ABSTRACT
The first vaccine against SARS-CoV-2 made available in Italy has been BNT162b2, the two-dose mRNA-based vaccine developed by Pfizer-BioNTech. The ASST Fatebenefratelli-Sacco hospital is located in one of the areas most affected by the pandemic, and to date over 2000 healthcare professionals have been injected with both vaccine doses. We have collected all spontaneous safety reports in which BNT162b2 was designated as the possible cause. We also have carried out a descriptive analysis of reports submitted in EudraVigilance in the same time-frame and compared our findings with those observed in clinical trials. We have identified several new and unexpected adverse reactions that will be helpful for reviewing the safety profile defined in the Summary of Product Characteristics for this vaccine.
Dear Editor,
The Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV-2) pandemic has resulted in the infection of over one hundred million people globally.Citation1 Compared to some other coronaviruses, SARS-CoV-2 has demonstrated a higher degree of lethality, probably because of its efficient person-to-person transmission and lack of population-level immunity. The disease might become endemic; therefore, the primary intervention strategy for the control of SARS-CoV-2 transmission and infection is a safe and effective vaccine.Citation2,Citation3
In Italy, since its first detection in February 2020, SARS-CoV-2 rapidly spread throughout the country, especially affecting the Lombardy Region and its capital Milan. To date, over 3 million Italians have been diagnosed with coronavirus disease 19 (COVID-19); the highest number of infected patients was reached on November 22nd 2020 with >800,000 confirmed cases.Citation4,Citation5
Despite the efficiency of the hospital organization and the strict containment strategies imposed by the government in order to slow the progression of the pandemic, over 100,000 deaths related to COVID-19 have been reported across the country.Citation5
On December 28th 2020, the first thousands of doses of BNT162b2 (Comirnaty), the mRNA-based vaccine developed by Pfizer-BioNTech and approved by EMA on December 21st, started to be administered to Italian healthcare professionals. Thus, they constitute the population to examine in order to first evaluate the safety of the vaccine.
ASST Fatebenefratelli-Sacco is a public health facility that comprises four hospital units located in Milan, plus several outpatients units covering almost one-quarter of the city healthcare needs. Up until March 8th 2021, over 2,000 employees have been administered both the first and the second dose upon informed consent: among these, 214 experienced suspected adverse drug reactions (ADRs) and reported them to the Pharmacovigilance Service in the form of Individual Case Safety Reports (ICSRs): the female/male ratio was 4.5 and mean age was 47.5 ± 15.6 years.
A total of 17 ICSRs (7.9% of total ADR cases) reported serious ADRs, mainly allergic reactions (n = 10; 4.7%), which occurred with a variety of symptoms, among which the most frequently reported ones were bradycardia, urticaria, airway obstruction, laryngeal edema, tachycardia, asthma and hypotension; they were all promptly resolved via intravenous administration of antihistamine drugs and corticosteroids. Other detected serious ADRs were hyperpyrexia (n = 6; 2.8%) and hypertensive crisis (n = 1; 0.5%).
In 65 cases (30.4%), unexpected ADRs (not reported in the Summary of Product Characteristics) were identified: the most common ones were paresthesia (n = 13; 6.1%), rash (n = 8; 3.7%), vomiting (n = 7; 3.3%), diarrhea (n = 6; 2.8%), dizziness (n = 7; 3.3%), blood pressure increased (n = 4; 1.9%), dysgeusia (n = 4; 1.9%), sore throat (n = 4; 1.9%), abdominal pain (n = 3; 1.4%) and cough (n = 3; 1.4%).
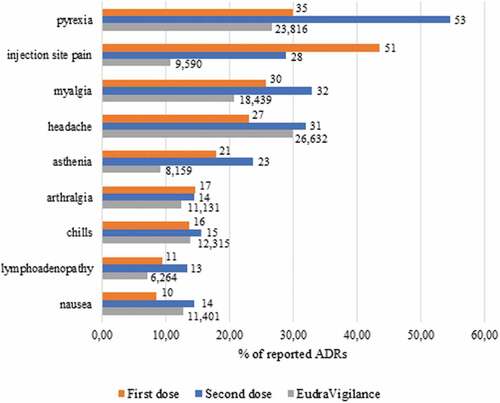
Details on the most commonly observed ADRs after each dose are listed in . Interestingly, after the first dose a wide range of ADRs were retrieved, whereas after the second dose, only the most common and expected ones occurred: this shows that each common ADR retrieved after the second dose represents a higher percentage of overall ADRs compared to the first one (e.g., pyrexia represents 29.9% of total ADRs reported after the first dose and 54.6% after the second one), apart from injection-site pain.
Only 4 subjects that had previously been diagnosed with COVID-19 infection reported ADRs, the symptoms of which did not significantly differ from those experienced by the rest of the sample, being hyperpyrexia, lymphadenopathy, chills and dyspnea.
We assessed the findings from the hospital setting we examined against evidence from a large and comprehensive context by carrying out an analysis on EudraVigilance, the spontaneous reporting system database maintained by EMA that collects ADR reports on all authorized medicines in the European Economic Area.
Selecting all ICSRs that reported Comirnaty as the only vaccine suspected in the occurrence of the ADR, a total of 89,181 reports submitted from December 10th 2020 to March 6th 2021 were retrieved (details on the most common ones are shown in ). Among these, 25,877 were classified as serious (29.0%). Since ADRs are recorded in EudraVigilance using the Medical Dictionary for Regulatory Activities (MedDRA®) Preferred Terms (PTs), we identified potential allergic reactions through a search of specific PTs (allergic reaction to excipient, allergy to vaccine, anaphylactic reaction, anaphylactic shock, drug hypersensitivity, hypersensitivity, type I hypersensitivity), which were indicated in 1,601 cases (1.8% of total reports).
Hyperpyrexia was identified in 565 (0.6%) of the retrieved ICSRs, and 196 (0.2%) of them reported hypertensive crisis.
We searched among the ICSRs submitted in EudraVigilance the ones reporting the same unexpected ADRs as we detected in our sample: the analysis allowed to retrieve 4,836 cases of dizziness (5.4%); diarrhea was reported 3,758 (4.2%) times, vomiting in 3,216 (3.6%), paresthesia in 2,901 (3.3%), rash in 1,914 (2.2%), cough in 1,611 (1.8%), dysgeusia in 757 (0.9%) and blood pressure increase in 603 (0.7%) cases.
Overall, serious ADRs represent a higher percentage of total reports submitted in EudraVigilance compared to our sample (29.0% vs. 7.9%): this discrepancy may be only apparent as the numbers of vaccinations we recorded in real life are small compared to that in EudraVigilance. However, those that were most frequently observed in our sample were also the most common among the EudraVigilance cohort. Unexpected reactions were reported at similar frequencies.
Our findings also confirm those emerged from clinical trials, which all delineated a safety profile characterized by short-term, mild-to-moderate ADRs, spontaneously regressing within days.
In a multinational, placebo-controlled pivotal efficacy trial that randomized 1:1 43,448 patients to receive 30 μg of BNT162b2 or placebo, pain at the injection site was the most common ADR (occurring in 83% of ≤55-year-old vaccine recipients after the first dose and in 78% after the second dose).Citation6 In another placebo-controlled dose-escalation phase I trial, 12 participants aged 18–55 years received 30 μg of BNT162b2 and pain at injection site was reported in 92% of cases after the first dose and 83% after the second one.Citation7
Other commonly observed adverse effects in the first trial were fatigue (47% and 59% after the first and second dose, respectively), headache (42% and 52%), pyrexia (4% and 16%), chills (14% and 35%), myalgia (21% and 37%) and joint pain (11% and 22%). The incidence of serious adverse events was low and was similar in the vaccine and placebo groups (27% and 12%, respectively).
These results were consistent with those of the second trial, in which severe systemic events (fatigue, headache, chills, myalgia and joint pain) were reported in small numbers of vaccine recipients. ADRs that were considered to be related to the vaccine were reported by 25% of the participants, and no serious adverse events were reported.
Among those unexpected ADRs that we found in our sample, only vomiting (1% after first dose and 2% after the second one) and diarrhea (11% and 10%) were commonly observed in the first trial, while there is no available information about their occurrence in the second one.
This kind of analysis has undisputable intrinsic limitations, including no definitive proof of causal relationship between exposure to the product and the reported event occurrence, the potential bias due to under-reporting, passive reporting, stimulated reporting and other confounding factors. Furthermore, the lack of a denominator in spontaneous reporting system databases does not allow absolute measures of risk estimation.Citation8
Despite these critical aspects, our study highlights the clinical relevance of post-marketing spontaneous reporting, since a cohort of individuals never tested previously is being injected with this vaccine: pivotal clinical trials did not include immune-compromised or previously infected individuals as did our cohort. Furthermore, comorbid patients were under-represented in the trial by Polack et al. (20.9% of BNT162b2 group had at least one Charlson comorbidity).
Noteworthy is that our analysis detected a few unexpected ADRs, which will have to be confirmed in forthcoming clinical trials. This issue emphasizes the key role of post-marketing monitoring, since Comirnaty is the first mRNA-based vaccine approved by EMA and its current safety profile delineated in clinical trials is based on interim analysis, being still ongoing. Furthermore, serious adverse events were reported with a lower relative frequency in our sample compared to those retrieved in EudraVigilance and in clinical trials.
Comparing all available evidence from clinical trials with findings emerged in a real-world context may provide significant insights on unsolicited adverse events and on their prompt management in clinical practice.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- Cohen SA, Kellogg C, Equils O. Neutralizing and cross-reacting antibodies: implications for immunotherapy and SARS-CoV-2 vaccine development. Hum Vaccin Immunother. 2021;17(1):84–87. doi:10.1080/21645515.2020.1787074 .
- Yadav T, Srivastava N, Mishra G, Dhama K, Kumar S, Puri B, Saxena SK. Recombinant vaccines for COVID-19. Hum Vaccin Immunother. 2020;16(12):2905–12. doi:10.1080/21645515.2020.1820808 .
- Vashishtha VM, Kumar P. Development of SARS-CoV-2 vaccines: challenges, risks, and the way forward. Hum Vaccin Immunother. 2020;3:1–15. doi:10.1080/21645515.2020.1845524 .
- Coronavirus 2019‐nCoV, CSSE. Coronavirus 2019‐nCoV Global Cases by Johns Hopkins CSSE. [accessed 2021 Feb 16]. https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6 .
- Dipartimento della Protezione Civile. COVID-19 Situazione Italia [accessed 2021 Feb 16]. https://opendatadpc.maps.arcgis.com/apps/opsdashboard/index.html#/b0c68bce2cce478eaac82fe38d4138b1 .
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C; C4591001 Clinical Trial Group. et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–15. doi:10.1056/NEJMoa2034577 .
- Walsh EE, Frenck RW Jr, Falsey AR, Kitchin N, Absalon J, Gurtman A, Lockhart S, Neuzil K, Mulligan MJ, Bailey R, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383(25):2439–50. doi:10.1056/NEJMoa2027906 .
- Carnovale C, Gringeri M, Battini V, Mosini G, Invernizzi E, Mazhar F, Bergamaschi F, Fumagalli M, Zuccotti G, Clementi E, et al. Beta-blocker associated hypoglycaemia: New insights from a real-world pharmacovigilance study. Brit Jnl Clinical Pharma. 2021;1–12... PMID: 33506522. doi:10.1111/bcp.14754 .