunization history (varicella vaccination), anti-VZV IgG antibodies, and varicella infection before and after the introduction of the NIP. The elementary school students were tested for anti-viral antibodies to measles, rubella, chickenpox, and mumps in school health checks in the first grade to prevent outbreaks of those viral diseases in elementary schools, and any additional supplemental vaccinations were recommended by school doctors. Written informed consent for blood examinations were obtained from parents or guardians.
The subjects of the current study were the students admitted to a private elementary school in Tokyo city in 2007, 2008, and 2017, and a private elementary school in Yokohama city in 2017 (). The elementary school in Tokyo city had 144 students in each grade, and that in Yokohama city had 108 students in each grade. There were 540 candidate subjects and of these, 528 students were enrolled in the present study. Parents of students were required to submit history of varicella and vaccination history for the routine health checks upon admission to elementary schools, at subsequent annual health checks and having varicella in schools according Japanese low and code of the schools. Titers of anti-VZV IgG antibodies, history of varicella vaccination, and history of varicella infection were extracted from school records. The present retrospective study included a cross-sectional study about varicella status among the students of the elementary schools in 2007–2008 and 2017, and a longitudinal study from admission to graduation of the school among the students admitted the elementary school in 2007 and 2008.
Table 1. Number of students
Serological examination
Serum samples from the students admitted to elementary schools in April of 2007 and 2008 were examined at the Kitasato Institute for Life Sciences, and those from the students admitted to elementary schools in April of 2017 were examined in a commercial laboratory test center (SRL, Japan). Anti-VZV IgG antibodies were measured by enzyme immunoassay (anti-VZV EIA antibody SEIKEN, DENKA SEIKEN, Tokyo, Japan) according to the manufacturer’s instructions. Standard sera were provided from DENKA SEIKEN (anti-VZV antibody control SEIKEN, DENKA SEIKEN, Tokyo, Japan). The absorbance of tested sera higher than that of a positive control was considered positive, and below that of the positive control was considered negative.
Definitions
BV in the present study was defined as clinically diagnosed VZV infection occurring more than 42 days after the vaccination. Prevalence ratio of varicella at admission to elementary school was evaluated .Citation15
Statistical analysis
Statistical analysis was performed using SPSS version 24 (SPSS Inc., Chicago, Illinois, United States). Age when students were infected with varicella was evaluated by the Kruskal–Wallis test with a Bonferroni correction. The positive rate of anti-VZV antibodies was evaluated by 95% confidence intervals (95% CI) and the chi-square test or Fisher’s exact test. VE was evaluated with 95% CI. Values of corrected P < .05 were considered significant.
Ethical approval
This retrospective observational study was carried out using the opt-out method. The study protocol was approved by the Research Ethics Committee of Keio University (approval No.:17–006).
Results
History of varicella and vaccination
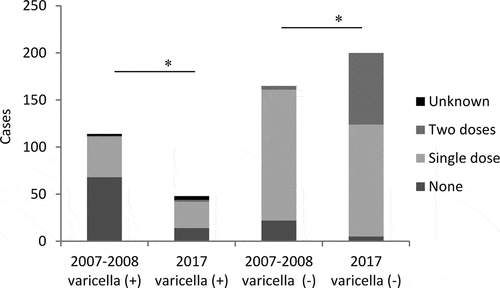
The number of students with a history of varicella decreased from 114/279 (41%) in 2007–2008 to 48/249 (19%, P < .01) in 2017 (). The number of students with a history of varicella without vaccination decreased from 68/114 (60%) in 2007–2008 to 14/48 (29%, P < .01) in 2017. The ratio of BV after a single dose vaccination increased from 43/114 (38%) in 2007–2008 to 28/48 (58%, P < .05) in 2017. The number of students developed BV after two doses of vaccine were 1/5 in 2007–2008 and 2/78 in 2017. Students who had a history of varicella within 6 weeks after varicella vaccination were 2/279 in 2007–2008 and 4/249 in 2017. The number of students who received a single dose of varicella vaccine without BV was 139 in 2007–2008, and 119 in 2017. The number of students who received two doses of varicella vaccine without BV was 4/279 in 2007–2008, and 76/249 in 2017. Only one student in 2017 received three doses of varicella vaccine. Twenty-two students in 2007–2008 and five in 2017 had no history of vaccination or natural infection.
Figure 1. History of varicella infection before admission to elementary school in students who received varicella vaccination and those who did not. Total number were 279 in 2007–2008, and 249 in 2017. The number of students with a history of varicella decreased from 114 (41%) in 2007–2008 to 48 (19%) in 2017. Six students had a history of varicella within six weeks after varicella vaccination were defined as unknown
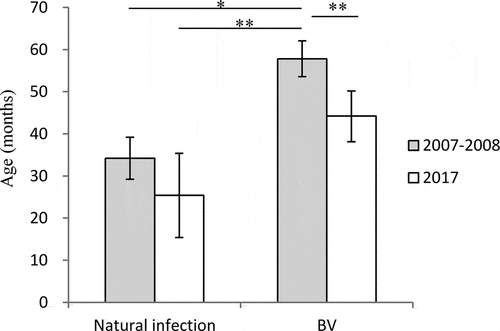
The mean age of students infected with varicella is shown in . The mean age of students with natural varicella infection was 34 months (95%CI: 29–39), and was 58 months (95%CI: 54–62) for BV after a single dose of vaccine in 2007–2008. The mean age for natural infection was 25 months (95%CI: 15–35), and was 44 months (95%CI: 38–50) for BV after a single dose of vaccine in 2017.
Prevalence of varicella and vaccination status
The vaccination doses and prevalence of varicella are shown in . Two students in 2007–2008 with a history of varicella before varicella vaccination were considered no vaccination. Two students in 2007–2008 and four in 2017 with a history of varicella within 6 weeks after varicella vaccination, and one in 2017 who received three doses of varicella vaccine were excluded from . The number of students who did not receive the vaccination decreased from 90/279 (32%, 95%CI: 27–38%) in 2007–2008 to 19/249 (7.6%, 95%CI: 4.7–12%, P < .01) in 2017. Those receiving a single dose vaccination accounted for 182/279 (65%, 95%CI: 59–71%) in 2007–2008 and 147/249 (59%, 95%CI: 53–65%, P < .01) in 2017. Students who received two vaccinations increased from 5/279 (1.8%, 95%CI: 0.6–4.1%) in 2007–2008 to 78/249 (31%, 95%CI: 26–38%, P < .01) in 2017. Prevalence ratio of varicella for a single dose to that in non-immunized group was 0.313 (95%CI: 0.235–0.416, P < .01) in 2007–2008, and 0.259 (95%CI: 0.168–0.397, P < .05) in 2017, and that of two doses was 0.265 (95%CI: 0.046–1.534, P < .05) in 2007–2008, and 0.035 (95%CI: 0.009–0.140, P < .01) in 2017.
Table 2. Vaccine effectiveness pre- and post- introduction of national immunization program
Serological status
The positive rates of IgG-EIA are shown in . Six students had a history of varicella within 6 weeks after vaccination, another six students received the vaccination after varicella infection, and one student who received three vaccinations were excluded from analysis. Antibody-positive rates among students with a varicella history without vaccination were 66/66 (100%, 95%CI: 95–100%) in 2007–2008, and 10/10 (100%, 95%CI: 69–100%) in 2017. In contrast, antibody-positive rates among students without a varicella history or vaccination were 12/22 (55%, 95%CI: 32–76%) in 2007–2008, and 0/5 (0%, 95%CI: 0–52%, P < .05) in 2017). Antibody-positive rates among students with a varicella history after administration of a single dose of vaccine were 40/43 (93%, 95%CI: 81–99%) in 2007–2008, and 22/28 (79%, 95%CI: 59–92%) in 2017. These were higher than among students without a varicella history who received a single dose of vaccine, and were 69/139 (50%, 95%CI: 41–58%, P < .01) in 2007–2008, and 34/119 (29%, 95%CI: 21–38%, P < .01) in 2017. In 2017, the antibody-positive rate among students without a varicella history who received a single dose of vaccine was 34/119 (29%, 95%CI: 21–38%), and that of students without a varicella history who received two doses of vaccine was 33/76 (43%, 95%CI: 32–55%, P < .05).
Table 3. Positivity for anti-VZV IgG antibodies and vaccination doses
Varicella infection in elementary schools
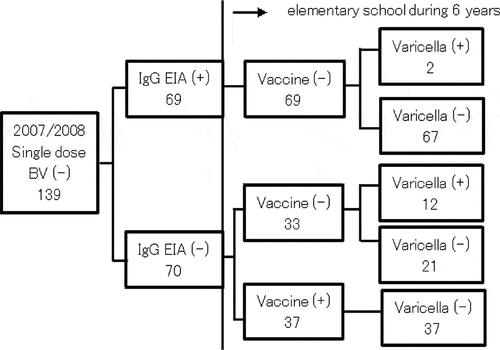
Varicella infection rates after admission to elementary school in students who received a single dose of vaccine upon admission in 2007 and 2008 are shown in . A total of 139 students in 2007–2008 who received a single dose of vaccine were without BV at admission to elementary school. IgG-EIA was positive in 69 students, and negative in 70 students. BV was observed in two among 69 students with positive antibodies, and 12 in 33 students without antibodies did not receive an additional vaccination. Thirty-seven students received second dose of vaccine after the examination of IgG-EIA. The median of distance from blood examination to having an additional vaccination was 3 months (interquartile range two to 5 months), and the students having an additional vaccination until 6 months after blood examination were 30/37. On the other hand, 37 students without antibodies received an additional vaccination after entering elementary school, and did not develop BV.
Discussion
Varicella vaccination was introduced in a NIP in October 2014 in Japan. In the present study, vaccination coverage with at least a single dose of vaccine increased for students from 2007 to 2008 to 2017. Only 31% of students received two doses of varicella vaccine in 2017, and students with a history of varicella decreased after introduction of the NIP. Students in 2017 were born between April 2010 and March 2011, and they were not the targeted age of two doses varicella vaccination of the NIP. All students in the first grade of elementary school will receive two doses of varicella vaccine in 2020, and after that, the number of children infected with varicella will markedly decrease in Japan.
In the present study, the mean age of natural infection with varicella was 34 months in 2007–2008, and 9 months earlier after the introduction of the NIP. That of BV after a single dose of varicella vaccine was 58 months in 2007–2008, and 14 months earlier after the introduction of the NIP. Several reports referred about the possibility of varicella age shift after the NIP.Citation9,Citation16 On the other hand, varicella vaccination also had indirect protection to the people without vaccination. In the USA, the reduction rate of varicella incidence from 2005 to 2006 to 2013–2014 was 76.8% for <1 year of age, 58.0% for 1–4 years, 89.3% for 5–9 years, 84.8% for 10–14 years, 35.0% for 15–19 years, and 25.0% for ≥20 years.Citation8 In Germany, the number of cases per medical practice per month decreased from 3.47 in 2005 to 0.43 in 2013, and a sharp decline of 90–94% was noted among 1–4 years-old children.Citation17 In Japan, varicella vaccination was introduced in a NIP for children 1–3 years of age, similar to the age in Germany, in 2014 and a single dose of varicella vaccine was introduced provisionally for those 3–4 years of age from Oct. 1, 2014 to Mar. 31, 2015. Japanese children over 9 years of age on Oct. 1, 2018 were not included in the NIP, and therefore herd immunity against varicella is not strong enough. Varicella vaccine effectiveness was 81.9% for one dose and 94.4% for two doses in Germany.Citation18 At least a single dose of varicella vaccine is necessary for school-aged children older than 10 years of age to prevent severe varicella infection, while the effectiveness of one dose of varicella vaccine decreased from 93% after first year since vaccination to 61% after the third year .Citation7,Citation19 The present study revealed an additional vaccination among elementary school students having single-dose vaccination was protected varicella infection in the school.
In the present study, the positive rate of anti-varicella IgG-EIA antibodies among students without a varicella history who received a single dose of varicella vaccine decreased in 2017 compared to 2006–2007, and that of antibodies among students who received two doses of varicella vaccine in 2017 was only 43%. On the other hand, the number of varicella cases decreased after the introduction of the NIP. One reason for this is likely because the reduction in contact with VZV weakened the boost of immunity induced initially by varicella vaccination and infection .Citation20–22 Recently, over-attenuation of immunogenicity of the Japanese varicella vaccine was suspected by some researchers. The vaccine used in Japan contains over 1000 PFU/dose of VZV. Kamiya et al.Citation23 reported that the mean viral titer of current varicella vaccines was 42,000–67,000 PFU/dose, the minimum titer was 23,000 PFU/dose and the maximum titer was 95,000 PFU/dose. Despite the high viral titer contained in the current vaccines, the seroconversion rate in the immune adherence hemagglutination test decreased from 93.6% in 1987–1997 to 76.2% in 2010–2011 .Citation23,Citation24 Ozaki et al.Citation25 reported that the seroconversion rate among people vaccinated with 0.1 ml of varicella vaccine containing 2600–6400 PFU was 25%. The reason for the reduction of immunogenicity of the varicella vaccine was unclear. Further epidemiological study is needed to assess for presence of immunogenicity of Japanese varicella vaccine.
The present study has some limitations. History of varicella vaccination and varicella were taken from guardian’s memory. In Japan, Maternal and Child Health Handbook was widely used to record the history of vaccination and viral infections. Parents of students were requested to submit the records of vaccination and history of major infectious diseases including varicella before and after admission to elementary school. Students with varicella was required to submit the medical certificate to the elementary schools.
The formal definition of BV was varicella caused by wild type strain that occurred more than 42 days after varicella vaccination. Varicella was diagnosed by clinical examination, not laboratory examination, in Japan. Watanabe et al.Citation26 reported that clinically diagnosed 11 of 42 BV cases in Japan was not caused by VZV. On the other hand, incorrect negative history among students without vaccination in 2007–2008 was 55% in the present study. Asymptomatic infection of varicella was possible to occur and mild varicella was occasionally misdiagnosed. Kavaliotis et al. reported that incorrect negative varicella history without vaccination ranged from 6% among 13–60 months of age to 48% among 120–168 months of age .Citation27
Humoral and cellular immunity are important in the prevention of varicella infection.Citation28 Anti-VZV IgG was measured in the present study. Ludwig et al.Citation29 reported that those vaccinated without anti-VZV IgG antibodies had cellular immunity. A total of 87% of children had cellular immunity 5 years after varicella vaccination.Citation30 The cellular immunity of students without anti-VZV IgG antibodies in the present study was not examined.
Anti-VZV IgG antibodies were detected by enzyme immunoassay (EIA) in the present study. The sensitivity of several commercial ELISA kits was lower than the fluorescent-antibody-to-membrane-antigen (FAMA) assay and glycoprotein-based enzyme immunoassay (gpELISA) .Citation31,Citation32 Otani et al.Citation33 reported that the positivity of EIA (anti-VZV EIA antibody SEIKEN) was similar to FAMA and gpELISA. IgG-EIA in the present study was measured by two different laboratories. EIA (anti-VZV EIA antibody SEIKEN) kit was standardized by manufacturer.
The lot numbers of varicella vaccine administered to students were not obtained in the present study. Kamiya et al.Citation23 reported that viral titers of varicella vaccines were not stable. Many lots of varicella vaccine were used in the periods investigated in the current study, and the influence of the viral titer of each varicella vaccine lot was unclear.
In conclusion, varicella vaccination coverage increased after the introduction of the NIP, and children with a history of varicella infection decreased in Japan. In addition, the positive rate of anti-varicella IgG-EIA antibodies among vaccinated children decreased after the introduction of the NIP. Catch up varicella vaccination to the school-age children without adaptation of the NIP decreased varicella infection in the school. The change of epidemiology of varicella after introduction of the NIP needs to be observed carefully to prevent varicella infection.
Disclosure of potential conflicts of interest
The corresponding author, TN, received research funds from Daiichi Sankyo Pharmaceutical Company, Tokyo, Japan. All other authors report no potential conflicts.
Acknowledgments
The authors acknowledge all participants in the study and all the staff at Health Center, Keio University.
Author contributions
YY conducted the data collection, analysis, and manuscript preparation. TM, FA, KU, MI, MT conducted the data collection and interpretation. YY and TN designed the study. YY performed the statistical analysis. TN conducted the data interpretation and manuscript preparation.
References
- Takahashi M, Otsuka T, Okuno Y, Asano Y, Yazaki T, Isomura S. Live vaccine used to prevent the spread of varicella in children in hospital. Lancet. 1974;304:1288–90. doi:10.1016/S0140-6736(74)90144-5.
- Marin M, Güris D, Chaves SS, Schmid S, Seward JF. Prevention of varicella: recommendations of the advisory committee on immunization practices (ACIP). MMWR Recomm Rep. 2007;56:1–40.
- European Centre for Disease Prevention and Control. Varicella vaccination in the European Union. 2015 Feb 4. [accessed 2018 Feb 5]. https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/Varicella-Guidance-2015.pdf.
- National Centre for Immunisation Research & Surveillance. Varicella-zoster (chickenpox) vaccines for Australian children. National Centre for Immunisation Research & Surveillance Fact sheet. 2015 Jul [accessed 2018 Feb 5]. http://www.ncirs.edu.au/assets/provider_resources/fact-sheets/varicella-fact-sheet.pdf.
- Guris D, Jumaan AO, Mascola L, Watson BM, Zhang JX, Chaves SS, Gargiullo P, Perella D, Civen R, Seward JF. Changing varicella epidemiology in active surveillance sites–United States, 1995-2005. J Infect Dis. 2008;197(Suppl 2):S71–5. doi:10.1086/522156.
- Lopez AS, Guris D, Zimmerman L, Gladden L, Moore T, Haselow DT, Loparev VN, Schmid DS, Jumaan AO, Snow SL. One dose of varicella vaccine does not prevent school outbreaks: is it time for a second dose? Pediatrics. 2006;117:e1070–7. doi:10.1542/peds.2005-2085.
- Marin M, Marti M, Kambhampati A, Jeram SM, Seward JF. Global varicella vaccine effectiveness: a meta-analysis. Pediatrics. 2016;137:e20153741. doi:10.1542/peds.2015-3741.
- Lopez AS, Zhang J, Marin M. Epidemiology of varicella during the 2-dose varicella vaccination program —United States, 2005–2014. MMWR Morb Mortal Wkly Rep. 2016;65:902–05. doi:10.15585/mmwr.mm6534a4.
- Varela FH, Pinto LA, Scotta MC. Global impact of varicella vaccination programs. Hum Vaccin Immunother. 2019;15:645–57. doi:10.1080/21645515.2018.1546525.
- Yoshikawa T, Kawamura Y, Ohashi M, Yoshikawa A, Ihira M, Yahata Y, Kamiya H, Tanaka-Taya K, Yoshikawa T. Universal varicella vaccine immunization in Japan. Vaccine. 2017;35:4936–41. doi:10.1016/j.vaccine.2017.07.090.
- Ozaki T, Asano Y. Development of varicella vaccine in Japan and future prospects. Vaccine. 2016;34:3427–33. doi:10.1016/j.vaccine.2016.04.059.
- National institute of infectious diseases. Survey of infectious disease. [accessed 2018 Feb 5]. https://www.niid.go.jp/niid/ja/survei/2085-idwr/ydata/7314-report-jb2016.html.
- Lopez AS, LaClair B, Buttery V, Zhang Y, Rosen J, Taggert E, Robinson S, Davis M, Waters C, Thomas CA, et al. Varicella outbreak surveillance in schools in sentinel jurisdictions, 2012–2015. J Pediatric Infect Dis Soc. 2019;8:122–27. doi:10.1093/jpids/piy010.
- Thomas CA, Shwe T, Bixler D, Del Rosario M, Grytdal S, Wang C, Haddy LE, Bialek SR. Two-dose varicella vaccine effectiveness and rash severity in outbreaks of varicella among public school students. Pediatr Infect Dis J. 2014;33:1164–68. doi:10.1097/INF.0000000000000444.
- Tamhane AR, Westfall AO, Burkholder GA, Cutter GR. Prevalence odds ratio versus prevalence ratio: choice comes with consequences. Stat Med. 2016;35:5730–35. doi:10.1002/sim.7059.
- Spoulou V, Alain S, Gabutti G, Giaquinto C, Liese J, Martinon-Torres F, Vesikari T. Implementing universal varicella vaccination in Europe: the path forward. Pediatr Infect Dis J. 2019;38:181–88. doi:10.1097/INF.0000000000002233.
- Hecht J, Siedler A. The epidemiology of varicella disease in Germany after introduction of a vaccination recommendation: analysis of mandatory and sentinel data between 2002 and 2014. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2017;60:118–26. doi:10.1007/s00103-016-2475-8.
- Rieck T, Feig M, An der Heiden M, Siedler A, Wichmann O. Assessing varicella vaccine effectiveness and its influencing factors using health insurance claims data, Germany, 2006 to 2015. Euro Surveill. 2017;22:30521. doi:10.2807/1560-7917.ES.2017.22.17.30521.
- Cenoz MG, Martínez-Artola V, Guevara M, Ezpeleta C, Barricarte A, Castilla J. Effectiveness of one and two doses of varicella vaccine in preventing laboratory-confirmed cases in children in Navarre, Spain. Hum Vaccin Immunother. 2013;9:1172–76. doi:10.4161/hv.23451.
- Marinelli I, van Lier A, de Melker H, Pugliese A, van Boven M. Estimation of age-specific rates of reactivation and immune boosting of the varicella zoster virus. Epidemics. 2017;19:1–12. doi:10.1016/j.epidem.2016.11.001.
- Gershon AA 1, LaRussa P, Steinberg S, Mervish N, SH L, Meier P. The protective effect of immunologic boosting against zoster: an analysis in leukemic children who were vaccinated against chickenpox. J Infect Dis. 1996;173:450–53. doi:10.1093/infdis/173.2.450.
- Vossen MT, Gent MR, Weel JF, de Jong MD, van Lier RA, Kuijpers TW. Development of virus-specific CD4+ T cells on reexposure to Varicella-Zoster virus. J Infect Dis. 2004;190:72–82. doi:10.1086/421277.
- Kamiya H, Asano Y, Ozaki T, Baba K, Kumagai T, Nagai T, Shiraki K. Varicella vaccine potency and stability during transport and delivery. Kansenshogaku Zasshi. 2011;85:161–65. doi:10.11150/kansenshogakuzasshi.85.161.
- Ozaki T, Nishimura N, Kajita Y. Experience with live attenuated varicella vaccine (Oka strain) in healthy Japanese subjects; 10-year survey at pediatric clinic. Vaccine. 2000;18:2375–80. doi:10.1016/S0264-410X(00)00016-5.
- Ozaki T, Nisimura N, Gotoh K, Funahashi K. A study for the necessity of virus titer of varicella vaccine presently used. Kansenshogaku Zashi. 2012;86:749–54. doi:10.11150/kansenshogakuzasshi.86.749.
- Watanabe M, Ochiai H, Ito M, Negoro M, Suga S, Ihara T. Laboratory diagnosis of breakthrough varicella in children. Pediatr Infect Dis J. 2017;36:560–63. doi:10.1097/INF.0000000000001475.
- Kavaliotis J, Petridou S, Karabaxoglou D. How reliable is the history of chickenpox? Varicella serology among children up to 14 years of age. Int J Infect Dis. 2003;7:274–77. doi:10.1016/S1201-9712(03)90106-8.
- Arvin AM. Varicella-zoster virus. Clin Microbiol Rev. 1996;9:361–81. doi:10.1128/CMR.9.3.361.
- Ludwig B, Kraus FB, Allwinn R, Keim S, Doerr HW, Buxbaum S. Loss of varicella zoster virus antibodies despite detectable cell mediated immunity after vaccination. Infection. 2006;34:222–26. doi:10.1007/s15010-006-5616-9.
- Zerboni L, Nader S, Aoki K, Arvin AM. Analysis of the persistence of humoral and cellular immunity in children and adults immunized with varicella vaccine. J Infect Dis. 1998;177:1701–04. doi:10.1086/517426.
- Sauerbrei A, Schäfler A, Hofmann J, Schacke M, Gruhn B, Wutzler P. Evaluation of three commercial varicella-zoster virus IgG enzyme-linked immunosorbent assays in comparison to the fluorescent-antibody-to-membrane-antigen test. Clin Vaccine Immunol. 2012;19:1261–68. doi:10.1128/CVI.00183-12.
- Sauerbrei A, Wutzler P. Serological detection of varicella-zoster virus-specific immunoglobulin G by an enzyme-linked immunosorbent assay using glycoprotein antigen. J Clin Microbiol. 2006;44:3094–97. doi:10.1128/JCM.00719-06.
- Otani N, Tanaka M, Maeda K, Gomi Y, Nakajima K, Tanimura S, Takesue Y, Shima M, Okuno T. Varicella zoster virus antibody detection: a comparison of four commonly used techniques. J Infect Chemother. 2016;22:225–28. doi:10.1016/j.jiac.2015.12.018.