ABSTRACT
The current study examines differences between COVID-19 vaccine intention and delay. A survey was administered to 585 US respondents in late November 2020 as part of an online longitudinal study. Respondents provided information on whether they would obtain a COVID-19 vaccine, once available, and how long they intended to wait before obtaining it. In the negative intention group, 3.4% reported waiting a few weeks, 34.0% waiting a few months, and 62.6% never getting vaccinated. In multivariable models, social norms were a significant and independent predictor of all vaccine delay and intention models. Vaccine delay was associated with low levels of worry about becoming infected with COVID-19, political conservatism, concerns about vaccine side effects, and low levels of believing a vaccine would be effective. Negative vaccine intentions were associated with worries about becoming infected with COVID-19, concerns about vaccine side effects, beliefs that the vaccines were developed too quickly, and low endorsement of the altruistic belief that older people should have vaccination priority. The study results highlight the importance of a multifactorial approach to assessing vaccine attitudes. The findings suggest that uptake programs should focus on enhancing pro-vaccine norms.
A widely accepted and effective vaccine program is critical for mitigating the COVID-19 pandemic. In a US national poll conducted in December 2020, approximately one-quarter (27%) of respondents stated that they probably or definitely would not get a COVID-19 vaccine, even if available for free and deemed safe by scientists.Citation1 This poll was administered after the Pfizer-BioNTech vaccine was reported to have a high efficacy of 95.0% and approved for use in multiple countries, and the Moderna vaccine had reached 94.5% efficacy and was approved for emergency use in the US.Citation2 These polling results show that many Americans are hesitant about getting vaccinated and suggest that achieving adequate levels of vaccine coverage in the US will be difficult. In order to promote widespread vaccine uptake, there is a critical need for a greater understanding of COVID-19 vaccine intentions and hesitancy.
Vaccine hesitancy, defined by the World Health Organization (WHO) as the “delay in acceptance or refusal of vaccines despite availability of vaccine services,” is a major obstacle to achieving widespread vaccination.Citation3–5 Vaccine hesitancy is often conceptualized as a spectrum ranging from refusal to acceptance and has been identified as a major determinant of vaccine uptake.Citation6 Several theoretical models of vaccine hesitancy have been proposed. Vaccine hesitancy has been conceptualized using decision-making and risk perception frameworks within domains of perceived benefits and risks, especially concerning side effects.Citation7 A review by Peretti-Watel et al.Citation8 posits two domains of vaccine hesitancy, one on decision-making based on individuals’ risk assessment and another on trust toward health authorities. Other researchers have developed typologies for vaccine hesitancy based on vaccine attitudes and behaviors.Citation9,Citation10 Much of the research on vaccine hesitancy has focused on parental attitudes toward their children’s vaccination; with COVID-19, however, the focus of the vaccine testing, due to trends in mortality rates, has been on adults.
Research by Henrich and HolmesCitation11 suggests that newer vaccines tend to generate greater levels of vaccine hesitancy, which is particularly concerning for the COVID-19 vaccines for several reasons. First, COVID-19 vaccines’ side effects may be a salient source of concern as the vaccines rely on novel approaches and vaccination mechanisms that are either relatively new or previously unused. Despite an excellent safety profile in Phase 3 clinical trials, the AstraZeneca trial was stopped to investigate a serious adverse event.Citation12 As different COVID-19 vaccines are rolled out, other adverse events will continue to be highlighted in the media and may influence COVID-19 vaccine attitudes. Another factor that may further influence public perception is the rapid development of COVID-19 vaccines.Citation13 Although safety and quality standards were upheld throughout the development and approval processes, modified standard clinical trial procedures, such as parallel clinical trial stages, may exacerbate public confusion or concerns. Additional factors may also contribute to vaccine hesitancy, such as the political polarization of the public health response to COVID-19, which has included downplaying disease severity and spreading misinformation in multiple countries.Citation14,Citation15 Political ideology and party affiliation have already been linked to engagment in COVID-19 prevention behaviors and may also play a role in COVID-19 vaccine behaviors.Citation16 Undoubtedly, COVID-19 misinformation prevalent on social media, including the spread of COVID-19 conspiracy theories, is likely to influence COVID-19 vaccine attitudes and behaviors.Citation17–22
In the current study, we investigate vaccine hesitancy among a sample of adults in the US using two dependent variables of vaccine intention and vaccine delay. Since vaccine hesitancy is multifaceted and understood to encompass several factors, we first studied the vaccine intention component of vaccine hesitancy, which is commonly done in the literature. This vaccine intention construct measures positive, neutral, or negative intention toward obtaining the vaccine, once available. There is no temporal component associated with the vaccine intention construct. Based on formative research, we also assessed a novel construct of “vaccine delay” as a potential contributor to vaccine hesitancy. We constructed this concept of vaccine delay, which has a temporal component, based on our expectation that some individuals would want to obtain the vaccine immediately, while others might plan to wait a few weeks, or months, and some may have no intention of ever getting the vaccine. Existing vaccines are typically administered on an immunization schedule, and the optionality of the COVID-19 vaccines – combined with limited supply and highly politicized nature – is unprecedented. Thus, we anticipated that, in addition to vaccine intention, characterizing vaccine delay would be important in differentiating vaccine-hesitant subgroups. To this end, we assess in this study how vaccine delay and vaccine intention correlate with various sociodemographic, behavioral, and perception-based factors hypothesized to be associated with vaccine hesitancy.
In this study, we used a risk perception framework.Citation9 This framework posits that perceived personal risks and benefits of vaccination strongly influence vaccine intentions. As part of an ongoing longitudinal study, we have developed a set of quantitative survey questions to assess COVID-19 vaccine concerns based on qualitative assessments of vaccine hesitancy. The qualitative assessments identified both factors that overlap with prior research on vaccine concerns (e.g. concern about vaccine side effects) and concerns that are more salient with the COVID-19 vaccines in particular (e.g. vaccines developed too quickly).Citation23–29 Additionally, we examined the vaccine’s perceived effectiveness, which previous research has identified as an important determinant of vaccine intentions.Citation23 We also assessed if altruistic beliefs, such as the belief that vulnerable groups should get the vaccine first, would cause some people to desire to delay vaccination.
We anticipated that respondents might also be influenced by vaccine attitudes among their close social networks due to the novel nature of COVID-19 vaccines and the complex development and approval processes. Relatively few studies have examined how social norms may influence vaccine intentions and behaviors. Only a few measures (mainly regarding HPV vaccinations) have also included assessments of social norms.Citation23 Prior research suggests that, through social comparison processes, peers may have a greater influence on novel and stressful activities, as compared to those behaviors that have become habitual or are less stressful.Citation30 Social norms also have a reciprocal influence on beliefs: political ideology, especially since 2016, has been linked to the establishment and dissolution of friendships.Citation31 Augmented by social media feeds tailoring to individual beliefs and partisan bias, increased social norms that support an individual’s belief can lead to the development of information silos, which further polarize these beliefs.Citation32 On the other hand, social norms can also modulate beliefs and behaviors through the influence of conformity.Citation33 Given that the establishment and dissolution of friendships have been linked to political ideology, especially since 2016, we were also interested in examining the relationship between COVID-19 vaccination social norms, political ideology, and vaccine intention.Citation31 Social norms might be linked to political ideology, with individuals affiliating with those who have similar political ideologies. Hence, if political ideologies are associated with vaccine attitudes, then social norms may be associated with both vaccine attitudes and political ideologies.
In this study, we constructed models assessing general COVID-19 vaccine intention and compared them to models assessing vaccine delay. We hypothesized that greater concern about side effects would be associated with longer anticipated waiting periods as well as more hesitant vaccine intention. As the pandemic response has become politically polarized in the US, we theorized that political conservatism would be associated with vaccine intentions across all groups. We also anticipated that social norms would moderate this association due to political ideology being a key basis for friendship patterns.Citation31
Methods
Study population
The study respondents were drawn from the four-wave longitudinal online COVID-19 Health and Well-Being survey. Respondents who completed the first survey were invited to participate in subsequent rounds of data collection. We administered the first survey from March 24th-27th 2020, the second from May 5th-14th, the third from July 22nd-30th, and the fourth from November 18th-29th, 2020. The fourth survey wave came after Pfizer-BioNTech (November 9) and Moderna (November 16) presented preliminary data indicating that their COVID-19 vaccines were over 90% effective. At the time, there was no definitive information provided to the public about when individuals would be able to obtain a COVID-19 vaccine.
The study participants were recruited through Amazon’s Mechanical Turk (MTurk). MTurk is an online forum where participants can complete surveys and other small tasks. MTurk has been extensively studied for its adequacy for survey research. The study populations recruited through MTurk are not nationally representative, but the platform outperforms other online opinion sampling on several dimensions.Citation34 Previous research has supported the reliability of data from MTurk participants.Citation35 We designed study protocols following best practices for MTurk.Citation36–38 Eligible participants were adults living in the United States who were English speaking and reading, had heard of the coronavirus or COVID-19, and who provided consent to participate in the study. Additionally, to enhance reliability, eligible participants had to pass attention and validity checks embedded in the surveyCitation39; these included repeated questions and questions with certain responses with an exceedingly low probability of being accurate. The median completion time was 20.75 minutes. In the baseline survey, participants were informed that this was a longitudinal study, and, if interested, that they would have the opportunity to participate in a future study. Participants were compensated 2.50 USD for completing the first survey and 4.00 USD for the fourth survey, which was equivalent to approximately 11.6 USD per hour. The Johns Hopkins Bloomberg School of Public Health IRB approved the study.
Measures
Independent variables: We developed survey items assessing COVID-19 vaccine concerns based on responses to the third survey wave, which included the open-ended question “Why would you not trust a vaccine for the coronavirus?” This question was asked only of participants who responded, “neither agree nor disagree,” “agree,” or “strongly agree” to the question, “I would not trust a vaccine for the coronavirus.” The most prevalent themes found in the responses were converted into survey items for the subsequent survey wave, including “I am concerned that the coronavirus vaccines are being developed too quickly,” “I am worried about having bad side effects if I got a coronavirus vaccine,” “I am concerned that a coronavirus vaccine will not be effective,” and “I am concerned that short cuts have been taken with coronavirus vaccine development because of political pressures.” We also assessed potentially altruistic reasons for vaccine hesitancy with the item: “More vulnerable people such as the elderly should have priority for a coronavirus vaccine.” Two measures of risk perception, susceptibility, and severity, were assessed with the items: “I am very worried about getting the coronavirus” and “If I got the coronavirus it’s likely that I would get very sick.” The response options for these questions were “strongly agree,” “agree,” “neither agree nor disagree,” “disagree,” and “strongly disagree.” We compared participants who responded either “strongly agree” or “agree” with those who responded “neither agree nor disagree,” “disagree,” and “strongly disagree.”
As the response to the COVID-19 pandemic in the US has become politically polarized, we included a measure of political ideology, assessed with the question, “Where would you place yourself on a scale running from “Very liberal” to “Very conservative?” The response categories were “Very Liberal,” Liberal,” Slightly Liberal,” “Moderate,” “Slightly Conservative,” “Conservative,” and “Very Conservative. We measured descriptive social norms of vaccine intention using the items: “The majority of my family members will get the coronavirus vaccine, when available” and “The majority of my friends will get the coronavirus vaccine, when available.” The response options were “strongly agree,” “agree,” “neither agree nor disagree,” “disagree,” and “strongly disagree.” These two variables were added together for a continuous measure of social norms, with higher scores indicating increased vaccine intention among family and friends.
The response categories for self-reported race/ethnicity included “White,” “Black,” “Asian,” “Hispanic,” “Mixed,” or “Other.” Due to the small sample size, we combined “Hispanic” and “Mixed” with the “Other” category. Family income was assessed and dichotomized, based on the median, at less than 60,000 USD versus 60,000 USD or more. Gender was determined by biological sex at birth. Educational attainment was dichotomized at the median as a Bachelor’s degree and higher versus Associate’s degree or less.
Dependent variables: The qualitative data, collected in the third round of data collection, also suggested that many individuals might delay vaccination because they were concerned about the vaccine. Consequently, to assess vaccine delay, we included the question: “When a coronavirus vaccine is available, when do you think you would get it? The response categories were “right away,” “wait a few weeks,” “wait a few months,” and “never.” This variable was used as one of the two dependent variables. Based on prior vaccine hesitancy surveys, we also included an outcome of vaccine intention based on the question: “I am very likely to get a coronavirus vaccine, when available.” The response options were “strongly agree,” “agree,” “neither agree nor disagree,” “disagree,” and “strongly disagree.” These items were trichotomized for analysis: positive intentions (strongly agree/agree), neutral intentions (neither agree nor disagree), and negative intentions (disagree/strongly disagree).
Analyses
To assess differences in vaccine delay, we used bivariate and multivariate multinomial regression models to compare respondents who planned to obtain the vaccine when available (1) immediately to respondents who planned to delay by (2) waiting a few weeks, (3) waiting a few months, or (4) never getting vaccinated. Models of vaccine intention compared (1) positive vaccine intentions to (2) neutral intentions, and (3) negative intentions.
We conducted descriptive statistics and then a correlation matrix among the vaccine concern questions. Next, multinomial regression models were examined. The multivariable models included all demographic variables. The criterion for retention of the other covariates in the multivariate multinomial model was p < .15.Citation40,Citation41 Only those demographic variables that were statistically significant were presented in the tables of the regression models. Based on our hypothesis that social norms might influence vaccine intentions and delay, we constructed two multivariate models for each outcome. The first model (Model 1) included all independent variables except for social norms of vaccine intentions, and the second model (Model 2) included social norms of vaccine intention as an additional covariate. Odds ratios and 95% confidence intervals were calculated, and the statistical significance was set at p-value <0.05. SPSS 27 and Stata 15 were used for analyses.
Results
Data for the current analyses were primarily collected in the fourth survey wave, administered between November 18 – 29, 2020 (N = 586). One respondent was missing data on the dependent variable and removed from the analyses. The baseline sample size was 809 and the fourth wave sample was 585 respondents with complete data for a 72% retention rate from wave one to wave four of data collection. We replaced two missing values for the political ideology scale with means. We first presented bivariate models. An initial analysis of the independent variables indicated a high (.642) Spearman’s correlation between the variable “I am concerned that the coronavirus vaccines are being developed too quickly” and “I am concerned that short cuts have been taken with coronavirus vaccine development because of political pressure.” Consequently, only the former variable was included in the multinomial regression models.
Descriptive findings are reported in . Approximately 22% of the sample reported that, once the vaccine was available, they would get a coronavirus vaccine “right away,” with 29.2% waiting a few weeks, 32.8% waiting a few months, and 16.2% reporting they would “never” obtain the vaccine. The mean age of survey respondents was 40.3 (with a standard deviation of 11.6). The majority of the sample (81.0%) identified as White, and just over half of the sample self-reported female sex at birth (57.4%). Approximately half (53.0%) of participants self-identified as slightly liberal, liberal, or very liberal, with 20.3% identifying as moderate and the remaining quarter (26.3%) of participants identifying as slightly conservative, conservative, or very conservative.
Table 1. Descriptive statistics
Half of the participants (52.1%) worried about getting COVID-19, and 38.3% felt it was likely that they would get very sick, if infected. 39.0% agreed with the statement that the “vaccine would prevent coronavirus infection.” Many participants expressed concerns about the COVID-19 vaccine, with 54.0% reporting that the vaccines were being developed too quickly, 64.1% endorsing that they might experience bad side effects, and 47.2% expressing concern that vaccines would not be effective. A majority of respondents agreed that vulnerable people should have priority (86.8%). Social norms of vaccine intention were high, with 57.8% and 57.9% reporting that the majority of their family and friends, respectively, planned to get vaccinated once a vaccine was available.
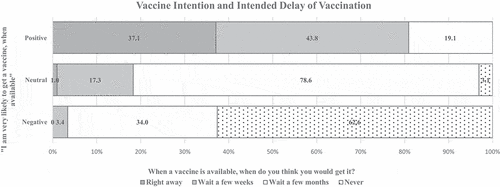
depicts vaccine delay by vaccine intention. Participants within all vaccine intention categories showed intention to delay vaccination, once a vaccine was available. In the positive vaccine intention group, 37.1% reported intention to get vaccinated right away, while 43.8% reporting wanting to wait a few weeks and 19.1% waiting a few months. Among the neutral vaccine intention group, 1.0% intended to get vaccinated right away, while 17.3% would wait a few weeks, 78.6% would wait a few months, and 3.1% would never get the vaccine. In the negative intention group, no participants reported willingness to get vaccinated right away, with 3.4% waiting a few weeks, 34.0% waiting a few months, and 62.6% never intending to get vaccinated.
reports results from the vaccine delay regression models. Bivariate analyses revealed that those who reported vaccine timing of “wait a few weeks,” “wait a few months,” or “never”, compared to those who would obtain an available vaccine right away, were less likely to believe that a vaccine would prevent COVID-19 infection and more likely to have concerns that vaccines were developed too quickly, would have bad side effects, and would not be effective. In addition, political affiliation (greater conservatism) was significantly related to waiting a few months or never getting a COVID-19 vaccine, compared with getting a vaccine right away. Furthermore, in bivariate analyses, participants who reported they would “never” get a vaccine were more likely to be female and less likely to believe that vulnerable people should have priority compared with those who would obtain an available vaccine right away. In bivariate analyses, social norms surrounding vaccine intention were strongly associated with the vaccine delay.
Table 2. Multivariable regression model of vaccine delay (N = 585)
In multivariable models of vaccine delay (), some predictors were associated with all timing categories of vaccine delay, while others were uniquely associated with certain timing of outcomes. Perceived susceptibility, or worry about getting COVID-19, was associated with decreased vaccine delay across all timing outcomes (Model 1: “Wait a few weeks” aOR=0.53, 95%CI=0.30,0.94; “Wait a few months” aOR=0.34, 95%CI=0.18,0.64; “Never” aOR=0.07, 95%CI=0.03,0.16). Belief that a vaccine would prevent COVID-19 infection was also associated with reduced vaccine delay for all timing outcomes (Model 1: “Wait a few weeks” aOR=0.40, 95%CI=0.19,0.85; “Wait a few months” aOR=0.16, 95%CI=0.08,0.35; “Never” aOR=0.07, 95%CI=0.03,0.16). Concern about bad side effects as associated with increased delay across timing outcomes (Model 1: “Wait a few weeks” aOR=2.56, 95%CI=1.40,4.69; “Wait a few months” aOR = 8.41, 95%CI=4.18,16.9; “Never” aOR=19.56, 95%CI=6.43,59.5). Concern that the vaccine will not be effective was associated with the increased odds of waiting a few weeks to get the vaccine (Model 1: aOR=2.24, 95%CI=1.23,4.09) and waiting a few months (Model 1: aOR=1.69, 95%CI=0.89,3.22) as compared to getting it right away. Concern that vaccines were being developed too quickly was associated with waiting a few months (Model 1: aOR=3.68, 95%CI=1.92, 7.07) and never getting the vaccine (Model 1: aOR=4.19, 95%CI=1.62,10.9) but not with waiting a few weeks. Believing vulnerable people should have priority was associated only with waiting a few weeks (Model 1: aOR=3.13, 95%CI=1.11,8.80). Demographics were also associated with vaccine delay, with participants reporting a more conservative ideology, more likely to report waiting a few months (Model 1: aOR=1.28, 95%CI=1.08,1.53) and never getting the vaccine (Model 1: aOR =1.54, 95%CI=1.22,1.95).
The addition of the social norms of vaccine intention as a covariate in multivariable models of vaccine delay (, Model 2) found that social norms were a significant and independent predictor of all vaccine delay models (Model 2: “Wait a few weeks” aOR=0.65, 95%CI=0.45,0.70; “Wait a few months” aOR=0.46, 95%CI=0.36,0.58; “Never” aOR=0.26, 95%CI=0.19,0.36). The addition of the social norms variable also attenuated several associations. In Model 2, belief that a vaccine would prevent COVID-19 infection was no longer associated with a reduced intention of waiting a few weeks. Further, conservative ideology and concern that vaccines would not be effective were no longer significantly associated with waiting a few months, and concerns that the vaccine was being developed too quickly were no longer a significant predictor of never getting vaccinated, compared to getting vaccinated right away.
reports results from multivariable regression models with vaccine intention as the primary outcome. Bivariate analyses indicated that those who were neutral (reported “neither likely nor unlikely”) and not likely to get vaccinated were more likely to report concerns about the vaccine and less likely to report that vulnerable people should have priority or endorce social norms of vaccine intention, compared to people who were likely to get a COVID-19 vaccine when it was available. Two factors were significant in the bivariate analysis for the negative intention model but not in the neutral model – people in the negative vaccine intention group were more likely to report conservative ideologies and less likely to believe that they were likely to get very sick if they got COVID-19, compared to participants who were likely to get vaccinated.
Table 3. Multivariable regression model of vaccine intention (N = 585)
Two vaccine concerns remained significant predictors in adjusted models of vaccine intention (, Model 1). Concern that the vaccines were being developed too quickly increased the odds of being in the neutral group (Model 1: aOR=4.09, 95%CI =2.23,7.51) and negative group (Model 1: aOR=4.87, 95%CI=2.41,9.83), compared to the likely intention group. Likewise, concern about bad side effects was associated with greater odds of neutrality (Model 1: aOR=3.45, 95%CI=1.74,6.86) and negative vaccine intention (Model 1: aOR=8.71, 95%CI=3.70, 20.5). The only concern that did not remain a significant predictor in adjusted models was the concern that the vaccine would not be effective. Two factors were shown to reduce neutral and negative vaccine intention and included belief that the vaccine would prevent COVID-19 infection (Model 1: “Neutral” aOR=0.25, 95%CI=0.14,0.44; “Negative” aOR=0.07, 95%CI=0.04,0.13), and belief that vulnerable people should have priority (Model 1: “Neutral” aOR=0.35, 95%CI=0.15,0.81; “Negative” aOR=0.20, 95%CI =0.09,0.47). Perceived susceptibility to COVID-19 infection reduced negative vaccine intentions (Model 1: aOR=0.15, 95%CI=0.08,0.29) but was not associated with the neutral intention. Conservative ideology was associated with greater odds of reporting negative vaccine intention (Model 1: aOR=1.34, 95%CI=1.12,1.60), compared to positive intention, but was not significantly associated with neutrality.
Multivariable models of COVID-19 vaccine intention, which included social norms (, Model 2), found that social norms of vaccine intention were a significant and independent predictor of “neutral” intention (Model 2: aOR=0.52, 95%CI=0.42,0.64) and negative vaccine intention (Model 2:aOR=0.34, 95%CI=0.27,0.44), compared to positive vaccine intention. Adding social norms did not change qualitatively adjusted findings with the exception of political ideology and belief that vulnerable people should be prioritized. In the models adjusted for social norms of vaccine intention, negative vaccine intention was no longer associated with increased odds of reporting conservative ideology compared to positive vaccine intention, and vulnerable people should be prioritized became marginally significant among the neutral group.
Discussion
The study findings show that both COVID-19 vaccine intention and delay are critical constructs for understanding vaccine hesitancy. Results show that desires to delay COVID-19 vaccination are evident in all vaccine intention groups. Additionally, this study indicates that concerns about COVID-19 vaccines are directly associated with both vaccine intention and vaccine delay. Further, COVID-19 vaccine concerns are prevalent in this study population, with the majority of the sample expressing various concerns about COVID-19 vaccines. Specifically, 54% reported concern that the vaccine was developed too quickly, 64% reported worries about bad side effects, and 57% reported concerns that shortcuts were taken in the vaccine development due to political pressures. Almost half of the sample (47%) was concerned that the vaccine would not be effective.
These findings are consistent with opinion poll data of vaccine concerns. The December 2020 Kaiser Family FoundationCitation1 poll found, among respondents who reported that they would not get vaccinated, that 59% were worried about side effects, 55% did not trust the government to make sure the vaccine was safe and effective, and 51% felt that politics had played too significant a role in the vaccine development process.
In addition to assessing individuals who do not plan to be vaccinated, as was the procedure in the Kaiser Family Foundation poll, our analyses further suggest that it is important to evaluate and address vaccine concerns even among those who do plan to get vaccinated. For example, among respondents who reported that they planned to only wait a few weeks to receive a COVID-19 vaccine, approximately one-quarter were still concerned about side effects and vaccine effectiveness.
The current analyses suggest that the constructs of COVID-19 vaccine intention and COVID-19 vaccine delay are not redundant and are both important measures in assessing vaccine hesitancy. Understanding substantially different responses regarding delay and intention is important to inform programs and interventions that promote vaccine uptake. Prior research has not focused on altruistic reasons for vaccine hesitancy, as supply has not historically been a major issue. Future research should assess in greater detail altruistic reasons for vaccine behaviors and attitudes.
These findings suggest that respondents are judging salient features of the vaccine itself (i.e. safety and efficacy). Additionally, risk perceptions, such as worries about contracting COVID-19 and social norms of vaccine intention, were significantly associated with vaccine intention and delay, indicating that factors besides concerns about the vaccine are also highly involved in general vaccine hesitancy. Although not presented in the tables, there was little indication that demographic characteristics of age, race, income, and level of education were associated with vaccine intentions. While these variables have been linked to differential vaccine access and intentions, our study had insufficient statistical power to gauge racial and ethnic differences accurately.Citation1,Citation6
Theoretically, concerns about side effects and vaccine efficacy may motivate individuals to wait for more data about COVID-19 vaccines before seeking to be vaccinated. However, in the current study, those who reported that they would “never” get the vaccine were most likely to endorse concerns about side effects and were significantly more inclined to disagree that a COVID-19 vaccine would be effective. It is important to understand the “never” group’s communication channels. This group may also distrust information about vaccines from mainstream sources of public health information, and hence it may be challenging to address their concerns about COVID-19 vaccines. Future research should examine what sources of information people who never intend to get a COVID-19 vaccine to help identify potential points of influence for future intervention or educational awareness campaigns.
COVID-19 vaccine social norms were consistently associated with vaccine intention and delay. Social norms may be a more proximal measure of affiliation and influence than political ideology. These results indicate that heightening pro-vaccination social norms surrounding vaccination may help to enhance vaccine intention. One approach to increase the influence of social norms is to make them more salient, which can be accomplished by encouraging and rewarding people to talk about and model pro-vaccine behaviors and beliefs. It may be effective for those who have already received vaccines to post information on social media and diffuse pertinent information through e-mails and conversations. Additionally, high-profile individuals or well-known public figures can publicly discuss their choice to obtain a vaccine and, if applicable, discuss or share their vaccination experiences. Also, individuals who have been vaccinated may also be able to more safely reach people who are less engaged in the healthcare system and less likely to obtain vaccine information from health professionals. Moreover, as one of the strongest correlates of never intending to obtain a COVID-19 vaccine was having concerns about the vaccine’s side effects, individuals who do not experience side effects or only mild transient side effects may be able to talk about their vaccine experiences and reduce vaccine hesitancy among their social network members.
The strong association between vaccine intention/delay and concerns about becoming infected with COVID-19 from the vaccine suggests that programs should acknowledge the severity of COVID-19 and not downplay its significance. The approval of a vaccine is a complicated process; however, it is imperative that educational campaigns are able to articulate this robust process and explain that “corners were not cut.” The strong association between perceptions that the vaccine would not be effective and decreased intention to be vaccinated may also require special focus. Individuals may view COVID-19 vaccines as having similar properties to the influenza vaccines, which vary by year in effectiveness, and recall times when they received the vaccine but still became infected. We do not know how SARS-CoV-2 may mutate over time, but the current level of protection afforded by several of the COVID-19 vaccines does allow us to emphasize that the vaccine is more effective than influenza vaccines. As COVID-19 vaccination programs are rolled out, various health conditions will be reported to be linked to the vaccine, as has occurred with prior vaccines. It is critical to set up a credible and transparent process to evaluate these claims.
Study limitations should be noted. We did not assess actual vaccine behaviors; in our construct of vaccine delay, “delay” still represents an intention rather than a behavior. In addition, vaccine attitudes are not the only predictors of vaccine behaviors, and attitudes may not predict behaviors if people are required to obtain a vaccine or cannot obtain one. We also did not assess all of the documented reasons for vaccine hesitancy, and new ones may arise as vaccine programs are implemented. For example, in the current study, the altruistic attitude of endorsing the sentiment that vulnerable groups should get the vaccine first was associated with intending to wait a few weeks before getting the vaccine but was not associated with vaccine delay of waiting a few months or not intending to get a COVID-19 vaccine. This dynamic may change once the vaccines become more widely available. Furthermore, the current analyses were cross-sectional because the COVID-19 vaccines had not been viable in previous rounds of data collection. The cross-sectional nature of the current analysis does not allow us to assess how intentions are associated with actual vaccine uptake. The survey responses are subject to social desirability and other response biases. Moreover, we did not have a representative sample, though the respondents were similar to demographic characteristics found in other online surveys. There was also an insufficient number of minorities, older adults, or those with health conditions that place them at risk for severe COVID-19 illness for sub-analyses of these important groups. Many vaccine hesitancy measures also assess whether individuals’ health-care providers recommended or encouraged vaccination. This latter topic was omitted from the current assessment, as a vaccine was not available at the time of the survey, and hence it was unlikely that health-care providers would have talked to their patients about a COVID-19 vaccination.
One of the drawbacks of vaccine hesitancy studies is that individuals may not be aware of key factors that influence their vaccine behaviors. Some of these are well-documented, such as access and cost, e.g. taking time off work.Citation26 Others, such as traveling to an unfamiliar and potentially uncomfortable setting, have been studied in less detail. It is critical to undertake intensive and detailed vaccine hesitancy and uptake studies, which go beyond surveys to engage in community-based and ethnographic studies, in order to ensure that researchers do not miss key factors that influence vaccine behaviors. Moreover, given the COVID-19 health disparities based on race and social class, it is imperative that vaccination campaigns strive to eliminate vaccine barriers with intense outreach and programs that bring vaccines to where people work and live.
The study findings do suggest it is beneficial to assess vaccine delay when assessing vaccine hesitancy and important to conduct sufficient formative research to understand and measure unique reasons for vaccine hesitancy, which may be due to features of the vaccine itself, lack of information and misinformation, as well as political and social pressures. In addition to context, it is also critical to assess changes in vaccine attitudes and ensure that the perspectives of minorities and vulnerable populations are included in vaccine hesitancy studies. Our study’s consistent association of social norms and vaccine intentions and delay suggests that vaccination campaigns should highlight social norms as a potential target for future research and intervention. In addition, media strategies and transparent reporting should be emphasized throughout the ongoing vaccination rollout and address concerns about the negative side effects, as this variable was strongly and consistently associated with vaccine delay and intention to be vaccinated.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
Study participants for their time and thoughtful responses.
Additional information
Funding
References
- Hamel L, Kirzinger A, Munana C, Brodie M. KFF COVID-19 vaccine monitor: December 2020. 2020 Dec 15. https://www.kff.org/coronavirus-covid-19/report/kff-covid-19-vaccine-monitor-december-2020/
- Corum J, Grady D, Wee SL, Zimmer C. Coronavirus vaccine tracker. 2020. https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html
- Friedrich MJ. WHO’s top health threats for 2019. JAMA. 2019 Mar 19;321(11):1041. doi:10.1001/jama.2019.1934. PMID: 30874765.
- Larson HJ, de Figueiredo A, Xiahong Z, Schulz WS, Verger P, Johnston IG, Cook AR, Jones NS. The state of vaccine confidence 2016: global insights through a 67-country survey. EBioMedicine. 2016 Oct;12:295–301. doi:10.1016/j.ebiom.2016.08.042.
- World Health Organization (WHO). Vaccine hesitancy: what it means and what we need to know in order to tackle it [PowerPoint slides]. World Health Organization (WHO). https://www.who.int/immunization/research/forums_and_initiatives/1_RButler_VH_Threat_Child_Health_gvirf16.pdf?ua=1.
- Dubé E, Laberge C, Guay M, Bramadat P, Roy R, Bettinger J. Vaccine hesitancy: an overview. Hum Vaccin Immunother. 2013 Aug;9(8):1763–73. doi:10.4161/hv.24657.
- Tucker Edmonds BM, Coleman J, Armstrong K, Shea JA. Risk perceptions, worry, or distrust: what drives pregnant women’s decisions to accept the H1N1 vaccine? Matern Child Health J. 2011 Nov;15(8):1203–09. doi:10.1007/s10995-010-0693-5.
- Peretti-Watel P, Larson HJ, Ward JK, Schulz WS, Verger P. Vaccine hesitancy: clarifying a theoretical framework for an ambiguous notion. PLoS Curr. 2015 Feb 25;7. doi:10.1371/currents.outbreaks.6844c80ff9f5b273f34c91f71b7fc289.
- Gust DA, Strine TW, Maurice E, Smith P, Yusuf H, Wilkinson M, Battaglia M, Wright R, Schwartz B. Underimmunization among children: effects of vaccine safety concerns on immunization status. Pediatrics. 2004;114(1):e16–22. doi:10.1542/peds.114.1.e16.
- Benin AL, Wisler-Scher DJ, Colson E, Shapiro ED, Holmboe ES. Qualitative analysis of mothers’ decision-making about vaccines for infants: the importance of trust. Pediatrics. 2006 May;117(5):1532–41. doi:10.1542/peds.2005-1728.
- Henrich N, Holmes B. The public’s acceptance of novel vaccines during a pandemic: a focus group study and its application to influenza H1N1. Emerg Health Threats J. 2009;2:e8. doi:10.3134/ehtj.09.008. PMID: 22460289.
- Phillips N, Cyranoski D, Mallapaty S. A leading coronavirus vaccine trial is on hold: scientists react. Nat Res J. 2020 Sep 09 [accessed 2020 Jan 21]. https://www.nature.com/articles/d41586-020-02594-w.
- Covid-19 vaccines [Internet]; 2020c [accessed, 2021 Jan 21]. https://www.nytimes.com/spotlight/coronavirus-vaccine?name=styln-coronavirus-vaccines®ion=TOP_BANNER&block=storyline_menu_recirc&action=click&pgtype=Article&impression_id=816e7d30-4c3d-11eb-9a55-253d51f22e93&variant=1_Show.
- Gollust SE, Nagler RH, Fowler EF. The emergence of COVID-19 in the US: a public health and political communication crisis. J Health Polit Policy Law. 2020 Dec 1;45(6):967–81. doi:10.1215/03616878-8641506.
- Lasco G. Medical populism and the COVID-19 pandemic. Glob Public Health. 2020 Oct;15(10):1417–29. doi:10.1080/17441692.2020.1807581.
- Pedersen MJ, Favero N. Social distancing during the COVID-19 pandemic: who are the present and future noncompliers? Public Admin Rev. 2020;80(5):805–14. doi:10.1111/puar.13240.
- Wilson SL, Wiysonge C. Social media and vaccine hesitancy. BMJ Glob Health. 2020 Oct;5(10):e004206. doi:10.1136/bmjgh-2020-004206. Epub 2020 Oct 23. PMID: 33097547.
- Puri N, Coomes EA, Haghbayan H, Gunaratne K. Social media and vaccine hesitancy: new updates for the era of COVID-19 and globalized infectious diseases. Hum Vaccin Immunother. 2020 Nov 1;16(11):2586–93. doi:10.1080/21645515.2020.1780846.
- Ahmed W, Vidal-Alaball J, Downing J, López Seguí F. COVID-19 and the 5G conspiracy theory: social network analysis of twitter data. J Med Internet Res. 2020 May 6;22(5):e19458. doi:10.2196/19458. PMID: 32352383.
- Islam MS, Sarkar T, Khan SH, Mostofa Kamal AH, Hasan SMM, Kabir A, Yeasmin D, Islam MA, Amin Chowdhury KI, Anwar KS, et al. COVID-19-related infodemic and its impact on public health: a global social media analysis. Am J Trop Med Hyg. 2020 Oct;103(4):1621–29. doi:10.4269/ajtmh.20-0812. PMID: 32783794.
- Romer D, Jamieson KH. Conspiracy theories as barriers to controlling the spread of COVID-19 in the U.S. Soc Sci Med. 2020 Oct;263:113356. doi:10.1016/j.socscimed.2020.113356. PMID: 32967786.
- Bierwiaczonek K, Kunst JR, Pich O. Belief in COVID-19 conspiracy theories reduces social distancing over time. Appl Psychol Health Well Being. 2020 Dec;12(4):1270–85. doi:10.1111/aphw.12223. PMID: 32864837.
- Yaqub O, Castle-Clarke S, Sevdalis N, Chataway J. Attitudes to vaccination: a critical review. Soc Sci Med. 2014 Jul;112:1–11. doi:10.1016/j.socscimed.2014.04.018. PMID: 24788111.
- Gidengil C, Chen C, Parker AM, Nowak S, Matthews L. Beliefs around childhood vaccines in the United States: a systematic review. Vaccine. 2019 Oct 23;37(45):6793–802. doi:10.1016/j.vaccine.2019.08.068. PMID: 31562000.
- Karafillakis E, Simas C, Jarrett C, Verger P, Peretti-Watel P, Dib F, De Angelis S, Takacs J, Ali KA, Pastore Celentano L, et al. HPV vaccination in a context of public mistrust and uncertainty: a systematic literature review of determinants of HPV vaccine hesitancy in Europe. Hum Vaccin Immunother. 2019;15(7–8):1615–27. doi:10.1080/21645515.2018.1564436.
- Schmid P, Rauber D, Betsch C, Lidolt G, Denker ML. Barriers of influenza vaccination intention and behavior - A systematic review of influenza vaccine hesitancy, 2005-2016. PLoS One. 2017 Jan 26;12(1):e0170550. doi:10.1371/journal.pone.0170550. PMID: 28125629.
- Dubé E, Gagnon D, MacDonald N, Bocquier A, Peretti-Watel P, Verger P. Underlying factors impacting vaccine hesitancy in high-income countries: a review of qualitative studies. Expert Rev Vaccines. 2018 Nov;17(11):989–1004. doi:10.1080/14760584.2018.1541406.
- National Academies of Sciences, Engineering, and Medicine. Vaccine access and hesitancy: part one of a workshop series: proceedings of a workshop—in brief. Washington (DC): The National Academies Press; 2020. doi:10.17226/25895.
- Xiao X, Wong RM. Vaccine hesitancy and perceived behavioral control: a meta-analysis. Vaccine. 2020 Jul 14;38(33):5131–38. doi:10.1016/j.vaccine.2020.04.076. PMID: 32409135.
- Gerber JP, Wheeler L, Suls J. A social comparison theory meta-analysis 60+ years on. Psychol Bull. 2018 Feb;144(2):177–97. doi:10.1037/bul0000127. PMID: 29144145.
- Buliga E, MacInnis C. How do you like them now?” expected reactions upon discovering that a friend is a political out-group member. J Soc Pers Relat. 2020;37(10–11):2779–801. doi:10.1177/0265407520939191.
- Chou WS, Oh A, Klein WMP. Addressing health-related misinformation on social media. Jama. 2018 Dec 18;320(23):2417–18. doi:10.1001/jama.2018.16865. PMID: 30428002.
- Pryor C, Perfors A, Howe P. Conformity to the descriptive norms of people with opposing political or social beliefs. PloS One. 2019;14(7):e0219464. doi:10.1371/journal.pone.0219464.
- Huff C, Tingley D. “Who are these people?” evaluating the demographic characteristics and political preferences of MTurk survey respondents. Research & Politics. 2015;2(3). doi:10.1177/2053168015604648.
- Follmer DJ, Sperling RA, Suen HK. The role of MTurk in education research: advantages, issues, and future directions. Educ Res. 2017;46(6):329–34. doi:10.3102/0013189X17725519.
- Chandler J, Shapiro D. Conducting clinical research using crowdsourced convenience samples. Annu Rev Clin Psychol. 2016;12(1):53–81. doi:10.1146/annurev-clinpsy-021815-093623. PMID: 26772208.
- Strickland JC, Stoops WW. The use of crowdsourcing in addiction science research: Amazon mechanical turk. Exp Clin Psychopharmacol. 2019;27(1):1–18. doi:10.1037/pha0000235. PMID: 30489114.
- Young JA, Young KM. Don’t get lost in the crowd: best practices for using Amazon’s mechanical turk in behavioral research. J Midwest Assoc Inf Syst. 2019;2:7. doi:10.17705/3jmwa.000050.
- Rouse SV. A reliability analysis of mechanical turk data. Comput Hum Behav. 2015;43:304–07. doi:10.1016/j.chb.2014.11.004.
- Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008 Dec 16;3:17. doi:10.1186/1751-0473-3-17. PMID: 19087314.
- Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989 Jan;129(1):125–37. doi:10.1093/oxfordjournals.aje.a115101.