ABSTRACT
By preventing infectious diseases, vaccines contribute substantially to public health. Besides, they offer great opportunities to investigate human immune responses. This is particularly true for live-attenuated virus vaccines which cause resolving acute infections and induce robust immunity. The fact that one can precisely schedule the time-point of vaccination enables complete characterization of the immune response over time, short-term and over many years. The live-attenuated Yellow Fever virus vaccine strain YF-17D was developed in the 1930’s and gave rise to the 17D-204 and 17DD vaccine sub-strains, administered to over 600 million individuals worldwide. YF vaccination causes a systemic viral infection, which induces neutralizing antibodies that last for a lifetime. It also induces a strong T cell response resembling the ones of acute infections, in contrast to most other vaccines. In spite of its use since 1937, learning how YF vaccination stimulates such strong and persistent immune responses has gained substantial knowledge only in the last decades. Here we summarize the current state of knowledge on the immune response to YF vaccination, and discuss its contribution as a human model to address complex questions on optimal immune responses.
1. The live-attenuated vaccine sub-strains 17D-204 and 17DD
Yellow Fever (YF) disease is caused by the Yellow Fever Virus (YFV) transmitted by mosquitoes belonging to the Aedes,Citation1,Citation2 Haemagogus, and Sabethes generaCitation3,Citation4 and is endemic to sub-Saharan African regions as well as tropical and subtropical regions of South America.Citation1,Citation5 YFV infection can cause subclinical to severe illness with acute hemorrhagic disease, including fever, hemorrhagic shock and multi-organ failure of liver, kidneys and heart.Citation3 While a majority of infected people develop no or only minor symptoms, an estimated 1 in 7 infected people enter a toxic phase, over which half of them do not survive.Citation6,Citation7 The liver is a major target organ, and liver dysfunction results in jaundice, hence the name “Yellow Fever”. YFV is the prototype virus of the family of flaviviridae. YFV is a single-stranded, positive-sense RNA virus that varies in size between 40 and 60 nm. The virus consists of three structural proteins (core C, membrane M and envelope E), and seven non-structural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B and NS5) that are necessary for viral replication. The virus particle is made of the genome (approximately 10ʹ800 nucleotides) surrounded by C protein, and the viral proteins (M- and E-proteins) embedding the virus envelope.Citation1,Citation5
There is no antiviral therapy to treat the disease but prophylaxis is efficient thanks to the vaccine strains 17D-204 and 17DD, live-attenuated viruses that are considered as highly efficient vaccines. The original 17D strain was developed in 1937 by Max Theiler and colleagues.Citation8 The virus was isolated from a cured African patient and passaged 176 times in mouse and chicken tissue. This process led to viral attenuation while maintaining the immunogenicity, giving rise to this highly efficient vaccine. This discovery was awarded with the Nobel Prize in Physiology or Medicine in 1951. Two sub-strains are currently used for vaccine production: 17D-204 and 17DD, originating from the 17D strain. These vaccine sub-strains show only subtle nucleotide variations (ca. 99.9% nucleotide sequence identity).Citation9,Citation10 The mutations observed in the gene encoding the E protein are thought to have a role in attenuation.Citation9,Citation11,Citation12 Both 17D-204 and 17DD sub-strains are regularly used and provide efficient protection against the disease.Citation13 To simplify, we use the short term “17D” whenever we mean the 17D-204, 17DD, or both vaccines.
In clinical practice, many vaccines that are made from viruses are inactivated vaccines (e.g. polio and influenza vaccines) which do not replicate in vivo. Even many live-attenuated viral strains (such as measles and oral polio vaccines) show usually only limited replication.Citation14,Citation15 In contrast, the live-attenuated YF-17D vaccine replicates substantially and therefore causes a systemic viral infection and is strongly immunogenic because the immune system has evolved to react to microbial invasion and multiplication.Citation16–20
The YF-17D vaccine represents an enormous success in terms of protection against Yellow Fever.Citation21 In addition to its exceptional efficacy, the YF-17D vaccine has an acceptable safety record. Viscerotropic and neurotropic serious adverse events were observed after YF vaccination and were defined as YF-17D vaccine-associated neurotropic and viscerotropic disease (YEL-AND and YEL-AVD, respectively).Citation22,Citation23 These events occur rarely, and there exist large differences in the quality of surveillance systems, making it difficult to report exact rates (approximatively 0.3 and 0.8 cases per 100ʹ000 doses for YEL-AVD and YEL-AND, respectively).Citation24–26 Unfortunately, these adverse events are often severe and may even be lethal.Citation27–30 For the development of novel vaccines, this type of risk moderates the risk–benefit balance toward avoidance of the use of live-attenuated viruses, in favor of synthetic vaccines. In the case of YF, the vaccine benefit is very high and synthetic vaccine alternatives are not available to date.
2. Immune responses to primary vaccination with YF-17D
In this section, we highlight the major findings on the cellular and humoral immune responses to YF-17D vaccination. We examine both the innate and the adaptive arms of the immune response, as summarized in . Given the scarcity of animal models for YFV immunobiology,Citation31,Citation32 the evidence on YF-17D vaccination largely originates from human studies (Supplemental Table 1).
Figure 1. Overview of the immune responses to YF-17D vaccination. Kinetics of viral replication and the innate & specific immune response, illustrating the acute response and the long-term persistence of immune memory consisting of neutralizing antibodies and memory T cells
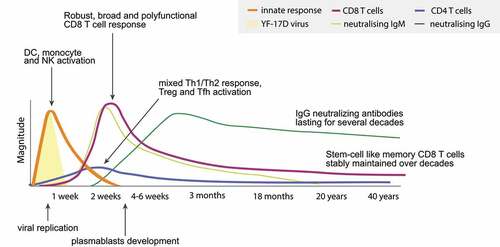
2.1. Innate immunity
2.1.1. Dendritic cells (DCs)
DCs are major professional antigen-presenting cells (APCs) inducing adaptive immunity.Citation33 Two major subsets of DCs have been identified: conventional DCs (cDCs) and plasmacytoid DCs (pDCs).
In vitro analysis showed that YF-17D is able to infect DCs and to activate various subsets of DCs via multiple Toll-Like Receptors (TLRs), including TLR2, 7, 8 and 9.Citation34–36 Infection of DCs seems to allow antigen processing and presentation.Citation35 In addition, YFV was shown to induce the secretion of type I and III IFNs from pDCs upon TLR7 ligation or cell contact.Citation37 It was hypothesized that the YF-17D vaccine contains sufficient amounts of individual TLR ligands, producing synergistically broad and polyvalent immune responses.Citation34 The frequency of circulating pDCs (CD123+) is transiently and significantly increased at day 7 post-vaccination (approximatively from 1% to 5%), while no changes were observed for the frequency of cDCs (CD11c+).Citation38 However, the latter are activated, rising to a peak at day 7 of CD11c+ HLA-DR+ DCs in peripheral blood.Citation39
2.1.2. Monocytes and macrophages
Monocytes are rapidly recruited to infected and inflamed tissues, where they differentiate into DCs and macrophages.Citation40 The percentage of macrophage-like (CD14+ CD16+) and activated monocytes (CD14+ CD16++) are slightly but significantly increased at day 7 post-vaccination with YF-17D compared to baseline (approximatively from 10% to 17% and 2.5% to 5%, respectively).Citation41 Activation of total monocytes is observed, as shown by the up-regulation of the activation marker CD86.Citation20 In addition, TNFα+ monocytes are increased at day 7 compared to baseline and are maintained over 30 days. Also, the frequency of IL-10+ monocytes was found to be increased at day 15 compared to baseline.Citation42
Macrophages are large phagocytes and are able to act as APCs.Citation40 One study showed that YF-17D is able to infect macrophages in vitro.Citation43,Citation44 Infection of macrophages might serve as a vehicle for dispersion of the virus to lymphoreticular tissues, where viral replication takes place.Citation44 Otherwise very little is known about macrophages in the context of YF-17D vaccination.
2.1.3. ILC
Innate lymphoid cell (ILC) is the collective term for a group of lymphoid cells lacking rearranged antigen-specific receptors of which the natural killer (NK) cells are the most well characterized.Citation45
It was shown that vaccination with YF-17D induces a robust NK cell response with increased expression of several markers such as Ki-67, CD69 and HLA Class II molecules.Citation18,Citation20,Citation46,Citation47 NK cells producing IFNγ and showing cytotoxic activity are also increased.Citation38,Citation42 There is no substantial change in NK cell numbers and NK subsets after YF-17D vaccination.Citation20 So far, only one study characterized the other known ILC subsets in the context of YF-17D vaccination, revealing that their total numbers transiently decreased 7 days after primary vaccination.Citation20
2.1.4. Granulocytes
Granulocytes have granules containing anti-microbicidal agents and are capable of ingesting foreign cells.Citation48 There are three types of granulocytes: neutrophils, eosinophils, and basophils.
Evidence shows that, whereas no changes in the percentages of circulating granulocytes are observed, both neutrophils and eosinophils are activated upon YF-17D vaccination.Citation41 Along the same line, TNFα+ neutrophils were increased at day 7 post-vaccination compared to baseline.Citation42 To our knowledge, there are no reports on basophils in the context of YF-17D vaccination. Overall, there are only few reports investigating granulocytes in the context of YF-17D vaccination as most studies analyzed peripheral blood mononuclear cells (PBMCs) that were isolated by Ficoll gradient centrifugation which eliminates granulocytes. Therefore, these cell types can only be assessed in fresh and whole blood samples, requiring immediate laboratory processing and analysis, which is often challenged by the logistics of longitudinal human studies.
2.2. Adaptive immunity
Unlike the responses of innate immune cells that are based on typical microbial patterns, the adaptive immune cells are highly specific for their target antigens. Adaptive responses are based primarily on the antigen-specific receptors expressed on the surfaces of T- and B-lymphocytes and become prominent only after several days, the time required for antigen-specific T and B cells to locate their cognate antigen, to undergo clonal expansion, and to differentiate into effector cells.
2.2.1. CD8 T cells
CD8 T cells (also called cytotoxic T cells) control viral infections by inducing apoptosis of infected cells through perforin- or Fas ligand-dependent pathways or producing antiviral cytokines such as IFNγ and TNFα.Citation49
In humans, total CD8 T cells rapidly expand and peak at 2 weeks after primary vaccination with YF-17D, showing expression of the proliferation marker Ki-67 and downregulation of the anti-apoptotic marker Bcl-2.Citation16,Citation17,Citation20,Citation50,Citation51 In addition, the CD8 T cells have an activated phenotype as revealed by the transient up-regulation of CD69, HLA-DR and CD38.Citation16,Citation20,Citation39,Citation50–55 This activation leads to clonal expansion, which is associated with differentiation to effector T cells that migrate throughout the body to defend from the infection.Citation56This effector response contracts by 4 weeks after vaccination, followed by the memory phase with its long-term surviving memory T cells.Citation57
The magnitude of the effector CD8 T cell response correlates directly with the viral serum titer, reflecting that the CD8 T cell proliferation is driven by viral expansion and the amount of antigen.Citation17 CD8 T cells may respond to epitopes from all 10 YF-17D proteins. Multiple proteins are targeted in each individual, with several epitopes per protein.Citation51,Citation53,Citation58 In particular, CD8 T cells specific for the immunodominant HLA-A2-restricted epitope NS4B214-222 (“A2/LLW”) form a large part of the total response in HLA-A2 positive individuals.Citation51 Surprisingly, A2/LLW-specific CD8 T cells can be detected already in unvaccinated HLA-A2 individuals, revealing an extraordinary high precursor frequency that relates to the dominance of these cells in the response to YF-17D vaccination.Citation59,Citation60 A2/LLW-specific CD8 T cells undergo an initial phase of expansion peaking at 2 weeks after YF-17D vaccination. These cells are strongly activated, expressing high levels of HLA-DR, CD38 and PD1.Citation20,Citation51 Upon peptide recognition in vitro, A2/LLW-specific CD8 T cells produce a variety of pro-inflammatory cytokines, such as IFNγ, TNFα, MIP-1β and IL-2.Citation51,Citation53,Citation59 They also express granzyme B and the degranulation marker CD107a, reflecting the cytotoxicity of these cells.Citation51,Citation53,Citation59
Following expansion, A2/LLW-specific CD8 T cells differentiate into central memory cells (CM: CCR7+ CD45RA-) and effector memory cells (EM: CCR7- CD45RA-; EMRA: CCR7-CD45RA+). These cells decrease over time, but remain detectable for decades.Citation59,Citation61 Strikingly, a stem cell-like memory population persists stably for at least 25 years, showing self-renewing capacity and rapid proliferation upon re-stimulation in vitro.Citation59 Although such long-term persistence of memory cells at stable frequencies over decades is expected in immune individuals, this has not been documented in any other human or animal model situation.
2.2.2. CD4 T cells
CD4 T cells play a central role in the adaptive immune system.Citation62 They are called T helper (Th) cells because they provide help to B cells for antibody production and to CD8 T cells for becoming efficient in killing infected cells. Upon antigen stimulation, CD4 T cells polarize toward different subsets depending on the nature of the cytokines present at the site of activation. While Th1 cells support cell-mediated immune responses, Th2 cells support humoral and allergic responses. In addition, T follicular helper (Tfh) cells play a central role in activating B cells leading to isotype switch and production of long-lasting neutralizing antibodies (nAbs). Regulatory T cells (Tregs) is another subset of CD4 T cells. They express CD25 and FOXP3. Tregs can suppress effector T cells and thus reduce inflammation and reactivity to self or non-self-antigens.
The overall CD4 T cell response to YF-17D is of lower magnitude compared to CD8 T cells but develops slightly earlier, peaking at day 7 post-vaccination with YF-17D.Citation20,Citation52–54 The YF-17D vaccine induces a mixed Th1/Th2 cytokine signature.Citation42,Citation55,Citation63 A recent study investigated the dynamics of Tfh cells and observed that the frequency of circulating Tfh cells is stable following primary YF-17D vaccination.Citation64 However, Tfh cells become activated early after primary vaccination. The Tfh response was found to be dominated by CXCR3+ CCR6- Tfh cells (Tfh1 subset), which increased 2 weeks post-vaccination, whereas CXCR3- CCR6+ Thf cells (Thf17 subset) were decreased. The frequency of Tregs increased early upon YF-17D vaccination and these cells became transiently activated.Citation53,Citation64,Citation65
Few studies aiming at identifying CD4 epitopes revealed that such epitopes are present within all YFV proteins.Citation58,Citation66–68 A study analyzed DRB1*03:01- and DRB1*15:01-restricted CD4 T cells ex vivo using fluorescent peptide-HLA tetramers, revealing transiently increased frequencies of these cells within the first two weeks after vaccination.Citation58 Interestingly, they could detect NS3145-161-specific CD4 T cells by tetramers even in an unvaccinated DRB1*15:01 individual.
2.2.3. B cells and antibody response
B cells mediate the humoral response, consisting of antibodies, i.e. antigen-specific immunoglobulins (Ig) directed against invasive pathogens.Citation69 Following cognate antigen encounter, B cells undergo differentiation. IgM is the first class of antibody made by a developing B cells, providing a rapid initial response. IgM secreting plasma cells do not have somatically mutated Ig genes and are short lived. In germinal centers, B cells receive help from CD4 T cells to proliferate, perform antibody class switch to produce IgG, IgA or IgE antibodies, and undergo affinity maturation.
Increased frequencies of activated B cells are observed 15 days after YF-17D vaccination.Citation20,Citation42,Citation52 Single-cell analysis showed that the early memory B cell response is mediated by classical IgM+ and switched memory B cells, whereas the late memory B cell response was dominated by atypical IgM+, IgD+ and switched memory B cells.Citation70 Plasmablasts, which secreted antibodies in larger quantities than B cells, showed increased frequencies 2 weeks after vaccination.Citation20,Citation39,Citation64,Citation70 However, although the frequency of plasmablasts almost doubled, this frequency remained low (below 1%) and only a minority of these cells produced antibodies with potent neutralizing activity.Citation70
Infection or vaccination often results in the production of nAbs, characterized by their capability to bind a virus in a manner that directly blocks its infectious action. The level of nAb titers is generally considered as the main correlate of protection from viral disease. However, because of ethical reasons that preclude challenging humans with wild-type virus, there is no direct correlate of protection in humans and no consensus on the cutoff for protection. Some laboratories set it at 50%, 80% or even 90% reduction of Plaque Forming Units of Virus in the 1:10 dilution in the varying serum-constant virus Plaque Reduction Neutralizing Test.Citation71–74 These methodological differences make the direct comparison of studies difficult.
Historically, the humoral response was the first laboratory parameter that was studied in the research of YF-17D vaccination. Several studies revealed that primary YF-17D vaccination leads to the production of nAbs in >98% of individuals from non-endemic regions.Citation20,Citation39,Citation54,Citation75 The YF-17D vaccine is outstanding for the nAbs raised, as they are not only frequent but also persist for very long, i.e. at least 30–40 years.Citation61,Citation75–77 However, the percentage of seropositive individuals after primary vaccination was considerably lower, down to ~75%, in vaccinees from endemic regions, reflecting the general notion that individuals in endemic regions require stronger or more frequent vaccination.Citation18,Citation75
3. Immune responses to YF-17D booster vaccination
Since 1959, booster vaccinations every 10 years was declared as required for the YF-17D vaccine. However, over the years, numerous studies were performed which identified only low numbers of vaccine failures and high seropositivity. Therefore, the WHO Strategic Advisory Group of Experts on immunization concluded in 2013 that a single dose the YF-17D vaccine was sufficient to provide lifelong protection against the YF disease.Citation75,Citation78 In 2016, the recommendation to remove the requirement for a 10-year booster dose was enacted. Despite the WHO recommendations, some countries, in particular endemic regions such as Brazil, questioned this decision and still require a booster dose every 10 years.Citation79,Citation80 The precise level of nAbs required for protection from YF disease remains unknown. Several studies observed an increase after booster vaccination.Citation20,Citation54,Citation61,Citation80 However, the titers after booster vaccination were significantly lower compared to the titers post-primary vaccination.Citation20,Citation54 It was suggested that the nAbs that are present prior to booster vaccination negatively influence the booster response through blockade of YF-17D replication.Citation20 The cellular memory response may further hamper viral replication. Several studies in individuals from non-endemic regions showed that the YF-17D virus remained undetectable in serum after booster vaccination, whereas viremia was detected in most individuals receiving the vaccine for the first time.Citation16,Citation17,Citation51 Importantly, several studies concluded that nAb titers also decline over time after vaccination.Citation54,Citation61,Citation77,Citation79–82 These observations, added to the aforementioned observation that primary vaccination induces less seroconversion in individuals from endemic regions, support that endemic regions may profit more from booster vaccinations as compared to non-endemic areas,Citation18,Citation83 as discussed further below.
Although the exact role of T cell-mediated immunity for protection remains to be elucidated, the latter is gaining increasing attention because T cells may contribute to protection and are likely reducing disease severity. Booster YF-17D vaccination induces minimal CD8 T cell responses compared to primary vaccination.Citation20,Citation54 This is visible in the limited activation of total CD8 T cells. Also, the magnitude of the A2/LLW-specific CD8 T cell responses is much lower to booster than primary vaccination.Citation20,Citation54 Nonetheless, booster YF-17D vaccination restored the level of EM CD8 T cells.Citation80 Although not substantially expanding, A2/LLW-specific CD8 T cells show significant up-regulation of activation and proliferation markers.Citation20 Interestingly, neither the frequency nor the activation of CD4 T cells was significantly increased upon booster vaccination,Citation20,Citation54,Citation55,Citation84 reflecting the notion that CD4 T cells generally expand less in response to immunization as compared to CD8 T cells. Together, these data suggest that the CD8 T cell adaptive response is mobilized upon booster vaccination even if not massively as in primary vaccination, reflecting effective immunity and likely also the effectiveness of nAbs that rapidly clear the YF-17D vaccine virus.
Finally, regarding the innate response to revaccination with YF-17D, there is no substantial change in cell frequencies and activation after booster YF-17D vaccination for NK cells, ILCs or monocytes.Citation20 Granulocytes and DCs have not yet been studied upon YF-17D booster vaccination.
4. Immune responses to YF-17D vaccination in specific populations
Most studies investigating vaccine efficacy were performed on healthy adults, while data on YF vaccination in individuals with specific immune status remain scarce. Of note, studies may involve individuals in endemic or non-endemic regions.
4.1. Individuals in endemic regions
An elegant study compared the immune responses of individuals in endemic (Uganda) and non-endemic (Switzerland) regions.Citation18 The number of individuals with detectable viremia at days 3 and 7 after YF-17D vaccination was higher in the non-endemic compared to the endemic group. The endemic group showed higher activation of the innate immune system, such as increased frequency of exhausted NK cells at baseline and after vaccination, as well as an increased frequency of proinflammatory monocytes at baseline in the endemic group compared to the non-endemic group. In addition, they observed a higher baseline activation of the adaptive immune system in endemic compared to non-endemic groups, such as higher frequencies of terminally differentiated CD8 T cells, activated CD4 and CD8 T cells, and plasmablasts. The cellular (frequency of YF-specific CD8 T cells) and the humoral (nAb titers) immune responses following YF-17D vaccination was impaired and showed decreased persistence in the endemic group. The increased immune activation at baseline in the endemic group was associated with lower magnitudes of cellular and humoral responses to YF-17D and with reduced memory persistence. One hypothesis is that exposure to more frequent infectious diseases can lead to sustained inflammation and immune activity. The causes and mechanisms of this baseline immune activation in the endemic group remain to be elucidated.
4.2. Children
The WHO recommends that all individuals aged 9 months or older and living in countries or areas at risk should receive yellow fever vaccination. Due to the increased incidence of YEL-AND, the YF-17D vaccine is contraindicated in infants under 6 months of age and is not recommended for those aged 6–8 months, except during epidemics when the risk of YFV transmission may be very high.Citation21
As the YF-17D vaccine is routinely given to infants at 9–12 months of age as part of the Expanded Programme on Immunization in endemic countries, infants constitute an important vaccination target in YF-endemic countries. A study reported that children of 23 months seroconvert at lower rates and develop weaker antibody titers compared to healthy adults.Citation85
Another study showed that 30 children seroconverted (PRNT ≥ 2.5 log10 mIU/mL), whereas 10 children remained seronegative 30 days after primary YF-17D vaccination. A proinflammatory microenvironment, as demonstrated by enhanced synthesis of IL-12 and TNF-α by neutrophils and monocytes, and decreased IL-4 production by CD4 T cells, was observed upon YF-antigen recall in seropositive compared to seronegative children after primary YF-17D vaccination.Citation86 These findings were corroborated by another study.Citation87 In addition, the nAb titers after YF-17D vaccination in children was associated with the overall signature of high cytokine production upon YF-antigen re-stimulation.Citation86 Seronegative children revaccinated 1 year after primary YF-17D vaccination became seropositive (PRNT ≥ 2.5 log10 mIU/mL). Upon such revaccination and induction of nAbs, the synthesis of IL-12 and TNF-α by neutrophils was increased.Citation86
A long-term longitudinal study followed the antibody response in children concomitantly vaccinated at 8–12 months of age under the Expanded Programme on Immunization schedule against yellow fever and measles. Humoral immunity after 2–6 years largely declined relative to the findings at 4 weeks after vaccination. However, it cannot be excluded that the drop in immunity was due to interference by the concomitantly administered live-virus vaccine against measles.Citation88
4.3. The elderly
One study compared nAb titers and viremia in young (18–28y) and elderly (60–81y) travelers vaccinated with YF-17D.Citation89 This study found that elderly subjects had a delayed antibody response and higher viremia levels. Indeed, ten days after YF-17D vaccination, the geometric mean titer was higher in young individuals compared to the elderly, whereas the difference was no longer statistically significant at day 28. Viremia was significantly more common in the elderly than in the younger participants with higher YF-17D RNA copy numbers in the elderly participants.
4.4. Pregnant women
The use of YF-17D vaccine during pregnancy has not been studied in a large prospective trial, and the WHO stated that the YF-17D vaccine should be avoided during pregnancy or breastfeeding. However, pregnant or nursing women may be vaccinated during epidemics or if travel to a country or area at risk of transmission is unavoidable.Citation21
Only one study assessed the immunogenicity of YF-17D in women at various stages of pregnancy during a YF outbreak in Nigeria in the 80’s.Citation90 The results showed that the antibody responses of these pregnant women were much lower than those of YF-vaccinated, non-pregnant women in a comparable control group. Safety assessment from limited data showed that there is no increased risk for major malformations,Citation91 nor an increased risk for fetal death,Citation92 however a higher rate of spontaneous abortion was observed.Citation93
4.5. Immunocompromised and autoimmune disease patients
In immune-compromised individuals and patients with autoimmune diseases (AID), the YF-17D vaccine is contraindicated due to a risk of uncontrolled viral replication and risk of adverse events. However, such individuals are sometimes vaccinated inadvertently or vaccinated after careful weighting of the risk of YEL-AVD versus the risk of acquiring yellow fever, providing some evidence about vaccine efficacy. For instance, nAbs were produced in 2 patients undergoing different immunosuppressive treatments.Citation94 In addition, 15 immune-compromised patients were found to mount protective nAbs (>80% virus neutralization with a 1:10 serum dilution) following YF-17D vaccination.Citation95
A controlled study showed that the geometric mean of nAb titers was not different between patients under different immunosuppressive drugs and healthy individuals. The detection of YF-specific CD4 and CD8 T cells was similar between the two groups. Furthermore, early-differentiated memory-like T cells persisted, associated with effective expansion upon re-encounter with antigen, suggesting that memory T cells had good potential.Citation96 A study conducted in patients with AID assessed the immunogenicity of the YF-17D vaccine. These patients seroconverted later than healthy individuals and seropositivity rate was lower after 28 days. The viremia peak was 5–6 days after vaccination in all groups but was lower in patients with AID.Citation97
Overall, these results highlight the importance of evaluating the immunogenicity of the YF-17D vaccine in specific populations and how the prior immune status influences the immune response to YF-17D vaccination. In addition, these studies pinpoint to important immune parameters at baseline or linked to immune status that interfere or hinder with the induction of optimal immune responses.
5. Immunogenicity of YF-17D fractional doses
Currently, manufacturers are limited in their production capacity and can only produce 80 million doses worldwide each year, although it has been estimated that 400 million doses are required for unvaccinated populations in high-risk countries.Citation98,Citation99 Despite the reserve stockpile of 6 million doses maintained by UNICEF, unpredictable YF outbreaks have recurrently resulted in vaccine stockpile depletion. To reduce the consequence of global shortage of YF-17D vaccine, the WHO was urged to the emergency solution of vaccinating with a fractional dose.Citation100,Citation101
Three randomized controlled non-inferiority clinical studies were conducted. First, the study from the Netherlands compared the intradermal administration of 0.1 ml fractional dose (1/5th of the standard dose) to subcutaneous administration of the standard dose of 0.5 ml. The volunteers involved in this study were young (20–50y) healthy men and women.Citation99,Citation102 The choice of the administration route arose from the hypothesis that intradermal injection would be highly immunogenic due to the direct targeting of antigen-presenting cells in the papillary dermis. Second, the study from Brazil compared several fractional doses (10ʹ447 IU, 3ʹ013 IU, 158 IU, and 31 IU) via regular route of administration (intramuscular or subcutaneous) compared to the standard dose (27ʹ476 IU). Only young male army recruits (18–20y) were enrolled in this study.Citation103–105 Third, the study performed in research centers in Uganda and in Kenya, with healthy female and male volunteers (18–59y), compared standard and fractional doses (1/5th of the standard dose) administrated subcutaneously.Citation106
The Dutch study showed that seroconversion (defined as serum dilution at which 80% viral neutralization occurred) induced by an intradermal 1/5th reduced dose and by the standard dose did not differ between 2 weeks and 1 year after vaccination.Citation102 A follow-up study reported that participants had nAbs at protective levels more than 10 years after fractional dose vaccination,Citation99
The Brazilian study showed that seroconversion (determined by a 50% viral plaque reduction by anti-YFV nAbs) was equivalent in participants vaccinated with 1/46 dilution (587 IU) or standard dose.Citation103 Additional investigation showed that peak of viremia was reduced and delayed at 1/46 dilution, while the serum cytokines were equivalent to standard dose.Citation105 The follow-up study of this group showed that seroconverted participants in reduced doses remained seropositive 8 years later.Citation104
The study conducted in Africa showed that most participants had high nAb titers and that the rate of seroconversion (determined as post-vaccination nAb titers at least 4 times of pre-vaccination, measured by 50% plaque reduction neutralization test) 28 days after YF-17D vaccination were non-inferior to standard dose (non-inferiority criteria defined as less than 10% decrease in seroconversion in fractional compared to standard dose 28 days after vaccination). Seroconversion rates and nAb titers remained high up to 1 year after YF-17D vaccination for both fractional and standard doses.Citation106
Based on these data, the WHO recommended that dose sparing should contain at least 1ʹ000 IU.Citation100 The number of vaccinated individuals in the described studies is too low to assess the rate of serious adverse events upon vaccination with fractional dose of YF-17D.
6. Profiling and modeling immune events during YF-17D vaccination
Because of its renown efficacy, the YF-17D vaccine has also been used to profile and model immune responses and to investigate associations between various immune parameters, with the overall goal to identify key immune determinants able predict immunogenicity and immunity, i.e. protection from YF disease.
6.1. Correlations between immune events during YF-17D vaccination
As mentioned above, although most vaccinated individuals develop long-lasting nAbs, the titers vary amongst individuals. In order to identify prognostic markers, it is important to identify parameters that correlate with nAb titers.
Using high-throughput technologies, Querec at al. identified the gene encoding for the B cell growth factor BLyS-BAFF (TNFRSF17) as a central positive predictor of the antibody response.Citation50 Furthermore, nAb titers were positively correlated with the frequency of A2/LLW-specific CD8 T cells at day 14.Citation18 In contrast, the nAb titers were negatively associated with baseline immune activation (baseline frequencies of activated naïve cells B cells, CD38+ tissue-like memory B cells, PD1+ memory CD8 T cells, and CD14+ CD16+ monocytes).Citation18 A negative association was also found between the nAb levels detected before revaccination and after boosting, again suggesting that preexisting nAbs inhibit the humoral response following booster vaccination.Citation18,Citation54,Citation80 The frequencies of Tfh1 cells at day 14 and day 28 positively correlated with nAb titers, whereas Tfh17 cells negatively correlated with nAb titers.Citation64 For CD8 T cells, both the magnitude of the response and the activation levels in CD8 T cells showed positive correlations with the viral load.Citation17,Citation20 As the virus load increased above a certain threshold, the magnitude of the CD8 T cell response saturated.Citation17,Citation20
Interestingly, lymphocyte levels transiently dropped in peripheral blood early after YF-17D vaccination. The T cell drop was restricted to cells expressing the chemokine receptor CCR7. Furthermore, the CD8 T cell drop positively correlated with the percentage of CD8 T cells co-expressing CCR7 and CD69,Citation107 suggesting that lymphocytes are trapped in lymphoid tissues. This T cell drop was negatively correlated to immunogenicity parameters, including T cell activation, the magnitude of the antigen-specific CD8 T cells, and nAbs.Citation107
A systems biology approach made many interesting observations of the molecular and cellular dynamics induced by YF-17D vaccination. For example, it revealed that gene signatures involved in glucose metabolism and the integrated stress response predicted the T cell response.Citation18 Future immune profiling studies could improve and predict the immunogenicity of emerging vaccines. However, while investigations on YF-17D vaccination have increased the mechanistic understanding of this efficient immune response, the individual observations made across studies do not yet provide a comprehensive unified picture (, Supplemental Table 1). This reveals the complexity of immune actors and parameters that determine an optimal immune response. Furthermore, whether the findings from YF-17D studies may be applicable to other immune responses remains to be elucidated.
6.2. Modeling T cell responses
With major technical advances to track immune responses, many basic questions on the kinetics of virus-specific immunity in humans can be addressed. In particular, the mechanisms regulating proliferation and differentiation of T cells remains unclear. Multidisciplinary studies combining experimental data and mathematical modeling are increasingly used to gain insights into this kinetics in the context of YF-17D vaccination.
The recent identification of YFV-specific stem cell-like memory CD8 T cells raised the question whether this subset is responsible for memory maintenance. New evidence supports the notion that stem cell-like memory T cells originate directly from naïve CD8 T cells,Citation108 whereas other studies concluded that memory CD8 T cells derive from effector cells.Citation19,Citation109 Several models of T cell differentiation have been proposed.Citation110 A recent elegant murine study formerly demonstrated that central memory T cells derive from rare stem-like memory CD8 T cells present during the acute response to viral infection.Citation111 Human data on YF-17D vaccination revealed the presence of CD8 T cell memory subsets already in the acute phase of the response, supporting models where memory cells arise very early without an obligatory transition through an effector stage.Citation112 Interestingly, the latter human data submitted to mathematical modeling suggest that the kinetics of stem cell-like memory T cells is compatible with their role in memory maintenance.Citation112,Citation113
The initiation of T cell responses occurs in the draining lymph nodes, where the cells are activated and then migrate to the tissues where they are required for immune defense. Unfortunately, obtaining human samples to delineate the spatial dynamics of T cells throughout the body is very limited. Nevertheless, computational analysis and available experimental data obtained from YF-17D vaccinated individuals can be used to address these questions. We believe that the identification of critical immune parameters, the understanding of the complex interplay, and the prediction of immunogenicity will benefit from such mathematical modeling. While well designed mouse studies may provide mechanistic proof, human studies evaluate whether such findings are translatable and explore the effects of the wide genetic and phenotypic heterogeneity.
7. Conclusion and perspectives
YF-17D vaccination is highly successful, inducing robust and long-term immune responses. It can be used as a model to answer longstanding questions of the immune system, and to identify the key determinants of optimal immunogenicity. Such insights may improve the knowledge and the rationale for the design of more powerful vaccines against microbial infections as well as for other medical interventions such as cancer immunotherapy.
Conflicts of Interest
The authors declare that they have no competing interests.
Supplemental Material
Download MS Word (161.5 KB)Acknowledgments
We thank the members of our laboratory for their numerous contributions. Worldwide, many scientists have made seminal contributions to this field which we could not all cite due to space limitations.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Pulendran B. Learning immunology from the yellow fever vaccine: innate immunity to systems vaccinology. Nat Rev Immunol. 2009;9(10):741–47. doi:10.1038/nri2629.
- Pulendran B, Oh JZ, Nakaya HI, Ravindran R, Kazmin DA. Immunity to viruses: learning from successful human vaccines. Immunol Rev. 2013;255(1):243–55. doi:10.1111/imr.12099.
- Dixon B. Microbe hunters—then and now. BMJ. 1996;313(7068):1340. doi:10.1136/bmj.313.7068.1340.
- Cardoso Jda C, de Almeida MAB, Dos Santos E, da Fonseca DF, Sallum MAM, Noll CA, Monteiro HADO, Cruz ACR, Carvalho VL, Pinto EV, et al. Yellow fever virus in haemagogus leucocelaenus and aedes serratus mosquitoes, Southern Brazil, 2008. Emerg Infect Dis. 2010;16(12):1918–24. doi:10.3201/eid1612.100608.
- Monath TP. Yellow fever vaccine. Expert Rev Vaccines. 2005;4(4):553–74. doi:10.1586/14760584.4.4.553.
- CDC. [accessed 2020 Oct 24]. https://www.cdc.gov/yellowfever/symptoms/index.html.
- WHO. [accessed 2020 Oct 24]. https://www.who.int/health-topics/yellow-fever#tab=tab_1.
- Theiler M, Smith HH. The use of yellow fever virus modified by in vitro cultivation For human immunization. J Exp Med. 1937;65(6):787–800. doi:10.1084/jem.65.6.787.
- Hahn CS, Dalrymple JM, Strauss JH, Rice CM. Comparison of the virulent Asibi strain of yellow fever virus with the 17D vaccine strain derived from it. Proc Natl Acad Sci USA. 1987;84(7):2019–23. doi:10.1073/pnas.84.7.2019.
- Barrett ADT, Teuwen DE. Yellow fever vaccine—how does it work and why do rare cases of serious adverse events take place? Curr Opin Immunol. 2009;21(3):308–13. doi:10.1016/j.coi.2009.05.018.
- Guirakhoo F, Zhang Z, Myers G, Johnson BW, Pugachev K, Nichols R, Brown N, Levenbook I, Draper K, Cyrek S, et al. A single amino acid substitution in the envelope protein of chimeric yellow fever-dengue 1 vaccine virus reduces neurovirulence for suckling mice and viremia/viscerotropism for monkeys. J Virol. 2004;78(18):9998–10008. doi:10.1128/JVI.78.18.9998-10008.2004.
- Lee E, Lobigs M. E protein domain III determinants of yellow fever virus 17D Vaccine strain enhance binding to glycosaminoglycans, impede virus spread, and attenuate virulence. J Virol. 2008;82(12):6024. doi:10.1128/JVI.02509-07.
- Ferreira CC, Campi-Azevedo AC, Peruhype-Magalhāes V, Costa-Pereira C, Albuquerque CPD, Muniz LF, Yokoy de Souza T, Oliveira ACV, Martins-Filho OA, da Mota LMH, et al. The 17D-204 and 17DD yellow fever vaccines: an overview of major similarities and subtle differences. Expert Rev Vaccines. 2018;17(1):79–90. doi:10.1080/14760584.2018.1406800.
- Hanlon P, Hanlon L, Marsh V, Byass P, Sillah H, Hayes R, Whittle HC, Greenwood BM. Serological comparisons of approaches to polio vaccination in the Gambia. Lancet. 1987;329(8536):800–01. doi:10.1016/S0140-6736(87)92818-2.
- Valsamakis A, Auwaerter PG, Rima BK, Kaneshima H, Griffin DE. Altered virulence of vaccine strains of measles virus after prolonged replication in human tissue. J Virol. 1999;73(10):8791–97. doi:10.1128/JVI.73.10.8791-8797.1999.
- Miller JD, van der Most RG, Akondy RS, Glidewell JT, Albott S, Masopust D, Murali-Krishna K, Mahar PL, Edupuganti S, Lalor S, et al. Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines. Immunity. 2008;28(5):710–22. doi:10.1016/j.immuni.2008.02.020.
- Akondy RS, Johnson PLF, Nakaya HI, Edupuganti S, Mulligan MJ, Lawson B, Miller JD, Pulendran B, Antia R, Ahmed R, et al. Initial viral load determines the magnitude of the human CD8 T cell response to yellow fever vaccination. Proc Natl Acad Sci USA. 2015;112(10):3050–55. doi:10.1073/pnas.1500475112.
- Muyanja E, Ssemaganda A, Ngauv P, Cubas R, Perrin H, Srinivasan D, Canderan G, Lawson B, Kopycinski J, Graham AS, et al. Immune activation alters cellular and humoral responses to yellow fever 17D vaccine. J Clin Invest. 2014;124(7):3147–58. doi:10.1172/JCI75429.
- Akondy RS, Fitch M, Edupuganti S, Yang S, Kissick HT, Li KW, Youngblood BA, Abdelsamed HA, McGuire DJ, Cohen KW, et al. Origin and differentiation of human memory CD8 T cells after vaccination. Nature. 2017;552(7685):362–67. doi:10.1038/nature24633.
- Bovay A, Nassiri S, Maby–El Hajjami H, Marcos Mondéjar P, Akondy RS, Ahmed R, Lawson B, Speiser DE, Fuertes Marraco SA. Minimal immune response to booster vaccination against Yellow fever associated with pre-existing antibodies. Vaccine. 2020;38(9):2172–82. doi:10.1016/j.vaccine.2020.01.045.
- Staples JE, Gershman M, Fischer M. Yellow fever vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recommendations Rep. 2010;59(RR–7):1–27.
- Martin M, Tsai TF, Cropp B, Chang GJJ, Holmes DA, Tseng J, Shieh W-J, Zaki SR, Al-Sanouri I, Cutrona AF, et al. Fever and multisystem organ failure associated with 17D-204 yellow fever vaccination: a report of four cases. Lancet. 2001;358(9276):98–104. doi:10.1016/S0140-6736(01)05327-2.
- McMahon AW, Eidex RB, Marfin AA, Russell M, Sejvar JJ, Markoff L, Hayes EB, Chen RT, Ball R, Braun MM, et al. Neurologic disease associated with 17D-204 yellow fever vaccination: a report of 15 cases. Vaccine. 2007;25(10):1727–34. doi:10.1016/j.vaccine.2006.11.027.
- Lindsey NP, Rabe IB, Miller ER, Fischer M, Staples JE. Adverse event reports following yellow fever vaccination, 2007-13. J Travel Med. 2016;23(5):taw045. doi:10.1093/jtm/taw045.
- de Menezes Martins R, Fernandes Leal MDL, Homma A. Serious adverse events associated with yellow fever vaccine. Hum Vaccin Immunother. 2015;11(9):2183–87. doi:10.1080/21645515.2015.1022700.
- Thomas RE, L. Lorenzetti D, Spragins W, Jackson D, Williamson T. Reporting rates of yellow fever vaccine 17D or 17DD-associated serious adverse events in pharmacovigilance data bases: systematic review. Curr Drug Saf. 2011;6(3):145–54. doi:10.2174/157488611797579258.
- Galler R, Pugachev KV, Santos CLS, Ocran SW, Jabor AV, Rodrigues SG, Marchevsky RS, Freire MS, Almeida LFC, Cruz ACR, et al. Phenotypic and molecular analyses of yellow fever 17DD vaccine viruses associated with serious adverse events in Brazil. Virology. 2001;290(2):309–19. doi:10.1006/viro.2001.1168.
- Belsher JL, Gay P, Brinton M, DellaValla J, Ridenour R, Lanciotti R, Perelygin A, Zaki S, Paddock C, Querec T, et al. Fatal multiorgan failure due to yellow fever vaccine-associated viscerotropic disease. Vaccine. 2007;25(50):8480–85. doi:10.1016/j.vaccine.2007.08.061.
- Pulendran B, Miller J, Querec T, Akondy R, Moseley N, Laur O, Glidewell J, Monson N, Zhu T, Zhu H, et al. Case of yellow fever vaccine–associated viscerotropic disease with prolonged viremia, robust adaptive immune responses, and polymorphisms in CCR5 and RANTES genes. J Infect Dis. 2008;198(4):500–07. doi:10.1086/590187.
- Bae H-G, Domingo C, Tenorio A, Ory F, Muñoz J, Weber P, Teuwen D, Niedrig M. Immune response during adverse events after 17D-derived yellow fever vaccination in Europe. J Infect Dis. 2008;197(11):1577–84. doi:10.1086/587844.
- Julander JG. Animal models of yellow fever and their application in clinical research. Curr Opin Virol. 2016;18:64–69. doi:10.1016/j.coviro.2016.03.010.
- Watson AM, Klimstra WB. T cell-mediated immunity towards yellow fever virus and useful animal models. Viruses. 2017;9(4):77. doi:10.3390/v9040077.
- Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu Y-J, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18(1):767–811. doi:10.1146/annurev.immunol.18.1.767.
- Querec T, Bennouna S, Alkan S, Laouar Y, Gorden K, Flavell R, Akira S, Ahmed R, Pulendran B. Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity. J Exp Med. 2006;203(2):413–24. doi:10.1084/jem.20051720.
- Barba-Spaeth G, Longman RS, Albert ML, Rice CM. Live attenuated yellow fever 17D infects human DCs and allows for presentation of endogenous and recombinant T cell epitopes. J Exp Med. 2005;202(9):1179–84. doi:10.1084/jem.20051352.
- Cong Y, McArthur MA, Cohen M, Jahrling PB, Janosko KB, Josleyn N, Kang K, Zhang T, Holbrook MR, et al. Characterization of yellow fever virus infection of human and non-human primate antigen presenting cells and their interaction with CD4+ T Cells. PLoS Negl Trop Dis. 2016;10(5):e0004709–e0004709. doi:10.1371/journal.pntd.0004709.
- Sinigaglia L, Gracias S, Décembre E, Fritz M, Bruni D, Smith N, Herbeuval J-P, Martin A, Dreux M, Tangy F, et al. Immature particles and capsid-free viral RNA produced by Yellow fever virus-infected cells stimulate plasmacytoid dendritic cells to secrete interferons. Sci Rep. 2018;8(1):10889. doi:10.1038/s41598-018-29235-7.
- Hou J, Wang S, Jia M, Li D, Liu Y, Li Z, Zhu H, Xu H, Sun M, Lu L, et al. A systems vaccinology approach reveals temporal transcriptomic changes of immune responses to the yellow fever 17D vaccine. J Immunol (Baltimore, Md : 1950). 2017;199(4):1476–89. doi:10.4049/jimmunol.1700083.
- Kohler S, Bethke N, Böthe M, Sommerick S, Frentsch M, Romagnani C, Niedrig M, Thiel A. The early cellular signatures of protective immunity induced by live viral vaccination. Eur J Immunol. 2012;42(9):2363–73. doi:10.1002/eji.201142306.
- Ginhoux F, Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol. 2014;14(6):392–404. doi:10.1038/nri3671.
- Martins MÂ, Silva ML, Elói-Santos SM, Ribeiro JGL, Peruhype-Magalhães V, Marciano APV, Homma A, Kroon EG, Teixeira-Carvalho A, Martins-Filho OA, et al. Innate immunity phenotypic features point toward simultaneous raise of activation and modulation events following 17DD live attenuated yellow fever first-time vaccination. Vaccine. 2008;26(9):1173–84. doi:10.1016/j.vaccine.2007.12.035.
- Silva ML, Martins MA, Espírito-Santo LR, Campi-Azevedo AC, Silveira-Lemos D, Ribeiro JGL, Homma A, Kroon EG, Teixeira-Carvalho A, Elói-Santos SM, et al. Characterization of main cytokine sources from the innate and adaptive immune responses following primary 17DD yellow fever vaccination in adults. Vaccine. 2011;29(3):583–92. doi:10.1016/j.vaccine.2010.08.046.
- Schlesinger JJ, Brandriss MW. Antibody-mediated infection of macrophages and macrophage-like cell lines with 17D-yellow fever virus. J Med Virol. 1981;8(2):103–17. doi:10.1002/jmv.1890080204.
- Wheelock EF, Edelman R. Specific role of each human leukocyte type in viral infections. J Immunol. 1969;103:429.
- Guia S, Narni-Mancinelli E. Helper-like innate lymphoid cells in humans and mice. Trends Immunol. 2020;41(5):436–52. doi:10.1016/j.it.2020.03.002.
- da Costa Neves PC, de Souza Matos DC, Marcovistz R, Galler R. TLR expression and NK cell activation after human yellow fever vaccination. Vaccine. 2009;27(41):5543–49. doi:10.1016/j.vaccine.2009.07.028.
- Marquardt N, Ivarsson MA, Blom K, Gonzalez VD, Braun M, Falconer K, Gustafsson R, Fogdell-Hahn A, Sandberg JK, Michaëlsson J, et al. The human NK cell response to yellow fever virus 17D is primarily governed by NK cell differentiation independently of NK cell education. J Immunol. 2015;195(7):3262. doi:10.4049/jimmunol.1401811.
- Geering B, Stoeckle C, Conus S, Simon H-U. Living and dying for inflammation: neutrophils, eosinophils, basophils. Trends Immunol. 2013;34(8):398–409. doi:10.1016/j.it.2013.04.002.
- Zhang N, Bevan MJ. CD8(+) T cells: foot soldiers of the immune system. Immunity. 2011;35(2):161–68. doi:10.1016/j.immuni.2011.07.010.
- Querec TD, Akondy RS, Lee EK, Cao W, Nakaya HI, Teuwen D, Pirani A, Gernert K, Deng J, Marzolf B, et al. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat Immunol. 2009;10(1):116–25. doi:10.1038/ni.1688.
- Akondy RS, Monson ND, Miller JD, Edupuganti S, Teuwen D, Wu H, Quyyumi F, Garg S, Altman JD, Del Rio C, et al. The yellow fever virus vaccine induces a broad and polyfunctional human memory CD8 + T cell response. J Immunol (Baltimore, Md : 1950). 2009;183(12):7919–30. doi:10.4049/jimmunol.0803903.
- Martins MA, Silva ML, Marciano APV, Peruhype-Magalhães V, Eloi-Santos SM, Ribeiro JGL, Correa-Oliveira R, Homma A, Kroon EG, Teixeira-Carvalho A, et al. Activation/modulation of adaptive immunity emerges simultaneously after 17DD yellow fever first-time vaccination: is this the key to prevent severe adverse reactions following immunization? Clin Exp Immunol. 2007;148(1):90–100. doi:10.1111/j.1365-2249.2006.03317.x.
- Blom K, Braun M, Ivarsson MA, Gonzalez VD, Falconer K, Moll M, Ljunggren H-G, Michaëlsson J, Sandberg JK. Temporal dynamics of the primary human T cell response to yellow fever virus 17D as it matures from an effector- to a memory-type response. J Immunol. 2013;190(5):2150. doi:10.4049/jimmunol.1202234.
- Kongsgaard M, Bassi MR, Rasmussen M, Skjødt K, Thybo S, Gabriel M, Hansen MB, Christensen JP, Thomsen AR, Buus S, et al. Adaptive immune responses to booster vaccination against yellow fever virus are much reduced compared to those after primary vaccination. Sci Rep. 2017;7(1):662–662. doi:10.1038/s41598-017-00798-1.
- Santos APD, Bertho ÁL, Dias DC, Santos JR, Marcovistz R. Lymphocyte subset analyses in healthy adults vaccinated with yellow fever 17DD virus. Mem Inst Oswaldo Cruz. 2005;100:331–37. doi:10.1590/S0074-02762005000300021.
- Weninger W, Manjunath N, Von Andrian UH. Migration and differentiation of CD8+ T cells. Immunol Rev. 2002;186(1):221–33. doi:10.1034/j.1600-065X.2002.18618.x.
- Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2(4):251–62. doi:10.1038/nri778.
- James EA, LaFond RE, Gates TJ, Mai DT, Malhotra U, Kwok WW. Yellow fever vaccination elicits Broad Functional CD4+T cell responses that recognize structural and nonstructural proteins. J Virol. 2013;87(23):12794–804. doi:10.1128/JVI.01160-13.
- Fuertes Marraco SA, Soneson C, Cagnon L, Gannon PO, Allard M, Maillard SA, Montandon N, Rufer N, Waldvogel S, Delorenzi M, et al. Long-lasting stem cell–like memory CD8 + T cells with a naïve-like profile upon yellow fever vaccination. Sci Transl Med. 2015;7(282):282ra48. doi:10.1126/scitranslmed.aaa3700.
- Bovay A, Zoete V, Dolton G, Bulek AM, Cole DK, Rizkallah PJ, Fuller A, Beck K, Michielin O, Speiser DE, et al. T cell receptor alpha variable 12-2 bias in the immunodominant response to Yellow fever virus. Eur J Immunol. 2018;48(2):258–72. doi:10.1002/eji.201747082.
- Wieten RW, Jonker EFF, van Leeuwen EMM, Remmerswaal EBM, Ten Berge IJM, de Visser AW, van Genderen PJJ, Goorhuis A, Visser LG, Grobusch MP, et al. A single 17D yellow fever vaccination provides lifelong immunity; characterization of yellow-fever-specific neutralizing antibody and T-cell responses after vaccination. PloS One. 2016;11(3):e0149871–e0149871. doi:10.1371/journal.pone.0149871.
- Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112(5):1557–69. doi:10.1182/blood-2008-05-078154.
- Gaucher D, Therrien R, Kettaf N, Angermann BR, Boucher G, Filali-Mouhim A, Moser JM, Mehta RS, Drake DR 3rd, Castro E, et al. Yellow fever vaccine induces integrated multilineage and polyfunctional immune responses. J Exp Med. 2008;205(13):3119–31. doi:10.1084/jem.20082292.
- Huber JE, Ahlfeld J, Scheck MK, Zaucha M, Witter K, Lehmann L, Karimzadeh H, Pritsch M, Hoelscher M, Sonnenburg F, et al. Dynamic changes in circulating T follicular helper cell composition predict neutralising antibody responses after yellow fever vaccination. Clin Trans Immunol. 2020;9(5):e1129. doi:10.1002/cti2.1129.
- de Wolf ACMT, van Aalst S, Ludwig IS, Bodinham CL, Lewis DJ, van der Zee R, van Eden W, Broere F. Regulatory T cell frequencies and phenotypes following anti-viral vaccination. PloS One. 2017;12(6):e0179942–e0179942. doi:10.1371/journal.pone.0179942.
- de Melo AB, Nascimento EJ, Braga-Neto U, Dhalia R, Silva AM, Oelke M, Schneck P, Sidney J, Sette A, Montenegro SM, et al. T-cell memory responses elicited by yellow fever vaccine are targeted to overlapping epitopes containing multiple HLA-I and -II binding motifs. PLoS Negl Trop Dis. 2013;7(1):e1938–e1938. doi:10.1371/journal.pntd.0001938.
- Koblischke M, Mackroth MS, Schwaiger J, Fae I, Fischer G, Stiasny K, Heinz FX, Aberle JH. Protein structure shapes immunodominance in the CD4 T cell response to yellow fever vaccination. Sci Rep. 2017;7(1):8907–8907. doi:10.1038/s41598-017-09331-w.
- Stryhn A, Kongsgaard M, Rasmussen M, Harndahl MN, Osterbye T, Bassi MR, Thybo S, Gabriel M, Hansen MB, Nielsen M, et al. A systematic, unbiased mapping of CD8(+) and CD4(+) T cell epitopes in yellow fever vaccinees. Front Immunol. 2020;11:1836–1836.
- LeBien TW, Tedder TF. B lymphocytes: how they develop and function. Blood. 2008;112(5):1570–80. doi:10.1182/blood-2008-02-078071.
- Wec AZ, Haslwanter D, Abdiche YN, Shehata L, Pedreño-Lopez N, Moyer CL, Bornholdt ZA, Lilov A, Nett JH, Jangra RK, et al. Longitudinal dynamics of the human B cell response to the yellow fever 17D vaccine. Proc Nat Acad Sci. 2020;117(12):6675. doi:10.1073/pnas.1921388117.
- Julander JG, Trent DW, Monath TP. Immune correlates of protection against yellow fever determined by passive immunization and challenge in the hamster model. Vaccine. 2011;29(35):6008–16. doi:10.1016/j.vaccine.2011.06.034.
- Lang J, Zuckerman J, Clarke P, Barrett P, Kirkpatrick C, Blondeau C. Comparison of the immunogenicity and safety of two 17D yellow fever vaccines. Am J Trop Med Hyg. 1999;60(6):1045–50. doi:10.4269/ajtmh.1999.60.1045.
- WHO Expert Committee on Biological Standardization. Forty-sixth report. World Health Organ Tech Rep Ser. 1998;872:i–vii, 1–90.
- Simões M, Camacho LAB, Yamamura AMY, Miranda EH, Cajaraville ACRA, da Silva Freire M. Evaluation of accuracy and reliability of the plaque reduction neutralization test (micro-PRNT) in detection of yellow fever virus antibodies. Biologicals. 2012;40(6):399–404. doi:10.1016/j.biologicals.2012.09.005.
- Gotuzzo E, Yactayo S, Córdova E. Efficacy and duration of immunity after yellow fever vaccination: systematic review on the need for a booster every 10 years. Am J Trop Med Hyg. 2013;89(3):434–44. doi:10.4269/ajtmh.13-0264.
- Poland JD, Calisher CH, Monath TP, Downs WG, Murphy K. Persistence of neutralizing antibody 30-35 years after immunization with 17D yellow fever vaccine. Bull World Health Organ. 1981;59:895–900.
- Niedrig M, Lademann M, Emmerich P, Lafrenz M. Assessment of IgG antibodies against yellow fever virus after vaccination with 17D by different assays: neutralization test, haemagglutination inhibition test, immunofluorescence assay and ELISA. Trop Med Int Health. 1999;4(12):867–71. doi:10.1046/j.1365-3156.1999.00496.x.
- Vaccines and vaccination against yellow fever. WHO position paper – june 2013. Wkly Epidemiol Rec. 2013;88(27):269–83.
- Campi-Azevedo AC, Costa-Pereira C, Antonelli LR, Fonseca CT, Teixeira-Carvalho A, Villela-Rezende G, Santos RA, Batista MA, Campos FM, Pacheco-Porto L, et al. Booster dose after 10 years is recommended following 17DD-YF primary vaccination. Hum Vaccin Immunother. 2016;12(2):491–502. doi:10.1080/21645515.2015.1082693.
- Campi-Azevedo AC, Peruhype-Magalhaes V, Coelho-Dos-Reis JG, Antonelli LR, Costa-Pereira C, Speziali E, Reis LR, Lemos JA, Ribeiro J, Bastos Camacho LA, de Sousa Maia ML, et al. 17DD yellow fever revaccination and heightened long-term immunity in populations of disease-endemic areas, Brazil. Emerg Infect Dis. 2019;25(8):1511–21. doi:10.3201/eid2508.181432.
- Staples JE, Barrett ADT, Wilder-Smith A, Hombach J. Review of data and knowledge gaps regarding yellow fever vaccine-induced immunity and duration of protection. Npj Vaccines. 2020;5(1):54. doi:10.1038/s41541-020-0205-6.
- Gómez SY, Ocazionez RE. Yellow fever virus 17D neutralising antibodies in vaccinated Colombian people and unvaccinated ones having immunity against dengue. Rev Salud Publica (Bogota). 2008;10(5):796–807. doi:10.1590/s0124-00642008000500012.
- Hepburn MJ, Kortepeter MG, Pittman PR, Boudreau EF, Mangiafico JA, Buck PA, Norris SL, Anderson EL. Neutralizing antibody response to booster vaccination with the 17d yellow fever vaccine. Vaccine. 2006;24(15):2843–49. doi:10.1016/j.vaccine.2005.12.055.
- Minervina AA, Pogorelyy MV, Komech EA, Karnaukhov VK, Bacher P, Rosati E, Franke A, Chudakov DM, Mamedov IZ, Lebedev YB, et al. Primary and secondary anti-viral response captured by the dynamics and phenotype of individual T cell clones. eLife. 2020;9:e53704. doi:10.7554/eLife.53704.
- Collaborative Group for Studies of Yellow Fever Vaccine. A randomised double-blind clinical trial of two yellow fever vaccines prepared with substrains 17DD and 17D-213/77 in children nine-23 months old. Mem Inst Oswaldo Cruz. 2015;110(6):771–80. doi:10.1590/0074-02760150176.
- Luiza-Silva M, Campi-Azevedo AC, Batista MA, Martins MA, Avelar RS, da Silveira Lemos D, Bastos Camacho LA, de Menezes Martins R, de Lourdes de Sousa Maia M, Guedes Farias RH, et al. Cytokine signatures of innate and adaptive immunity in 17DD yellow fever vaccinated children and its association with the level of neutralizing antibody. J Infect Dis. 2011;204(6):873–83. doi:10.1093/infdis/jir439.
- Campi-Azevedo AC, Araújo-Porto LPD, Luiza-Silva M, Batista MA, Martins MA, Sathler-Avelar R, da Silveira-lemos D, Camacho LAB, de Menezes Martins R, de Lourdes de Sousa Maia M, et al. 17DD and 17D-213/77 yellow fever substrains trigger a balanced cytokine profile in primary vaccinated children. PLoS One. 2012;7(12):e49828. doi:10.1371/journal.pone.0049828.
- Domingo C, Fraissinet J, Ansah PO, Kelly C, Bhat N, Sow SO, Mejía JE. Long-term immunity against yellow fever in children vaccinated during infancy: a longitudinal cohort study. Lancet Infect Dis. 2019;19(12):1363–70. doi:10.1016/S1473-3099(19)30323-8.
- Roukens AH, Soonawala D, Joosten SA, de Visser AW, Jiang X, Dirksen K, de Gruijter M, van Dissel JT, Bredenbeek PJ, Visser LG. Elderly subjects have a delayed antibody response and prolonged viraemia following yellow fever vaccination: a prospective controlled cohort study. PLoS One. 2011;6(12):e27753. doi:10.1371/journal.pone.0027753.
- Nasidi A, Monath TP, Vandenberg J, Tomori O, Calisher CH, Hurtgen X, Munube GR, Sorungbe AO, Okafor GC, Wali S. Yellow fever vaccination and pregnancy: a four-year prospective study. Trans R Soc Trop Med Hyg. 1993;87(3):337–39. doi:10.1016/0035-9203(93)90156-K.
- Cavalcanti DP, Salomão MA, Lopez-Camelo J, Pessoto MA. Early exposure to yellow fever vaccine during pregnancy. Trop Med Int Health. 2007;12(7):833–37. doi:10.1111/j.1365-3156.2007.01851.x.
- Suzano CE, Amaral E, Sato HK, Papaiordanou PM; Campinas Group on Yellow Fever Immunization during Pregnancy. The effects of yellow fever immunization (17DD) inadvertently used in early pregnancy during a mass campaign in Brazil. Vaccine. 2006;24(9):1421–26. doi:10.1016/j.vaccine.2005.09.033.
- Nishioka Sde A, Nunes‐Ara£jo FRF, Pires WP, Silva FA, Costa HL. Yellow fever vaccination during pregnancy and spontaneous abortion: a case-control study. Trop Med Int Health. 1998;3(1):29–33. doi:10.1046/j.1365-3156.1998.00164.x.
- de Jong W, de Man RA, Dalm VASH, Reusken CBEM, Goeijenbier M, van Gorp ECM. Yellow fever vaccination for immunocompromised travellers: unjustified vaccination hesitancy? J Travel Med. 2019;26(6). doi:10.1093/jtm/taz015.
- Wieten RW, Jonker EFF, Pieren DKJ, Hodiamont CJ, van Thiel PPAM, van Gorp ECM, de Visser AW, Grobusch MP, Visser LG, Goorhuis A, et al. Comparison of the PRNT and an immune fluorescence assay in yellow fever vaccinees receiving immunosuppressive medication. Vaccine. 2016;34(10):1247–51. doi:10.1016/j.vaccine.2016.01.037.
- Wieten RW, Goorhuis A, Jonker EFF, de Bree GJ, de Visser AW, van Genderen PJJ, Remmerswaal EBM, Ten Berge IJM, Visser LG, Brobusch MP, et al. 17D yellow fever vaccine elicits comparable long-term immune responses in healthy individuals and immune-compromised patients. J Infect. 2016;72(6):713–22. doi:10.1016/j.jinf.2016.02.017.
- Valim V, Machado KLLL, Miyamoto ST, Pinto AD, Rocha PCM, Serrano EV, Dinis VG, Gouvêa SA, Dias JGF, Campi-Azevedo AC, et al. Planned yellow fever primary vaccination is safe and immunogenic in patients with autoimmune diseases: a prospective non-interventional study. Front Immunol. 2020;11(1382). doi:10.3389/fimmu.2020.01382.
- Collins ND, Barrett ADT. Live attenuated yellow fever 17D vaccine: a legacy vaccine still controlling outbreaks in modern day. Curr Infect Dis Rep. 2017;19(3):14–14. doi:10.1007/s11908-017-0566-9.
- Roukens AHE, van Halem K, de Visser AW, Visser LG. Long-term protection after fractional-dose yellow fever vaccination. Ann Intern Med. 2018;169(11):761–65. doi:10.7326/M18-1529.
- World Health, O. Fractional dose yellow fever vaccine as a dose-sparing option for outbreak response: WHO Secretariat information paper. Geneva, Switzerland: World Health Organization; 2016.
- Nnaji CA, Shey MS, Adetokunboh OO, Wiysonge CS. Immunogenicity and safety of fractional dose yellow fever vaccination: a systematic review and meta-analysis. Vaccine. 2020;38(6):1291–301. doi:10.1016/j.vaccine.2019.12.018.
- Roukens AH, Vossen AC, Bredenbeek PJ, van Dissel JT, Visser LG. Intradermally administered yellow fever vaccine at reduced dose induces a protective immune response: a randomized controlled non-inferiority trial. Plos One. 2008;3(4):e1993. doi:10.1371/journal.pone.0001993.
- Martins RM, Maia MDLS, Farias RHG, Camacho LAB, Freire MS, Galler R, Yamamura AMY, Almeida LFC, Lima SMB, Nogueira RMR, et al. 17DD yellow fever vaccine. Hum Vaccin Immunother. 2013;9(4):879–88. doi:10.4161/hv.22982.
- de Menezes Martins R, Maia MDLS, de Lima SMB, de Noronha TG, Xavier JR, Camacho LAB, de Albuquerque EM, Farias RHG, da Matta de Castro T, Homma A, et al. Duration of post-vaccination immunity to yellow fever in volunteers eight years after a dose-response study. Vaccine. 2018;36(28):4112–17. doi:10.1016/j.vaccine.2018.05.041.
- Campi-Azevedo AC, de Almeida Estevam P, Coelho-dos-Reis JG, Peruhype-Magalhães V, Villela-Rezende G, Quaresma PF, Maia MDLS, Farias RHG, Camacho LAB, Freire MDS, et al. Subdoses of 17DD yellow fever vaccine elicit equivalent virological/immunological kinetics timeline. BMC Infect Dis. 2014;14(1):391. doi:10.1186/1471-2334-14-391.
- Juan-Giner A, Kimathi D, Grantz KH, Hamaluba M, Kazooba P, Njuguna P, Fall G, Dia M, Bob NS, Monath TP, et al. Immunogenicity and safety of fractional doses of yellow fever vaccines: a randomised, double-blind, non-inferiority trial. Lancet. 2021;397(10269):119–27. doi:10.1016/S0140-6736(20)32520-4.
- Bovay A, Speiser DE, Fuertes Marraco SA. Early drop of circulating T cells negatively correlates with the protective immune response to yellow fever vaccination. Hum Vaccin Immunother. 202 Dec 1; 16(12):3103–3110. doi:10.1080/21645515.2020.1750249..
- Restifo NP, Gattinoni L. Lineage relationship of effector and memory T cells. Curr Opin Immunol. 2013;25(5):556–63. doi:10.1016/j.coi.2013.09.003.
- Abdelsamed HA, Moustaki A, Fan Y, Dogra P, Ghoneim HE, Zebley CC, Triplett BM, Sekaly R-P, Youngblood B. Human memory CD8 T cell effector potential is epigenetically preserved during in vivo homeostasis. J Exp Med. 2017;214(6):1593–606. doi:10.1084/jem.20161760.
- Kaech SM, Cui W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat Rev Immunol. 2012;12(11):749–61. doi:10.1038/nri3307.
- Pais Ferreira D, Silva JG, Wyss T, Fuertes Marraco SA, Scarpellino L, Charmoy M, Maas R, Siddiqui I, Tang L, Joyce JA, et al. Central memory CD8+ T cells derive from stem-like Tcf7hi effector cells in the absence of cytotoxic differentiation. Immunity. 2020;53:985–1000.e11. doi:10.1016/j.immuni.2020.09.005.
- Marraco SAF, Bovay A, Nassiri S, Maby-El Hajjami H, Ouertatani-Sakouhi H, Held W, Speiser DE. The human CD8 T stem cell-like memory phenotype appears in the acute phase in yellow fever virus vaccination. bioRxiv. 2019;808774.
- Costa Del Amo P, Lahoz-Beneytez J, Boelen L, Ahmed R, Miners KL, Zhang Y, Roger L, Jones RE, Fuertes Marraco SA, Speiser DE, et al. Human TSCM cell dynamics in vivo are compatible with long-lived immunological memory and stemness. PLoS Biol. 2018;16(6):e2005523. doi:10.1371/journal.pbio.2005523.
