ABSTRACT
Objective: We characterize public values regarding vaccinomics, which aims to improve vaccine safety and effectiveness using genomics.
Methods: Panel survey (2020) of ≥18-year-olds with embedded animation introduced vaccinomics. Sociodemographic, health, and vaccination-related items were adapted from validated scales. Novel items measured trust in public health authorities, vaccinomics-related values, and preferences for federal funding: vaccinomics compared with vaccine issues and chronic diseases. Beginning and end of survey confidence in vaccine safety was measured to assess potential changes. Data were weighted to the U.S. Census. Vaccinomics-related concerns were stratified by sociodemographic characteristics, vaccine hesitancy status (composite outcome), reported serious vaccine reactions, and trust in public health authorities (PHA). Log binomial regression models estimated associations between these variables and agency to make vaccine-related decisions.
Results: Most (70.7%, N = 1,925) respondents expected vaccinomics would increase their vaccine confidence compared to now. Agreement was highest among those without serious vaccine reaction experience (unexperienced: 74.2% versus experienced: 62.3%), with high trust in PHA (high: 83.3% versus low: 57.4%), and low vaccine hesitancy among parents of teenagers (low: 78.8% versus high: 62.5%) and adults without minor children (low: 79.8% versus high: 60.6%; all p < .01). Belief that vaccination was an individual’s choice was associated with reported serious reactions (adjusted Prevalence Ratio (aPR): 1.16; 95% CI: 1.07, 1.25) and low trust (aPR: 0.91; 0.84, 0.98). Beginning versus end of survey vaccine safety perceptions were similar.
Conclusion: Federal funding, communications, and policies should assure the public that vaccinomics will not remove their decision–making power and engender trust in PHA.
Background
Vaccinomics may improve vaccine development and use by applying advances in immunology, systems biology, and genomics to the study of vaccine response and identification of vaccine candidates.Citation1–4 Here, vaccinomics encompasses adversomics, a subfield focused on the application of genomics to the study of adverse events following immunization (AEFI) temporally, not necessarily causally, associated with vaccination.Citation1,Citation2,Citation5 Vaccinomics may address genomic differences in immune response and vaccine reactions, including due to sex, race, and specific genetic loci .Citation2,Citation6–15 Vaccine development and use may improve due to vaccinomics through the identificaiton of new vaccine candidates, enhanced understanding of immune response, and idenfication of genetic factors associated with increased risk of adverse reactions, leading to prediction and prevention of these events.Citation1–4 Public values, preferences, and concerns should inform vaccinomics policy and development. Vaccinomics may lead to changes in vaccine research and development, clinical trials, licensure, recommendations for use, policies, injury compensation, and communications.
Vaccinomics may impact vaccine hesitancy, one of the top 10 global health threats according to the World Health Organization (WHO).Citation16 Vaccine hesitancy is the delay or refusal of available vaccines.Citation17 In the U.S., 77% of parents report at least one vaccine-related concern, including that children receive too many vaccines at once, vaccines cause autism, and vaccine preventable diseases are not serious.Citation18 Vaccine hesitancy has fueled recurring outbreaks of measles, almost costing the U.S. its measles elimination status in 2019,Citation19 and poor human papillomavirus and influenza vaccine coverage.Citation20
Vaccinomics could improve vaccine safety through idenfication and inclusion of individuals with specific genetic variants in clinical trials, ultimately leading to more personalized vaccine schedules and increased vaccine confidence.Citation1,Citation2 Due to uncertainty surrounding genomics’ predictive power, or by discussing heterogeneity in vaccine response, vaccinomics could raise or amplify vaccine-realted concerns. Similar to other genomic-enabled technologies, policy safeguards need to protect genetic subgroups and prevent vaccinomics from increasing vaccine hesitancy.
Formative work was conducted with vaccine policymakers and community members in Boulder, Colorado and Baltimore, Maryland.Citation21 In 2017, we convened a group of academic vaccinologists and representatives of federal agencies involved in vaccines, including the National Institutes of Health, Food and Drug Administration, Centers for Disease Control and Prevention, and Health Resources and Services Administration (National Vaccine Injury Compensation Program) to discuss what vaccinomics-related policy issues might emerge, and where public input would be useful. To inform survey development, we conducted community meetings in 2018 to elicit public values and the rationale behind participants’ thinking. Participants supported vaccinomics but worried it could lead to stigmatization/discrimination through confidentiality breaches and that genetically-based vaccine prioritization would reduce their ability to make vaccine-related decisions. Regardless, the prospect of vaccinomics leading to more personalized vaccine schedules especially appealed to those who reported experiencing AEFIs.Citation21
In this study, we aimed to characterize public values around vaccinomics policy issues regarding prioritization, stigma/discrimination, funding and implementaion, genetic testing, and vaccine confidence. We aimed to quantify if vaccinomics-related concerns varied within the U.S. population by region, vaccine hesitancy level, perceived AEFI experience, and sociodemographic factors.Citation15,Citation16
Methods
Recruitment and consent
Survey respondents were recruited using a double opt-in process between January 22nd and February 11th 2020 from approximately 10 million members of the Qualtrics® web panel.Citation22 All consented before taking the survey. Quotas based on the American Community Survey,Citation23 Current Population Survey,Citation24 and 2010 CensusCitation25 were included so that respondents would reflect the nation’s sociodemographic distribution. Due to difficulty enrolling individuals with minority race/ethnicity, from the West, and in the lowest income, age, and education brackets, quotas were ignored when recruiting the final 400 (approximate) of 1,925 respondents.
Survey content
Novel items measured vaccinomics–related values, funding and implementation priorities, genetic testing use, and trust in public health authorities.Citation26,Citation27 We adapted validated vaccine hesitancy, sociodemographic, and personal health items.Citation23–25,Citation28–31 Respondents were randomized with 50:50 probability to receive positively or negatively worded vaccinomics items to minimize the effects of agreement bias. Items from preexisting scales were presented in a single version, like how they had previously been fielded. Survey content was revised for clarity and specificity after pretesting was conducted in November and December 2019. Twenty out of 131 pretest respondents, recruited by Qualtrics using similar methods as in this survey, completed cognitive phone interviews. Each received a 20 USD Amazon gift card after the interview.
Vaccinomics-related values and funding priorities
Vaccinomics was introduced to respondents via an embedded, four-minute-long animation (https://tinyurl.com/vaccinomics). Literature reviews and federal regulations influenced the phrasing of other novel items. Hypothetical scenarios framed survey items. Respondents were instructed to imagine that an infectious disease, potentially lethal, is spreading easily and quickly. Due to limited vaccine supplies, not everyone can get vaccinated. Respondents were
informed that “more contagious” individuals are more likely than average to get someone else sick and using genes/DNA, they might be identifiable. Questions about prioritization, altruism, and agency to make vaccine-related decisions followed. Respondents rated their agreement with “If there was a short supply of vaccine, it would make sense for the people ‘more susceptible’ to infection to get it first” and for prioritizing “more contagious” individuals. Respondents reported the likelihood (extremely unlikely/unlikely/likely/extremely likely) of acting altruistically: “If your genes/DNA showed you to be ‘more contagious’ or likely to get other people sick, how likely would you be to get vaccinated to protect other people?”
Two outcomes represented potential opposition to vaccine prioritization: 1) expecting to be angry if not prioritized for vaccination and 2) strongly agreeing/agreeing that vaccination is an individual’s choice. We posed: “If you were told you were NOT going to be among the first groups vaccinated during an infectious disease outbreak because your genetics showed you were not “more contagious” or “more susceptible” to infection, how might you react?” Respondents indicated they would 1) be angry because they would want the vaccine, 2) be okay with this decision, 3) not care because they would not want the vaccine anyway, or 4) other. Respondents also indicated 4-point Likert Scale agreement with: “Getting vaccinated should be an individual’s choice, even if they are ‘more contagious’ or ‘more susceptible.’“
Next, respondents were instructed to imagine a new and serious contagious disease emerged and that the vaccine caused paralysis or death in 1 person in 1 million people vaccinated. The risk of paralysis or death could be reduced to be closer to 0 people in 1 million people vaccinated by using vaccinomics to identify at risk individuals and advising them against vaccination.
Respondents indicated whether their vaccine confidence “would increase if the U.S. government spent more money studying how safe vaccines are now and telling the public the results” and whether the “U.S. government should invest in making vaccines more effective instead of trying to reduce the risk of very serious and rare events, like paralysis and death after getting a vaccine.”
Following these scenarios, the implications of vaccinomics were assessed regarding informed decision-making for oneself and one’s child. Respondents rated their agreement with: “Vaccinomics is likely to … ” “ … help other people,” “ … help me,” and “Vaccinomics would make me have more confidence in vaccines than I do now, and “I would support vaccinomics even if it made vaccine schedules more complex.” Most response options used a 4-point Likert Scale.
Funding and implementation priorities
Respondents’ federal funding priorities were recorded based on the information we provided about vaccinomics and their existing knowledge of public health needs: vaccinomics versus vaccine issues (safety and efficacy; new and free vaccines) and select chronic diseases (cancer, heart disease, and diabetes). Separately for children and adults, respondents indicated if vaccinomics should be used to make vaccines safer and more effective, identify those most likely to have serious and dangerous reactions to vaccines, identify those most likely to be more contagious/susceptible to infection, that genes/DNA should not be used for vaccine–related decisions, or none the above.
Current vaccines and public health authorities
Vaccine attitude and belief measures were adapted from validated scales.Citation29–31 Items for parents of children ≤10 years old were adapted from the Parent Attitudes About Childhood Vaccines (PACV) scale.Citation29,Citation30 Two sets of items were adapted from the Vaccination Confidence Scale (VCS) for parents of teenagers and adults without minor children.Citation31 The number of items and response options were reduced to simplify administration.Citation29–31 To assess the impact of asking questions about vaccine safety and vaccinomics on safety perceptions, respondents rated their agreement with the statement “vaccines are very safe” at the beginning and end of the survey.Citation31 Twenty items on trust in public health authorities (“trust”) were developed through formative work and a literature review.Citation26,Citation27 Influenza vaccination history was also measured.
Serious vaccine reactions and genetic testing
Respondents reported if they or anyone they knew experienced serious vaccine reactions, including “permanent disability, hospitalization, life-threatening illness, or death” (yes/no/don’t know).Citation32 To evaluate respondents’ willingness to participate in vaccinomics, we asked about their past use of genetic testing and hypothetical biobank participation. Respondents indicated if they had their genes/DNA tested in a “doctor’s office or through an in–home test like 23andme, ancestry.com, or one purchased in a pharmacy.” If yes, they indicated where the test was conducted (home or a medical office) and if applicable, with whom results were shared (medical providers, family, or friends).
Regarding future genetic testing related to vaccinomics, respondents rated their agreement with: “I would have my genes/DNA tested if it would help my doctor or another healthcare provider know which vaccines are best for ME.” Respondents reported their level of concern regarding “the security of my gene or DNA test result,” that “my health insurance company would learn my gene or DNA test result,” that “the U.S. government would learn my gene/DNA test result,” and “using genes/DNA in vaccine decisions could increase race/ethnic discrimination.”
Sociodemographic and personal health information
Items measuring sociodemographic information,Citation23–25 the ages of respondents’ youngest children,Citation33 personal health statusCitation28 were adapted from validated scales.
Data analyses
Data were weighted to the 2010 Census by region, Hispanic ethnicity, and race to facilitate making population-level inferences.Citation25 The distributions of survey weights were visualized using histograms and the weighted data were compared to the Census.Citation25
Items measuring vaccine attitudes and beliefs (vaccine hesitancy) were scored and combined into an overall composite score for each respondent (range 0 to 100; higher value indicated higher hesitancy) using a linear transformation.Citation29 Individual vaccine-related statements were scored so that hesitant responses equaled 2, don’t knows equaled 1, and non-hesitant responses equaled 0. The distributions of the composite scores were visualized with histograms. The median in one of the three age groups (parents of children ≤10 years–old, parents of 11-17-year-olds, and adults without minor children) equaled zero, preventing dichotomization at that point. For all age groups, vaccine hesitancy scores were dichotomized at the weighted mean (low versus high hesitancy). The overall and stratified prevalence of low and high vaccine hesitancy was assessed separately by age group and by vaccinomics-related items. A two-sided t-test for survey data estimated the probability that the difference in mean vaccine safety score at the beginning versus end of the survey did not equal 0.
The binary trust variable (low versus high) was derived from a linear score of 14 items, dichotomized at the median, for consistency with prior analyses.Citation34 For vaccinomics items with positive and negative wording, the distributions of each pair were compared. The scale of the negatively worded items was reversed, and responses were combined with the positively worded items for analysis.
Using survey estimation procedures and Taylor-linearized variance estimates,Citation35 univariate and bivariable tabulations were conducted to characterize the associations between vaccinomics-related policy issues and sociodemographic factors, parent status, vaccine hesitancy, trust in public health authorities, and perceived experience with or knowing someone who reported a serious vaccine reaction (“serious reaction”). Vaccinomics variables measured on a 4-point Likert Scale were dichotomized (strongly agree/agree versus strongly disagree/disagree; extremely likely/likely versus extremely unlikely/unlikely) for stratified tabulations and to facilitate multivariable risk factor analyses. Binary comparisons where the difference between two groups was ≥10% were expected to be meaningful to policymakers and are noted in the Results.
Post hoc analyses characterized support opposition to vaccinomics. The proportion of respondents who indicated they would get vaccinated to protect others was cross–tabulated with the proportion who were vaccinated against influenza in 2019-2020 to determine if their behavior matched their claims that they would get vaccinated to help others. Influenza vaccination status was measured first. Additional post hoc analyses characterized agency around vaccine-related decisions. Two outcomes were explored: 1) I would be angry because I would want the vaccine versus a combination of all other response options and 2) agreement that vaccination is an individual’s choice. Prevalence ratios for potential opposition to prioritization were estimated by sociodemographic factors, parent status, trust, vaccine hesitancy (by age group and parity: parents of young children, parents of teenagers, and adults without minor children), and serious reaction experience using the glm procedure for survey data, family(binomial), link(log). Factors associated with either outcome at p < .10 in unadjusted regression models were included in a saturated, adjusted model.
Associations between trust and factors associated with vaccine hesitancy (age, education, and household income);Citation36,Citation37 and vaccine response (gender) were explored.Citation2,Citation6,Citation7 Interaction terms for associations at p < .05 (trust-age and trust-education) were included in saturated regression models. Backwards stepwise regression was used to identify parsimonious models with p ≤ .05. Respondents who answered “prefer not to answer” regarding their income or education were excluded from adjusted models in which these variables were included. Adjusted models explored the association vaccine hesitancy and expecting to feel angry among adults without minor children, not other age groups due to a lack of association in unadjusted models. Since vaccine hesitancy was unassociated with the individual’s choice outcome in unadjusted models, it was excluded from all adjusted models.
This study had 96.9% power to detect a difference of 10% in the prevalence estimates between two groups when the proportion in the reference group was 0.50 and there were 3 times as many respondents in one group versus the other. Two-sided p-values were estimated using general tests of association. All analyses were conducted using Stata®, Version 16.Citation38
Ethical review
The Johns Hopkins Bloomberg School of Public Health Institutional Review Board deemed this study “exempt.”
Results
Characteristics of the study population
Among 52,853 invited individuals, 10,100 individuals entered the survey, and 1,925 individuals completed the survey (response rate 3.6%). The weighted study population was 50.6% female, 61.8% White, non–Hispanic; 36.0% 18- 34 years old, 62.9% had a child <18 years old, and 44.7% had a household annual income ≤$49,999. The distribution of vaccine attitudes and beliefs was right-skewed, where most had few concerns (). High vaccine hesitancy prevalence was 35.6% among parents of young children, 27.6% among parents of teenagers, and 37.7% among adults without minor children. Respondents were geographically diverse (). Less than a third of respondents previously underwent genetic testing (27.3%). Perceived experience with, or knowing someone who had, a serious vaccine reaction (“serious reaction”) was common (19.7%). Most (85.7%) who experienced serious reactions would be extremely likely/likely to participate in a biobank, and 81.9% would likely participate due to biobanks’ independent ethics oversight ().
Figure 1. A. Vaccine hesitancy among parents of young children 10 years old and younger, B. Vaccine hesitancy among parents of children 11 to 17 years old, and C. Vaccine hesitancy among parents without minor children
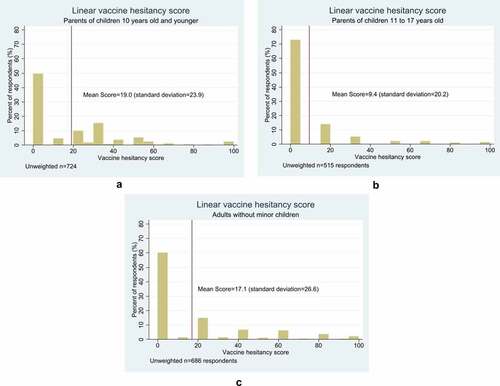
Table 1. Sociodemographic characteristics of the study population: weighted and unweighted
Table 2. The overall frequency and proportion of the study sample experienced with serious reactions to vaccines, genetic testing experience, and support for biobanks
Broad implications of vaccinomics
Most respondents expected vaccinomics to help others (82.6%), themselves (75.8%), and inform their vaccine-related decisions for themselves (91.4%) and their children (92.7%; ). Older respondents were more likely than younger respondents to expect vaccinomics to help others (≥55-years–olds: 90.6% versus 18-34-years-olds: 75.8%) and themselves (≥55-year–olds: 80.9% versus 18-34-year-olds: 70.4%; ). Regarding vaccinomics’ potential to improve vaccine-related decisions for oneself, low more than high vaccine hesitancy among parents of teenagers (97.7% versus 82.8%) and adults without minor children (96.1% versus 81.0%) was associated with agreement. High trust (high:97.4% versus low: 86.0%) was also associated with expecting vaccinomics to improve vaccine-related decision making. Among parents of young children, low vaccine hesitancy was associated with expecting vaccinomics to inform child–related vaccine decisions (low hesitancy: 95.9% versus high hesitancy: 85.9%; ). All comparisons between groups had p < .01).
Table 3. The overall frequency and proportion of participants’ responses to questions about the ethical and policy implications of vaccinomics
Table 4. Among people who agree/strongly agree with hypothetical scenarios, the frequency and proportion of participants’ concerns about the ethical and policy implications of vaccinomics: stratified by sociodemographic characteristics
Table 5. Among people who agree/strongly agree with hypothetical scenarios, the frequency and proportion of participants’ concerns about the ethical and policy implications of vaccinomics: stratified by experience with a serious vaccine reaction, vaccine hesitancy, and trust in public health authorities
Among all respondents, most expected vaccinomics to help others (82.6%), help themselves (75.8%), and increase their confidence in current vaccines (70.7%; ). Older age was also associated with expecting vaccinomics to help oneself (≥55: 80.9% vs. 18–34: 70.4%; ). Expecting vaccinomics to increase one’s confidence was more common among those with a household annual income ≥$150,000 (80.7%) versus ≤$49,000 (68.2%). Respondents without serious reaction experience (unexperienced: 70.0% versus experienced: 57.6%), with low vaccine hesitancy (parents of teenagers – low: 78.8% versus high: 62.5% and adults without minor children – low: 75.3% versus high: 60.6%), age ≥55 (90.6%) was associated with expecting vaccinomics to help others, compared to age 18-34 years-old (75.8%; all p < .01; ).
Even if vaccinomics leads to more complex vaccine schedules, 71.2% of respondents indicated they would support vaccinomics (). There was ≥14% variance between groups among those with high versus low trust (89.1% versus 63.5%), without versus with serious reaction experience (75.4% versus 59.9%), among parents of teenagers with low versus high vaccine hesitancy (77.2% versus 63.1%) and adults without minor children (77.5% versus 61.3%; ).
Most respondents (76.8%) reported they would undergo genetic testing if it would help their healthcare provider know which vaccines were best for them (). Low vaccine hesitancy was associated with an increased likelihood of undergoing genetic testing among parents with young children (low: 79.1% versus high: 70.5%), parents of teenagers (low: 83.5% versus high: 62.2%), and adults without minor children (low: 84.4% versus high: 67.4%). High trust (low: 63.5% versus high: 89.1%) and lack of vaccine reaction experience (without: 80.9% versus with: 64.8%; ) were also associated with higher willingness to undergo genetic testing.
Privacy
Approximately half of respondents indicated they would be concerned about the security of their genetic test result (56.3%) and that their health insurance company (45.5%) or that the U.S. government would learn their results (47.0%). Less than a quarter of respondents worried their genetic test result would prevent them from getting a vaccine (22.0%; ); however, concerns varied by education level (graduate degree: 28.1% versus college experience: 18.7%; ), experience with serious reactions (with: 33.7% versus without: 18.3%) and trust (low: 30.5% versus high 13.8%; ).
Discrimination
Although concern that one’s DNA test result could lead to race/ethnic discrimination was low (29.0%; ), results varied by level of trust (low: 38.9% versus high: 19.9%), experience with serious vaccine reactions (experienced: 42.8% versus unexperienced: 24.9%; ), and race/ethnicity (White, non-Hispanic: 28.1%, Black, non-Hispanic: 38.7%, Other: 28.6%; data not shown).
Prioritization
If vaccines were in short supply, three-quarters of respondents strongly agreed/agreed that more susceptible (74.5%) and contagious (75.7%) individuals should be prioritized for vaccination (). Respondents without serious reaction experience (unexperienced: 78.8% versus experienced: 59.4%) and those with high trust (high: 84.4% versus low: 63.3%) also supported prioritization. Analyses regarding prioritizing more contagious individuals were similar. Additionally, parents of teenagers with low vaccine hesitancy (80.9%) were more likely to support prioritizing more contagious individuals than those with high vaccine hesitancy (69.0%; ).
Table 6. The overall frequency and proportion of participants’ responses to questions about vaccine prioritization, screening tests, and government spending to improve vaccine safety
Table 7. Among people who agree/strongly agree with hypothetical scenarios, the frequency and proportion of participants’ concerns related to vaccine prioritization and screening: stratified by experience with a serious vaccine reaction, vaccine hesitancy, and trust in public health authorities
Being labeled “more susceptible” or “more contagious”
Less than half of respondents indicated “It would bother me if my doctor identified me as “more contagious” or “more susceptible” (41.3%), or that “Identifying people as “more contagious” will hurt people” (38.9%; ). Those without serious reaction experience (unexperienced: 38.1% versus experienced: 50.7%) and low trust (low: 47.3% versus high: 35.9%) were more likely than others to indicate they would be bothered by the “more susceptible” and “more contagious” labels. When asked if these labels would hurt others, compared to oneself, the results were similar, but agreement that it would be bothersome differed by perceived serious reaction experience (without: 36.2% versus with: 45.1%) and level of trust (low: 49.1% versus high: 30.1%; ).
Most respondents agreed (63.0%) vaccination should be an individual’s choice and 23.2% expected to be angry if not prioritized for vaccination (). Overall, agreement with vaccination being an individual’s choice was higher among those with serious reaction experience (experienced: 73.9% versus unexperienced: 60.4%), and higher education (graduate degree: 72.2% versus high school: 62.9%; and Supplementary Table 1).
Over half of respondents (56.2%) indicated they would get vaccinated to protect others if they were identified as being more contagious. In a post–hoc analysis, 62.8% of those who would be extremely likely/likely to get vaccinated had been vaccinated against influenza during the 2019–2020 season.
Using vaccinomics to improve vaccine safety
Support for genetic screening tests to identify individuals at increased risk of paralysis and death was high (64.7%), and 66.9% said their vaccine confidence would increase if the U.S. spent more to study vaccine safety and informed the public of the results (). Those without serious vaccine reaction experience (unexperienced: 67.8% versus experienced: 57.0%) and high trust (high: 70.8% versus low: 58.2%) also supported screening tests ().
Funding and implementation
More than 85.0% of participants indicated vaccinomics should get more or an equal amount of money compared to current chronic disease (research and development for breast and prostate cancer, diabetes, and heart disease) and vaccine priorities (improved safety and effectiveness, buying vaccines for poor children, and supporting the use of vaccines in poor countries; ). Most respondents (71.0%) strongly agreed/agreed that the U.S. government should invest in making vaccines more effective rather than trying to reduce the occurrence of rare and serious events, like paralysis and death ().
Table 8. The overall frequency and proportion of how respondents prefer the U.S. government prioritize funding and implement vaccinomics
Respondents indicated vaccinomics should be similarly implemented for children and adults, respectively, to improve vaccine safety and effectiveness (78.6%, 72.1%), identify individuals at risk of serious reactions (61.0%, 61.7%), and identify those likely to be more contagious (54.0%, 54.3%) or more susceptible (50.6%, 50.2%). Fewer than 15.0% opposed using genetic information in vaccine decisions for children (14.3%) or adults (13.7%; ).
Vaccine safety
Among all participants, the mean level of agreement that vaccines are very safe increased by 0.10 (p < .01) from the beginning of the survey to the end of the survey (range 0-3; higher score indicates higher agreement).
Agency
Trust and experience with a vaccine reaction were associated with feeling angry if not prioritized and believing vaccination is an individual’s choice (p < .05). Vaccine hesitancy was only associated with expecting to feel angry among adults without minor children ().
Table 9. Potential associations between opposition to vaccinomics and prioritization: expecting to feel angry if not prioritized for vaccination and strongly agreeing/agreeing that vaccination is an individual’s choice
Table 10. Potential associations between expected opposition to vaccinomics and prioritization: expecting to feel angry if not prioritized for vaccination and strongly agreeing/agreeing that vaccination is an individual’s choice
Angry if not prioritized
In unadjusted regression models that included all respondents, experience with serious reactions, age, education, household income, parent status, trust, region, race/ethnicity were associated with expecting to feel angry if not prioritized for vaccination because of vaccinomics. Vaccine hesitancy was associated with expecting to feel angry at p < .10 among adult without minor children, but not among respondents with children <18 years old (p > .1, ). In adjusted analysis of adults without minor children, experience with serious reactions, low trust in public health authorities, and high vaccine hesitancy were associated with expecting to feel angry if not prioritized (all p ≤ 0.03). Respondents with perceived serious reaction experience were more likely than those without this experience to indicate they would be angry if not prioritized, after controlling for level of vaccine hesitancy and trust (Prevalence Ratio [PR]: 1.84, 95% CI: 1.25, 2.70; p < .01). High vaccine hesitancy (PR: 0.49; 95% CI: 0.32, 0.73; p < .01) and low trust (PR: 0.67, 95% CI: 0.47, 0.96; p = .03) were associated with lower prevalence of expecting to feel angry, controlling for other variables in the model ().
Vaccination is an individual’s choice
In unadjusted regression models, serious reactions, age, parent status, household income, trust, region, and vaccine hesitancy among parents of young children were associated with higher prevalence of agreement that vaccination is an individual’s choice (all p ≤ 0.10, ). Vaccine hesitancy was unassociated with individual’s choice among parents of teenagers and adults without minor children. In adjusted analyses of all respondents, the prevalence of agreement was higher among those with serious reaction experience, low trust, a high school education, and parents of children <18 years old. After controlling for other variables in the model, respondents who had experience with a serious vaccine reaction were 16% (95% CI: 7%, 25%; p < .01) and those with low trust in public health authorities were 9% (95% CI: 2%, 16%; p = .01) more likely to strongly agree/agree that vaccination is an individual’s choice compared to other respondents ().
Discussion
Respondents strongly supported funding for and implementing vaccinomics, suggesting that vaccinomics – if it improves vaccine development, effectiveness, and safety – may bolster vaccine confidence. Respondents indicated vaccinomics should get at least as much funding as current federal priorities. A minority of respondents with low trust in public health authorities strongly opposed genetically-based vaccine prioritization; increasing trust in public health authorities might engender trust in vaccinomics and genetically-based vaccine prioritization.
This survey was conducted before the WHO declared COVID-19 a pandemic, or it dominated the U.S. news.Citation39 The scenarios presented in the survey, where some people might be “more contagious” or “more susceptible” to infection and that vaccine may be in short supply, are now more realistic. Due to the pandemic, most Americans have had their daily routines upended and experienced a vaccine shortage firsthand– less than 15% of each racial/ethnic group has been vaccinated, with vaccination rates twice as high among Whites compared to Blacks and three times as high among Whites compared to Hispanics.Citation40 Though high trust in public health authorities was associated with increased support for vaccinomics and vaccine prioritization, these associations may differ now. Public disputes between President Trump, other politicians, and public health experts may have dampened trust in public health authorities, similar to how trust in government has been on the decline for fifty years.Citation41 Interest in vaccinomics, despite the potential for more complex vaccine schedules, may be higher now that vaccines have been billed as the key to returning to normal, or lower, due to a perception that COVID-19 vaccine development and evaluation was rushed. In a rapid systematic review of surveys conducted between January and October 2020, the most commonly cited reasons respondents expected to refuse COVID-19 vaccines were concerns about side effects, safety, and effectiveness.Citation42
Serious vaccine reactions
A higher proportion of respondents than expected, based on the well-established vaccine safety profiles, indicated they experienced or knew someone who had a serious vaccine reaction.Citation43,Citation44 Respondents may have reported experience with milder, yet concerning, events than defined in the survey. Perceived experience with life-threating events was associated with opposition to vaccine prioritization. If vaccinomics improves perceptions of vaccine safety, this may lead to greater vaccine uptake.
Based on formative work, we expected those who reported experiencing serious reactions and high vaccine hesitancy to be most supportive of vaccinomics. Instead, most respondents supported vaccinomics regardless of their vaccine hesitancy status or experience with serious reactions. These findings may be due to differences in data collection methods. In the formative study, experience with AEFI may have motivated attendance, making our formative study participants less representative of the U.S. population compared to survey respondents. Though some of the vaccine hesitancy items used were validated in prior online studies, the PACV was designed for in-person administration.Citation29,Citation30,Citation45 Administering PACV items online may reduce their sensitivity to identify highly vaccine hesitant individuals.
Stigma/discrimination
Underrepresentation of minorities and methodological differencesCitation21 may explain why concerns about racial/ethnic discrimination were less prevalent in the survey than expected. In the formative study, when someone articulated concerns about stigma/discrimination, this may have triggered agreement in others, which did not occur in the survey. Although we set enrollment quotas for racial/ethnic minorities based on national-level estimates, quotas for Hispanics and Asians were unfilled. Public awareness of systemic racism has increased since data were collected through the prominence of the Black Lives Matters movement and preponderance of COVID-19 deaths among Black and Hispanic communities. Raising awareness of existing nondiscrimination legislation, emphasizing that prioritization will not be by race/ethnicity, and implementing policies that thwart racial/ethnic discrimination by insurance companies may encourage vaccinomics participation.Citation40,Citation46
Strengths and limitations
Two methodologic issues deserve reflection: the validity of the survey and the representativeness of the study population. The 15-item PACV and 5-item PACV Short Scale were developed for use in medical offices to screen parents of young children.Citation29,Citation30 The Vaccination Confidence Scale was developed for online administration, but with a 10-point Likert Scale rather than the 4-point scale used here.Citation31 Although the internal validity of included vaccine hesitancy items had previously been demonstrated,Citation30 they may not have had high external validity when used online. Adaption of existing items likely gave the survey higher validity than if we had created novel ones. Additionally, the embedded animation highlighted more of vaccinomics’ and adversomics’ potential benefits than negative consequences so that respondents could individually identify potential harms. In the funding priorities exercise, respondents may have been biased by having just learned about vaccinomics. We did not educate them about chronic disease burdens or the other vaccine issues to which they compared vaccinomics’ funding. This may have led to agreement bias.
Regarding representativeness, this survey likely excluded the 10% of the U.S. population without e-mail accounts or Internet access in the U.S.,Citation47,Citation48 and given the length of the survey and embedded video, those with slow or inconsistent Internet access would may have been excluded. However, the survey was accessible on mobile devices and data were weighted to the Census to facilitate making national-level inferences.Citation25 This was not a probability–based sample and some recruitment quotas were unmet. Weighted data were comparable to the 2010 Census and 2015-2016 NHANES results for four common items, despite methodological differences.Citation25,Citation28 This suggests that inferences from our survey can be extrapolated nationwide.Citation25 Although weighting inflates variance estimates compared to a simple random sample,Citation49 we found strong statistical associations. Cross-sectional surveys are limited by only collecting data at one point in time; these results may not be transportable over time if respondents’ trust in public health authorities is sensitive to recent news, like the COVID-19 pandemic, prioritization of COVID-19 vaccines, or Black Lives Matters movement.
Conclusion
Respondents strongly supported vaccinomics and indicated it may improve their vaccine confidence. Federal funding, communications, and policies need to engender trust in public health authorities and vaccinomics. Assurances that vaccinomics will not remove the public’s agency to make vaccine-related decisions may bolster participation. Research on what level of trust motivates vaccine acceptance is needed.
Declaration of competing interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: A Sutherland, RJ Limaye, M Blunt, J Brewer, B Carleton, TA Holroyd, G Geller, and J Kahn have no conflicts and report no financial disclosures. JE Gerber received support from RTI International for manuscript revisions. DA Salmon received consulting and/or research support from Janssen, Merck, and Walgreens.
Supplemental Material
Download ()Acknowledgments
We thank our respondents for their time and contributions.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2021.1911217.
Additional information
Funding
References
- Poland GA, Kennedy RB, McKinney BA, Ovsyannikova IG, Lambert ND, Jacobson RM, Oberg AL. Vaccinomics, adversomics, and the immune response network theory: individualized vaccinology in the 21st century. Semin Immunol. 2013;25(2):89–103. doi:10.1016/j.smim.2013.04.007.
- Poland GA, Ovsyannikova IG, Kennedy RB. Personalized vaccinology: a review. Vaccine. 2018;36(36):5350–57. doi:10.1016/j.vaccine.2017.07.062.
- Poland GA, Jacobson RM, Ovsyannikova IG. Trends affecting the future of vaccine development and delivery: the role of demographics, regulatory science, the anti-vaccine movement, and vaccinomics. Vaccine. 2009;27(25–26):3240–44. doi:10.1016/j.vaccine.2009.01.069.
- Poland GA, Ovsyannikova IG, Jacobson RM, Smith DI. Heterogeneity in vaccine immune response: the role of immunogenetics and the emerging field of vaccinomics. Clin Pharmacol Ther. 2007;82(6):653–64. doi:10.1038/sj.clpt.6100415.
- World Health Administration. Adverse events following immunization (AEFI). Global Vaccine Safety; 2018 [accessed 2019 Oct 29]. https://www.who.int/vaccine_safety/initiative/detection/AEFI/en/.
- Dhakal S, Klein SL, Coyne CB. Host factors impact vaccine efficacy: implications for seasonal and universal influenza vaccine programs. J Virol. 2019;93(21). doi:10.1128/JVI.00797-19.
- Klein SL, Marriott I, Fish EN. Sex-based differences in immune function and responses to vaccination. Trans R Soc Trop Med Hyg. 2015;109(1):9–15. doi:10.1093/trstmh/tru167.
- Halsey NA, Griffioen M, Dreskin SC, Dekker CL, Wood R, Sharma D, Jones JF, LaRussa PS, Garner J, Berger M, et al. Immediate hypersensitivity reactions following monovalent 2009 pandemic influenza A (H1N1) vaccines: reports to VAERS. Vaccine. 2013;31(51):6107–12. doi:10.1016/j.vaccine.2013.09.066.
- Griffioen M, Halsey N. Gender differences in immediate hypersensitivity reactions to vaccines: a review of the literature. Public Health Nurs. 2014;31(3):206–14. doi:10.1111/phn.12073.
- Zent O, Arras-Reiter C, Broeker M, Hennig R. Immediate allergic reactions after vaccinations–a post-marketing surveillance review. Eur J Pediatr. 2002;161(1):21–25. doi:10.1007/s00431-001-0853-0.
- Zerbo O, Modaressi S, Goddard K, Lewis E, Bok K, Gans H, and Klein NP. Parental risk factors for fever in their children 7-10 days after the first dose of measles-containing vaccines. Hum Vaccin Immunother. 2020;16(4):875–880. doi:10.1080/21645515.2019.1675458.
- Tartof SY, Tseng HF, Liu AL, Qian L, Sy LS, Hechter RC, Michael Marcy S, Jacobsen SJ. Exploring the risk factors for vaccine-associated and non-vaccine associated febrile seizures in a large pediatric cohort. Vaccine. 2014;32(22):2574–81. doi:10.1016/j.vaccine.2014.03.044.
- Vestergaard M, Hviid A, Madsen KM, Wohlfahrt J, Thorsen P, Schendel D, Melbye M, Olsen J. MMR vaccination and febrile seizures: evaluation of susceptible subgroups and long-term prognosis. JAMA. 2004;292(3):351–57. doi:10.1001/jama.292.3.351.
- Klein NP, Lewis E, McDonald J, Fireman B, Naleway A, Glanz J, Jackson LA, Donahue JG, Jacobsen SJ, Weintraub E, et al. Risk factors and familial clustering for fever 7-10 days after the first dose of measles vaccines. Vaccine. 2017;35(12):1615–21. doi:10.1016/j.vaccine.2017.02.013.
- Feenstra B, Pasternak B, Geller F, Carstensen L, Wang T, Huang F, Eitson JL, Hollegaard MV, Svanström H, Vestergaard M. Common variants associated with general and MMR vaccine-related febrile seizures. Nat Genet. 2014;46(12):1274–82. doi:10.1038/ng.3129.
- World Health Organization. Ten threats to global health in 2019;2019. https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019
- World Health Organization. Report of the SAGE working group on vaccine hesitancy; 2014.
- Kennedy A, LaVail K, Nowak G, Basket M, Landry S. Confidence about vaccines in the United States: understanding parents’ perceptions. Health Aff (Millwood). 2011;30(6):1151–59. doi:10.1377/hlthaff.2011.0396.
- Patel M, Lee AD, Clemmons NS, Redd SB, Poser S, Blog D, Zucker JR, Leung J, Link-Gelles R, Pham H, et al. National update on measles cases and outbreaks - United States, January 1-October 1, 2019. MMWR Morb Mortal Wkly Rep. 2019;68(40):893–96. doi:10.15585/mmwr.mm6840e2.
- Williams WW, Lu P-J, O’Halloran A, Kim DK, Grohskopf LA, Pilishvili T, Skoff TH, Nelson NP, Harpaz R, Markowitz LE, et al. Surveillance of vaccination coverage among adult populations - United States, 2015. MMWR Surveill Summ. 2017;66(11):1–28. doi:10.15585/mmwr.ss6611a1.
- Gerber JE, Brewer J, Limaye RJ, Sutherland A, Geller G, Spina CI, Salmon DA. Ethical and policy implications of vaccinomics in the United States: community members' perspectives. Hum Vaccin Immunother. 2021:1–12..
- Qualtrics. Qualtrics XM; 2020 [accessed 2020 Mar 7]. https://www.qualtrics.com.
- United States Census Bureau. American community survey (ACS). 2018 [accessed 2021 April 29]. https://www.census.gov/programs-surveys/acs.
- United States Census Bureau. Current population survey (CPS). 2019 [accessed 2021 April 29]. https://www.census.gov/programs-surveys/cps.html.
- United States Census Bureau. Decennial census tables; 2010 [accessed 2020 Mar 26]. https://www.census.gov/programs-surveys/decennial-census/data/tables.2010.html.
- Salmon DA, Moulton LH, Omer SB, deHart MP, Stokley S, Halsey NA. Factors associated with refusal of childhood vaccines among parents of school-aged children: a case-control study. Arch Pediatr Adolesc Med. 2005;159(5):470–76. doi:10.1001/archpedi.159.5.470.
- National Vaccine Advisory Committee. White paper on the United States vaccine safety system, Version 3.0; 2011. https://www.hhs.gov/sites/default/files/NVAC-White-Paper-Vaccine-Safety-System.pdf
- National Center for Health Statistics. NHANES 2015-2016 questionnaire data [accessed 2020 Mar 26]. https://wwwn.cdc.gov/nchs/nhanes/search/datapage.aspx?Component=Questionnaire&CycleBeginYear=2015.
- Opel DJ. PACV short scale, Gerber J editors. Opel DJ; 2018.
- Opel DJ, Taylor JA, Zhou C, Catz S, Myaing M, Mangione-Smith R. The relationship between parent attitudes about childhood vaccines survey scores and future child immunization status: a validation study. JAMA Pediatr. 2013;167(11):1065–71. doi:10.1001/jamapediatrics.2013.2483.
- Gilkey MB, Magnus BE, Reiter PL, McRee A-L, Dempsey AF, Brewer NT. The vaccination confidence scale: a brief measure of parents’ vaccination beliefs. Vaccine. 2014;32(47):6259–65. doi:10.1016/j.vaccine.2014.09.007.
- U.S. Food and Drug Administration. Code of federal regulations title 21, volume 5 (21CFR312.32); 2017. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=312.32
- Lofquist D, Lugaila T, O’Connell M, Feliz S. Households and families: 2010. In: Bureau USC, editor. U.S. Census Bureau; 2012.
- Holroyd TA, Limaye RJ, Gerber JE, Rimal RN, Brewer J, Musci RJ, Sutherland A, Geller G, Salmon DA. Development of a scale to measure trust in public health authorities: Prevalence of trust and association with vaccines. The Journal of Health Communication. In press.
- StataCorp LLC. Survey data reference manual, in release 16. College Station (Texas): Stata Press; 2019. p. 215.
- Salmon DA, Dudley MZ, Glanz JM, Omer SB. Vaccine hesitancy: causes, consequences, and a call to action. Vaccine. 2015;33(Suppl 4):D66–71. doi:10.1016/j.vaccine.2015.09.035.
- Salmon DA, Dudley MZ, Glanz JM, Omer SB. Vaccine hesitancy: causes, consequences, and a call to action. Am J Prev Med. 2015;49(Suppl 6):S391–8. doi:10.1016/j.amepre.2015.06.009.
- StataCorp LLC. Stata 16; 2019 [accessed 2019 Nov 1. https://www.stata.com/.
- World Health Organization. WHO director-general’s opening remarks at the media briefing on COVID-19-11 March 2020; 2020 Mar 11 [accessed 2020 Mar 28]. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19—11-march-2020.
- Ndugga N, Pham O, Hill L, Artiga S, Mengistu S. Latest Data on COVID-19 Vaccinations Race/Ethnicity Online: Kaiser Family Foundation; 2021 [updated 2021 Feb 18; cited 2021 Feb 20]. https://www.kff.org/coronavirus-covid-19/issue-brief/latest-data-on-covid-19-vaccinations-race-ethnicity/.
- Pew Research Center. Public trust in government: 1958-2019; 2019 Apr 11 [accessed 2020 Mar 27]. https://www.people-press.org/2019/04/11/public-trust-in-government-1958-2019/.
- Lin C, Tu P, Beitsch LM. Confidence and receptivity for COVID-19 vaccines: a rapid systematic review. Vaccines (Basel). 2020;9(1). doi:10.3390/vaccines9010016.
- Dudley MZ, Salmon DA, Halsey NA, Orenstein WA, Limaye RJ, O’Leary ST, Omer SB. The clinician’s vaccine safety resource guide: optimizing prevention of vaccine-preventable diseases across the lifespan. Cham (Switzerland): Springer Nature; 2018.
- Salmon DA, Proschan M, Forshee R, Gargiullo P, Bleser W, Burwen DR, Cunningham F, Garman P, Greene SK, Lee GM, et al. Association between Guillain-Barré syndrome and influenza A (H1N1) 2009 monovalent inactivated vaccines in the USA: a meta-analysis. Lancet. 2013;381(9876):1461–68. doi:10.1016/S0140-6736(12)62189-8.
- Oladejo O, Allen K, Amin A, Frew PM, Bednarczyk RA, Omer SB. Comparative analysis of the Parent Attitudes about Childhood Vaccines (PACV) short scale and the five categories of vaccine acceptance identified by Gust et al. Vaccine. 2016;34(41):4964–68. doi:10.1016/j.vaccine.2016.08.046.
- Prince AER. Political economy, stakeholder voices, and saliency: lessons from international policies regulating insurer use of genetic information. J Law Biosci. 2018;5(3):461–94. doi:10.1093/jlb/lsz001.
- Pew Research Center. Internet/broadband fact sheet. [ Webpage]; 2019 Jun 12 [accessed 2021 Feb 20]. https://www.pewresearch.org/internet/fact-sheet/internet-broadband/.
- Tankovska H. E-mail usage in the United States - statistics & facts; 2021 Feb 17 [accessed 2021 Feb 20]. https://www.statista.com/topics/4295/e-mail-usage-in-the-united-states/.
- Korn EL, Graubard BI. Analysis of health surveys. Vol. 323. John Wiley & Sons; 2011. https://onlinelibrary.wiley.com/doi/book/10.1002/9781118032619
