ABSTRACT
Age-appropriate vaccination is crucial for infants, protecting them from vaccine-preventable diseases. Delaying in starting initial immunization may result in incomplete or non-vaccination in early life. However, limited vaccine coverage data are available regarding the starting age of vaccination. In this study, we determined the factors associated with the delay in infant immunization. We carried out a cross-sectional study at three maternal-child health clinics in Dhaka city. Mothers visited these clinics for their infant immunization were surveyed with structured questionnaires. A multivariate logistic regression model was used to estimate the significant influencing factors on untimely vaccination. A total of 548 mother-infant pairs were surveyed. 46.5% of mothers did not receive Tetanus (TT) vaccines, and mothers who had a previous pregnancy were less likely to receive TT-vaccine (p < .01). 41.2% of infants did not receive BCG vaccines within 1-week of birth. Mothers working outside the home showed a negative impact on BCG vaccination (p < .05). Among the infants’ born in-clinic facilities, 39% were BCG unvaccinated, and 69% had c-section delivery. The median age of infants for starting vaccination was 6.57 wks (95% CI: 6.43–7.14); however, 17.3% infants received vaccination at ≥8 wks of age. Mother’s schooling-years and infant normal body-weight positively associated with vaccination at <8 wks, whereas sickness after birth increased the age to start vaccination program recommended at 6 wks. Our analysis suggests the need for specific interventions based on potential maternal determinants, such as educating mothers about the timing and the importance of infant immunization, and addressing programmatic barriers to timely vaccination among infants in Bangladesh.
Introduction
Immunization has proven to be one of the major contributors to improved public health as it prevents infectious diseases. Infant vaccination programs are considered the most successful and cost-effective health interventions to improve survival in early life.Citation1,Citation2 Since 1974, the Expanded Program on Immunization (EPI) set a goal to make immunization available for protecting every child in the world by 1990.Citation3 Despite the success of EPI, vaccine-preventable diseases in low and middle-income countries (LMIC) have remained a public health concern because of delayed or incomplete vaccine delivery and low vaccination coverage.Citation4 World Health Organization (WHO) recommends TT vaccination through EPI during pregnancy, which has become a standard approach to antenatal care (ANC) to prevent mother and infant mortality from tetanus. In Bangladesh, limited data are available regarding maternal TT vaccination coverage. According to the national health survey data, 64% of pregnant women received ANC from medically trained providers,Citation5 presumably a smaller proportion of pregnant women received TT vaccines since vaccines are provided only from the structured clinic facilities.
Considering the high tuberculosis burden in Bangladesh, the BCG vaccine is provided at birth according to national vaccine guidelines. BCG also contributes to improving the overall survival benefits of infants in developing countries, which is considered as the nonspecific beneficial effect of this vaccine. WHO’s Strategic Advisory Group of Experts on Immunization (SAGE) reports that BCG vaccination at birth prevented neonatal sepsis and respiratory infections and reduced neonatal mortality by 48%.Citation6,Citation7 In Bangladesh, overall BCG coverage by 12–23 months of age is 98%.Citation5 However, a recent survey in the rural area shows that only 2% of neonates receive BCG by the first week of life, and no one receives BCG at birth, although 23% of them were born in rural health facilities.Citation8 No data is available in urban areas where the health facilities are less accessible.
In Bangladesh, vaccination coverage by 2 years of age is >90%,Citation5 but the age of vaccine receipts relative to the recommended official schedule is not well documented. A range of factors influences whether an infant receives a vaccine at an appropriate age. However, the main obstacles for age-appropriate vaccination relate to immunization services, parental knowledge, and attitudes.Citation9 The most frequently cited factors were access to health services, staff attitudes and practices, reliability of services, false contraindications, parents’ practical knowledge of vaccination, conflicting priorities, fear of side effects, and parental beliefs.Citation9–12 Not all determinants are equally applicable in all settings; it differs considerably with demographic characteristics. Accordingly, it is recommended that each country should develop a strategy to increase vaccination at an appropriate age.
Reports from low and middle-income countries suggest that successful delivery of the third dose of DPT/PENTA coverage at 6 months of age occurs at 36%.Citation13 Thus, the age of the infants receiving the first dose of DPT/PENTA most likely would be higher than the recommended age of 6 weeks in many low-income countries, including Bangladesh. This information is particularly relevant because although maternally derived transplacental antibodies help to protect infants from infections during early infancy, this protection eventually wanes, and active immunization is required to protect infants from childhood vaccine-preventable infections.Citation14 Delay in starting the immunization program in early life increases the risk of vaccine-preventable illnesses. Thus, the benefits of the immunization programs depend on the beginning age of vaccine receipt being close to the recommended schedule.
In this cross-sectional study, we surveyed mothers and their infants visiting urban clinics to receive the first dose of DPT/PENTA vaccine and assessed the factors associated with the delay in starting initial vaccination. The information from this study will help policymakers to understand the potential factors influencing delays in starting infant immunization in urban populations.
Methods
Study setting and population
We surveyed the three largest maternal and child health clinics, located at the largest concentration of slums in Dhaka city, having an urban slum population of over 1 million. These clinics were Azimpur Maternity Clinic (MCHTI), Infertility care & Research Center (ICRC), and Urban Maternity Hospital which was previously known as Marie Stopes Clinic (MSC). Study participants predominantly lived in the Mohammadpur, Lalbagh, Kamrangir Char, and Hazaribagh area. All these clinics provide prenatal care at minimal or no cost and have infant immunization facilities. MCHTI is a public clinic, while ICRC and MSC are operated as non-government organizations. According to the clinic registry, there were ~400 babies born per month in each of these clinics. Because of subsidized rates, pregnant women and their families prefer these clinics for antenatal/postnatal care and infant immunization. According to the national immunization policy, if a pregnant woman has not previously been vaccinated, or if her immunization status is unknown, she should receive two doses of tetanus toxoid (TT) vaccine 1 month apart with the second dose given at least 2 wks before delivery. Newborn babies are allowed to receive BCG vaccine within 2 wks of birth; if they fail, then BCG is given at 6 wks with pentavalent vaccines (PENTA). PENTA is a combination of five different vaccine antigens: Hepatitis B (HBV), Haemophilus influenza type b (Hib), Tetanus toxoid, Diphtheria-toxoid, and whole-cell Pertussis. In the national EPI, three doses of PENTA are provided to the infants at one-month intervals beginning at 6 wks of age (i.e., at 6 wks, 10 wks, and 14 wks of age). Newborns receive a Health Card that keeps information regarding birthdate, birth weight, and vaccination schedule as per recommended age.
Study design and data collection
We carried out a cross-sectional survey targeting infants attending the clinics with their mothers for the first vaccine dose after on-birth BCG vaccination. These infants were considered as “starting the vaccination program recommended at 6 wks”. Voluntary consent to participate provided by the caregiver was the only inclusion criteria necessary for infants to participate in the study. Field staff checked the Health Cards to ensure vaccination status. There were no exclusion criteria. In these clinics, infant immunization facilities operate from 9 a.m. to 1 p.m. on weekdays. Any caregiver arriving at one of the clinics for their infant’s vaccination over the 6-month survey period was welcome to participate in the study. Field staff informed the participants about the study aim in the local language. After obtaining verbal consent, the survey team gathered the following information from the infant’s Health Card: date of birth, sex, place of birth, date of the administered BCG vaccine. We used structured questionnaires for the mothers to provide information about the birth order, mode of delivery, history of infections (diarrhea/respiratory distress) since birth, mother’s age, antenatal tetanus (TT) vaccination, residence, education, and so on. We recorded episodes of diarrhea or respiratory distress, and considered the episode as a disease, if the infant visited a physician, as reported by caregivers. We also included infants in the survey where the caregiver had lost the infant’s birth card. All data were collected consecutively for 6 months, from January to June 2015.
Statistical analysis
Data were entered into EpiInfo 7.1.1.14 database (Atlanta, GA, USA) and analyzed using Stata 13.0 (StataCorp LLC, TX, USA). Categorical variables were presented using proportions and continuous variables described as means (standard deviations) or medians (Inter-Quartile Range). Data were examined for normality (using a Shapiro-Wilk statistic >0.96) and equal variance. Data for the infant’s age of vaccination recommended at 6 wks required square-inverse-cubic transformations for normality. The one-way analysis of variance (ANOVA) was used to determine whether there were any significant differences in the key characteristics of the mother-infant pairs in the three clinics surveyed. Student’s t-tests were used to examine the differences in the age of first vaccination (after on-birth BCG vaccination) between different categorized variables, e.g. birth weight, on-birth BCG vaccination, and episode as a disease since birth. Wilcoxon’s rank-sum test was used for other continuous variables. The chi-square test of association evaluated relationships between categorical variables. Simple linear regression was used to estimate the relationship between the infant’s age of vaccination with different explanatory variables; if the association for a particular variable was significant (p < .05), they were included in the multivariate analysis. Since there was no standard definition for delayed vaccination, we defined vaccination delay as anything greater than 2 weeks post the recommended vaccination age (6 wks), as per previous studies.Citation13,Citation15 We categorized the age of our study infants visiting the clinic after on-birth BCG vaccination to start the vaccination program recommended at 6 wks, into two categories: <8 wks and ≥8 wks. Multivariate logistic regression was used to analyze factors associated with vaccination delayed by 2 weeks. Adjusted odds ratios (ORs) with 95% CIs were calculated to determine the association. P-value < .05 was considered statistically significant.
Results
Of the randomly selected 548 mother-infant pairs, 41.8%, 38.0%, and 20.3% were from MCHTI, ICRC, and MSC, respectively. Mother’s characteristics, e.g., age, education, occupation, antenatal TT-vaccination, and type of delivery were similar in the three clinics. Infant characteristics, e.g., sex, sickness since birth, BCG vaccination, low birth weight (LBW) were also equivalent. The proportion of infants ≥8 wks of age who visited the clinic to start “the vaccination program recommended at 6 wks” after on-birth BCG vaccination was slightly lower in MSC compared to the other two clinics (p = .085); however, the mean age of the infants from these clinics was similar. Twenty mothers forgot to bring the infant’s birth card, while 6 mothers lost the card. We collected data from them through an interview. Mother’s characteristics are presented in ; most of the mothers were <30 y, and half of them with their first baby. Four mothers had age <18 y, and we took verbal consent from their husbands. Approximately 10% of mothers worked outside of the home. Half of the surveyed mothers did not receive antenatal TT vaccine in the immediate previous pregnancy. Mothers who had a previous pregnancy were less likely to receive prenatal TT vaccine (p < .01). Neither maternal education level nor occupation is associated with antenatal TT vaccination. There was no association between antenatal TT vaccination and low birth weight or incidence of infection during the early infancy of their infants.
Table 1. Characteristics of mothers
Descriptive information of the infants visiting clinics to start “the vaccination program recommended at 6 wks” (after on-birth BCG vaccination) under EPI is in . Of the 548 infants surveyed, 64% had c-section delivery, similar to the national average of newborns in urban health facilities.Citation5 16% of infants had low birth weight (LBW, weighing < 2,500 g at birth) also similar to the national average.Citation5 Less than 10% of infants had a history of diarrhea and/or breathing difficulties since birth before visiting the clinics for vaccination. 41% of infants did not receive a BCG vaccine within 1 week of birth, and two-third of mothers did not know about the on-birth BCG vaccination program (). As expected, a considerably higher proportion of infants born in the clinics received BCG vaccines within 1 week compared with infants born at home (p < .01). Among the infants born in the clinic, 39% did not receive BCG within 1 week of birth, of them, more than two-thirds were born through c-section delivery. Mothers working outside the home had an impact on BCG vaccination. Among the mothers who stayed home, 60% of their infants received the BCG vaccine, while it was 46% for the mothers working outside the home (p < .05). There was an estimated 43% decline in infants of mothers working outside the home to receive BCG at birth. There was no association between BCG vaccination within the first week of birth and other covariates, including antenatal TT vaccination, maternal schooling years, previous pregnancy, infant sex, and mode of delivery.
Table 2. Characteristics of infants
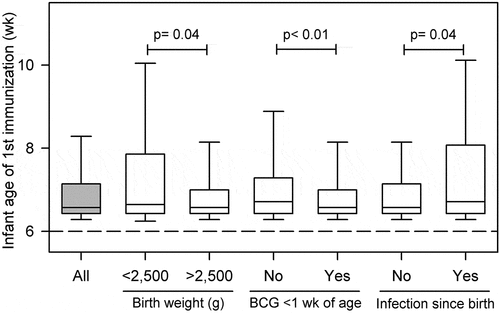
The median age of the infants starting the vaccination program was 6.57 weeks (95% CI: 6.43–7.14), and other than on-birth BCG vaccines, infants did not receive any other vaccines before visiting the clinic to start “the vaccination program recommended at 6 wks”. 17.3% of infants were ≥8 weeks of age and mothers of these infants were mostly unaware of the age of vaccination to begin with or forgot the due date of vaccination for their infants (). In addition, we identified several maternal conditions associated with the delayed immunization; maternal schooling years negatively associated with the infant’s age of vaccination (β± SE: −3.83 ± 1.62; p = .018). Thus, infants of mothers with higher education levels were more likely to receive the first vaccine much closer to the recommended age. Infants with normal birth weight (>2,500 g), infants who received birth-dose BCG, and infants free from infections since birth had a significantly higher likelihood of receiving the vaccines at the recommended age of 6 wks (). Comparing the relative importance of the multiple predictors, we found that infants from mothers with one-year higher schooling year or having an infant with normal birth weight, decreased the risk of receiving vaccine at ≥8 weeks of age by 11% and 55%, respectively, whereas infants that experienced infection after birth had >2.5 times the odds of being vaccinated at ≥8 wks of age (). Infants that received birth-dose BCG had a significantly lower (p = .042) incidence of diarrhea or respiratory distress in the first 2 months of life.
Table 3. Multivariate logistic regression analysis of the factors related to the starting age of infants received vaccines at ≥8 wks
Discussion
Inadequate and incomplete vaccinations are significant public health problems in Bangladesh. Along with these, delaying the start of infant immunization has also become a hindrance to minimizing the morbidity and mortality of infants from vaccine-preventable diseases.Citation16 Despite the high vaccination coverage observed by 2-years of age in Bangladesh, we found the proportion of neonates having received the BCG vaccine within 1 week of birth was only 59% in urban settings (), while it was found 2% in rural areas.Citation8 We observed that mothers working outside the home were one of the major contributing factors for delayed BCG vaccination. In other studies, there are several additional factors reported to be associated with delayed vaccination, namely, living an extended distance from vaccination delivery points, low maternal education,Citation17 a perception that vaccination is unsuitable for very early age, and traditional beliefs not to take the baby out of the home in the early days of life.Citation8 In Bangladesh, the available health facilities had increased significantly from 9% in 2004 to 37% in 2014, and this improvement presumably increased the opportunity for BCG vaccination at birth since the vaccines are only offered in-clinic facilities. However, in this study, we found that among the infants born in urban clinic facilities, 39% were BCG unvaccinated, and 69% of them had a c-section delivery. Mother’s lack of knowledge or ignorance about birth-dose BCG and the mother’s perception that newborns might be sick because of very early age vaccination were the main reasons for the low coverage. Similarly, in rural areas, mother’s understanding of small infant size at birth is found to be negatively associated with timely vaccination.Citation18 However, in our study, classic determinants for on-time infant vaccination such as the mother’s age, education level, previous pregnancy, infant sex, mode of delivery, and household sizes were not associated with on-birth BCG vaccination, which may not be significant in urban settings but maybe substantial in pre-urban and/or rural areas as reported earlier.Citation19,Citation20
In this study, 17.3% of infants were ≥8 weeks of age at the time of starting vaccination. Mothers’ schooling years and infants with normal birth weight significantly improved the infant’s opportunity to receive vaccines at <8 weeks of age. This study, as well as data from other low- and middle-income countries, indicate that infants born with low birth weight (LBW)Citation21 have an increased risk of vaccine-preventable diseasesCitation22–24 because of compromised passive immunity obtained from mothers.Citation25 Infants with LBW can make sub-optimal immune responses to the direct vaccination,Citation26 but the overall protective efficacy and safety of LBW infants are similar to the infants with normal birth weight.Citation26 Therefore, vaccination of LBW infants at the same chronological age is highly recommended. Infections, e.g., diarrhea and/or respiratory distress since birth, could increase the infant age of vaccination by more than 2.5 times. However, it is recommended that infants should receive vaccines with low-grade fever, cold, runny nose, cough, ear infection, and mild diarrhea. Therefore, periodic training for the health care providers regarding newborn and infant vaccination and the level of allowable sickness for the infants to receive vaccines is essential to improve health information for the mothers visiting the clinic.
We also found that 54% of mothers received the TT vaccine during pregnancy (). The Bangladesh demographic dataset does not include TT vaccination coverage during pregnancy, but they report that 64% of pregnant women receive antenatal care (ANC) from medically trained providers.Citation5 Since antenatal TT vaccines are provided only in permanent hospital facilities and not all qualified providers have access to the vaccine, our findings regarding the prevalence of antenatal TT vaccination in our study population are very much consistent with the national average. The national dataset also shows that as birth order increases the proportion of pregnant women seeking ANC goes down,Citation5 which is also consistent with our study that mothers who had previous pregnancies were significantly less likely to receive antenatal TT vaccines. Globally, neonatal tetanus deaths have decreased from 200,000 in 2000 to 49,000 in 2013 and account for only 1% of neonatal deaths globally.Citation27,Citation28 Despite moderate level antenatal TT vaccine coverage, Bangladesh achieved maternal-neonatal tetanus elimination status in 2008, indicating the contribution of improved access to skilled birth attendance throughout the delivery process along with hygienic delivery and cord care practices. In the 2014 national survey, 42% of births were assisted by medically trained providers, which had increased by 2.6 times since 2004.Citation5 Nevertheless, it is essential to strengthening vaccine delivery during pregnancy since it has a much higher potential for infection prevention in high-risk groups even beyond tetanus control. Several vaccine-preventable diseases can cause significant morbidity and mortality in pregnant women, neonates, and infants. In this study, approximately one-fifth of infants in the urban area received their first vaccine dose at ≥8 weeks of age, and in the rural area of Bangladesh, it has been estimated at more than two-third of infants (Hanifi SMA et al. unpublished). Thus, a significant proportion of infants in early life remained unprotected from vaccine-preventable diseases.
A major strength of our study is the study set-up and the large population size. This study was carried out within a short timeframe in the three largest public health facilities of the urban slum area at Dhaka city. Thus, this study’s results reflect a snapshot of the actual scenario of the infant’s vaccination status in early life in this area. A well-structured questionnaire and interviews with the mothers or caregivers provided an in-depth understanding of the factors contributing to delayed vaccination in infants. Our previous study in the same community showed that the study populations were very much similar in their socioeconomic status.Citation29 However, the limitation of this study is that this was a hospital-based study, and other associated factors such as availability of transport, distance to the nearest health facility, and caregiver’s illness that could influence the timeliness of vaccination were not assessed. Another limitation is that the study was conducted in 2015, but the applicability of the study findings for public health policy is still important.
Several studies report interference of maternal antibodies in generating optimal immunity in infants. In our previous research, we found that maternal antibodies, regardless of antenatal vaccination or natural exposure, show similar inhibitory effects on antigen-specific antibody-secreting plasma cell development in response to active immunization of the infantsCitation30 but maternal vaccination does not compromise the minimal cutoff level of the protective antibody titer in infants.Citation30 As a result, expanding the maternal immunization program to include other vaccines for preventable diseases in developing countries can prevent diseases in both the pregnant woman and fetus during a high-risk period in neonatal lives and protect the infants in early life. Since 2011, routine tetanus, diphtheria, and pertussis (Tdap) immunizations of pregnant women have already been implemented in the US and UK; consequently, hospitalizations and deaths from pertussis have reduced significantly in infants at less than 2 months of age.Citation31,Citation32 Therefore, action should be taken to increase ANC and maternal TT immunization. The concerned health department should improve the utilization of prenatal care follow-up, which will increase antenatal immunization coverage.
Conclusions
Proper health education for mothers is needed to address issues regarding the level of allowable sickness for the newborns and infants to receive vaccines. In addition, proper education and training of health care providers in rural/urban health care facilities to promote mothers to seek ANC, vaccination information, and the registering timeline for vaccination is needed to maintain on-time infants’ vaccination. Besides, considering the success of TT immunization programs to prevent maternal and neonatal tetanus, maternal immunization with other vaccines could be a promising strategy for preventing vaccine-preventable diseases in pregnant women and infants during very early infancy before starting the active immunization. Further research is necessary to address the safety and efficacy of the maternal immunization program in low- and middle-income countries.
List of abbreviations
| ANC | = | Antenatal care |
| BCG | = | Bacillus Calmette-Guérin vaccine |
| NIP | = | National Immunization Program |
| PENTA | = | vaccine (Diphtheria, Pertussis, Tetanus, Hepatitis B and Haemophilus influenzae type b vaccine) |
| TT | = | Tetanus toxoid |
Authors’ contributions
MJA designed the study, analyzed the data, interpreted the results, and drafted the manuscript. MNAA and AK analyzed the data and wrote the manuscript. SMA conceptualized, designed and supervised the study, analyzed the data, interpreted the results, drafted and approved the final version of the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors have no competing interests.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Ethics approval and consent to participate
Verbal consent was obtained from all participating mothers visited in the clinics to start the vaccination of their infants following an explanation of the study in the local language.
Acknowledgments
The authors gratefully acknowledge the participation of all mothers.
Additional information
Funding
References
- WHO U, World Bank. State of the world’s vaccines and immunization. WHO, UNICEF, World Bank; 2009.
- Breiman RF, Streatfield PK, Phelan M, Shifa N, Rashid M, Yunus M. Effect of infant immunisation on childhood mortality in rural Bangladesh: analysis of health and demographic surveillance data. Lancet. 2004;364:2204–11. doi:10.1016/S0140-6736(04)17593-4.
- Keja K, Chan C, Hayden G, Henderson RH. Expanded programme on immunization. World Health Stat Q. 1988;41:59–63.
- Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, Jha P, Campbell H, Walker CF, Cibulskis R. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375(9730):1969–87. doi:10.1016/S0140-6736(10)60549-1.
- Bangladesh DHS. National Institute of Population Research and Training (NIPORT), Mitra and Associates, and ICF International. Bangladesh demographic and health survey 2014. Dhaka (Bangladesh, and Rockville, Maryland, USA): NIPORT, Mitra and Associates, and ICF International; 2016.
- Higgins JP, Soares-Weiser K, Lopez-Lopez JA, Kakourou A, Chaplin K, Christensen H, Martin NK, Sterne JAC, Reingold AL. Association of BCG, DTP, and measles containing vaccines with childhood mortality: systematic review. BMJ. 2016;355:i5170. doi:10.1136/bmj.i5170.
- Aaby P, Roth A, Ravn H, Napirna BM, Rodrigues A, Lisse IM, Stensballe L, Diness BR, Lausch KR, Lund N, et al. Randomized trial of BCG vaccination at birth to low-birth-weight children: beneficial nonspecific effects in the neonatal period? J Infect Dis. 2011;204(2):245–52. doi:10.1093/infdis/jir240.
- Hanifi SMA, Das S, Rahman M. Bangladeshi neonates miss the potential benefits of early BCG vaccination. Int J Epidemiol. 2018;47:348–49. doi:10.1093/ije/dyx223.
- Taylor JA, Darden PM, Brooks DA, Hendricks JW, Wasserman RC, Bocian AB. Association between parents’ preferences and perceptions of barriers to vaccination and the immunization status of their children: a study from Pediatric Research in Office Settings and the National Medical Association. Pediatrics. 2002;110:1110–16. doi:10.1542/peds.110.6.1110.
- Favin M, Steinglass R, Fields R, Banerjee K, Sawhney M. Why children are not vaccinated: a review of the grey literature. Int Health. 2012;4:229–38. doi:10.1016/j.inhe.2012.07.004.
- Gust DA, Strine TW, Maurice E, Smith P, Yusuf H, Wilkinson M, Battaglia M, Wright R, Schwartz B. Underimmunization among children: effects of vaccine safety concerns on immunization status. Pediatrics. 2004;114:e16–22. doi:10.1542/peds.114.1.e16.
- Smith LE, Amlot R, Weinman J, Yiend J, Rubin GJ. A systematic review of factors affecting vaccine uptake in young children. Vaccine. 2017;35:6059–69. doi:10.1016/j.vaccine.2017.09.046.
- Clark A, Sanderson C. Timing of children’s vaccinations in 45 low-income and middle-income countries: an analysis of survey data. Lancet. 2009;373:1543–49. doi:10.1016/S0140-6736(09)60317-2.
- Van Den Berg JP, Westerbeek EA, Berbers GA, Van Gageldonk PG, Van Der Klis FR, Van Elburg RM. Transplacental transport of IgG antibodies specific for pertussis, diphtheria, tetanus, haemophilus influenzae type b, and Neisseria meningitidis serogroup C is lower in preterm compared with term infants. Pediatr Infect Dis J. 2010;29:801–05. doi:10.1097/INF.0b013e3181dc4f77.
- Yadav K, Srivastava R, Kumar R, Chinnakal P, Rai SK, Krishnan A. Significant vaccination delay can occur even in a community with very high vaccination coverage: evidence from Ballabgarh, India. J Trop Pediatr. 2012;58:133–38. doi:10.1093/tropej/fmr059.
- Nakatudde I, Rujumba J, Namiiro F, Sam A, Mugalu J, Musoke P. Vaccination timeliness and associated factors among preterm infants at a tertiary hospital in Uganda. PLoS One. 2019;14:e0221902. doi:10.1371/journal.pone.0221902.
- Miyahara R, Jasseh M, Gomez P, Shimakawa Y, Greenwood B, Keita K, Ceesay S, D’Alessandro U, Roca A. Barriers to timely administration of birth dose vaccines in The Gambia, West Africa. Vaccine. 2016;34:3335–41. doi:10.1016/j.vaccine.2016.05.017.
- Vasudevan L, Labrique AB, Mehra S, Wu L, Levine O, Feikin D, Klemm R, Christian P, West KP. Maternal determinants of timely vaccination coverage among infants in rural Bangladesh. Vaccine. 2014;32(42):5514–19. doi:10.1016/j.vaccine.2014.06.092.
- Rainey JJ, Watkins M, Ryman TK, Sandhu P, Bo A, Banerjee K. Reasons related to non-vaccination and under-vaccination of children in low and middle income countries: findings from a systematic review of the published literature, 1999-2009. Vaccine. 2011;29:8215–21. doi:10.1016/j.vaccine.2011.08.096.
- Weiss WM, Winch PJ, Burnham G. Factors associated with missed vaccination during mass immunization campaigns. J Health Popul Nutr. 2009;27:358–67. doi:10.3329/jhpn.v27i3.3378.
- Lee AC, Katz J, Blencowe H, Cousens S, Kozuki N, Vogel JP, Adair L, Baqui AH, Bhutta ZA, Caulfield LE. National and regional estimates of term and preterm babies born small for gestational age in 138 low-income and middle-income countries in 2010. Lancet Glob Health. 2013;1:e26–36. doi:10.1016/S2214-109X(13)70006-8.
- Langkamp DL, Davis JP. Increased risk of reported pertussis and hospitalization associated with pertussis in low birth weight children. J Pediatr. 1996;128:654–59. doi:10.1016/S0022-3476(96)80131-4.
- Shinefield H, Black S, Ray P, Fireman B, Schwalbe J, Lewis E. Efficacy, immunogenicity and safety of heptavalent pneumococcal conjugate vaccine in low birth weight and preterm infants. Pediatr Infect Dis J. 2002;21:182–86. doi:10.1097/00006454-200203000-00003.
- Baxter D. Impaired functioning of immune defenses to infection in premature and term infants and their implications for vaccination. Hum Vaccin. 2010;6:494–505. doi:10.4161/hv.6.6.12008.
- Okoko JB, Wesumperuma HL, Hart CA. The influence of prematurity and low birthweight on transplacental antibody transfer in a rural West African population. Trop Med Int Health. 2001;6:529–34. doi:10.1046/j.1365-3156.2001.00741.x.
- Baxter D. Vaccine responsiveness in premature infants. Hum Vaccin. 2010;6:506–11. doi:10.4161/hv.6.6.12083.
- World Health O. Maternal and Neonatal Tetanus Elimination (MNTE). 2017.
- Khan R, Vandelaer J, Yakubu A, Raza AA, Zulu F. Maternal and neonatal tetanus elimination: from protecting women and newborns to protecting all. Int J Womens Health. 2015;7:171–80. doi:10.2147/IJWH.S50539.
- Ahmad SM, Raqib R, Qadri F, Stephensen CB. The effect of newborn vitamin A supplementation on infant immune functions: trial design, interventions, and baseline data. Contemp Clin Trials. 2016;39:269–79. doi:10.1016/j.cct.2014.09.004.
- Ahmad SM, Alam J, Afsar NA, Huda N, Kabir Y, Qadri F, Raqib R, Stephensen CB. Comparisons of the effect of naturally acquired maternal pertussis antibodies and antenatal vaccination induced maternal tetanus antibodies on infant’s antibody secreting lymphocyte responses and circulating plasma antibody levels. Hum Vaccin Immunother. 2016;12:886–93. doi:10.1080/21645515.2015.1136759.
- Sukumaran L, McCarthy NL, Kharbanda EO, Vazquez-Benitez G, Lipkind HS, Jackson L, Klein NP, Naleway AL, McClure DL, Hechter RC. Infant hospitalizations and mortality after maternal vaccination. Pediatrics. 2018. doi:10.1542/peds.2017-3310.
- Gentile A, Juarez MDV, Lucion MF, Martinez AC, Romanin V, Areso S, Mistchenko A. Bordetella pertussis (Bp) disease: before (2003-2011) and after (2013-2016) maternal immunization strategy in a pediatric hospital. Vaccine. 2018;36:1375–80. doi:10.1016/j.vaccine.2018.01.091.