ABSTRACT
The COVID-19 pandemic has disrupted life throughout the world. Newly developed vaccines promise relief to people who live in high-income countries, although vaccines and expensive new treatments are unlikely to arrive in time to help people who live in low-and middle-income countries. The pathogenesis of COVID-19 is characterized by endothelial dysfunction. Several widely available drugs like statins, ACE inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) have immunometabolic activities that (among other things) maintain or restore endothelial cell function. For this reason, we undertook an observational study in four Belgian hospitals to determine whether in-hospital treatment with these drugs could improve survival in 959 COVID-19 patients. We found that treatment with statins and ACEIs/ARBs reduced 28-day mortality in hospitalized COVID-19 patients. Moreover, combination treatment with these drugs resulted in a 3-fold reduction in the odds of hospital mortality (OR = 0.33; 95% CI 0.17–0.69). These findings were in general agreement with other published studies. Additional observational studies and clinical trials are needed to convincingly show that in-hospital treatment with statins, ACEIs/ARBs, and especially their combination saves lives.
Introduction
The global COVID-19 pandemic has caused massive social, economic and political distress. Many of those infected with the SARS-CoV-2 virus, especially children and younger adults, remain asymptomatic. In contrast, severe COVID-19 largely affects those who are older, especially those with underlying co-morbidities.Citation1
The global response to the COVID-19 pandemic has been focused on traditional public health measures (e.g., social distancing, wearing masks, etc.) and the rapid development of new vaccines and treatments. Although these efforts promise some relief, COVID-19 will still kill thousands of people worldwide in the months ahead.
The pathogenesis of COVID-19 is characterized (among other things; see below) by endothelial dysfunction.Citation2,Citation3 Several inexpensive drugs like statins, angiotensin converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) have immunometabolic activities that maintain or restore endothelial cell function.Citation4 For this reason, we have undertaken an observational study to determine whether in-hospital treatment with these drugs could improve COVID-19 patient survival.
The pathogenesis of COVID-19
The pathogenesis of COVID-19 is slowly coming into view and has been extensively reviewed.Citation2,Citation5–22 COVID-19 is characterized by severe inflammation, altered interferon, complement and epigenetic responses and dysregulation of innate and adaptive immunity. These changes can lead to acute respiratory distress syndrome (ARDS) and multi-organ failure,Citation5–9 a reflection of renin-angiotensin and endothelial dysfunction that is central to COVID-19’s pathogenesis.Citation2,Citation3,Citation10–16 Endothelial damage is directly responsible for microvascular immunothrombosis and thromboembolism seen in the lungs and other organs of many COVID-19 patients who have died.Citation17–22
Treating COVID-19
Many studies of new treatments for severe COVID-19 have focused on new or repurposed antiviral drugs.Citation23–27 Compared with patients with mild disease, virus loads are higher in those with severe or fatal disease.Citation28,Citation29 When clinical deterioration develops, however (usually in the second week of illness), virus loads are generally lower than when symptoms first developed.Citation30,Citation31
Most studies of antiviral treatments of hospitalized COVID-19 patients have been disappointing. One of the largest studies was the Solidarity Trial conducted by the World Health Organization (WHO). None of the drugs tested, including remdesivir, improved COVID-19 patient survival.Citation32 Convalescent plasmaCitation33 and monoclonal antibody preparationsCitation34 have also been tested in hospitalized patients with severe disease. Like antiviral treatments, the trial results have been similarly disappointing. Moreover, the inevitability of SARS-CoV-2 mutations (e.g., 501Y.V2., B.1.1.1.7) jeopardizes the future effectiveness of antibody treatments (and even vaccines) that target the spike protein of the virus.Citation35–37
Another approach to treating COVID-19 (and other pandemic diseases) focuses on targeting the host response to infection, not the virus.Citation38 The most prominent drug tested thus far has been dexamethasone, one of several inexpensive corticosteroids that have long been studied for treating patients with sepsis and ARDS. In a randomized controlled trial (RCT), dexamethasone improved survival in COVID-19 patients requiring mechanical ventilation or oxygen treatment but failed to improve survival in those not requiring oxygen treatment.Citation39 A meta-analysis by a WHO working group concluded that corticosteroid treatment improved survival in COVID-19 patients with severe disease.Citation40 Nonetheless, questions have been raised about the utility of corticosteroids in these patients.Citation41
Another approach to treating the host response has used monoclonal antibodies (usually tocilizumab) that antagonize IL-6. Two RCTs have shown that tocilizumab treatment modestly reduced COVID-19 mortalityCitation42,Citation43 but other studies have shown it did not improve survival.Citation44–46 Its utility for COVID-19 treatment remains uncertain.Citation47
Among other drugs considered candidates for host response treatment are statins, ACEIs, and ARBs.Citation4,Citation48 Like dexamethasone, these drugs are produced as inexpensive generics, are familiar to practicing physicians, and are widely available in resource-poor countries. They have pleiotropic activities on inflammation, affect innate and adaptive immunity, and actively counteract endothelial dysfunction.Citation3,Citation4,Citation48–52 Considered individually, these drugs are safe when given to patients with acute critical illness.Citation53,Citation54 Cardiovascular investigators have long known that they are more effective when given in combination than when they are given by themselves.Citation55 More than a decade ago, statins were proposed to treat pandemic influenzaCitation56 and in 2014 a statin/ARB combination was used to treat Ebola patients in Sierra Leone.Citation4,Citation57 In early 2020, combination treatment was proposed for COVID-19 patients.Citation58
Several studies have described risk factors (e.g., age, co-morbidities) for mortality among patients hospitalized with COVID-19, although most reports provide little detail on the effects of in-hospital treatment.Citation59 We sought to determine the effectiveness of in-hospital treatment with statins alone, ACEIs/ARBs alone, or a combination of statins and ACEIs/ARBs in reducing 28-day mortality in COVID-19 patients.
Methods
We undertook a retrospective observational case-control study of the effectiveness of inpatient treatment with statins and/or ACEIs/ARBs on 28-day hospital mortality in 959 COVID-19 patients admitted consecutively to four Belgian hospitals during the first pandemic wave (1 March to 31 July 2020). We used the anonymized and standardized records of the minimal datasets for each hospital. The ethics committees of the four hospitals gave their approvals for the study.
Using propensity scoresCitation60–62 we matched all treated and untreated patients (PSM; 1:1) according to age, sex, hospital size, and underlying co-morbidities (ischemic heart disease, heart failure, stroke, hypertension, chronic kidney disease, diabetes, COPD, asthma, and nicotine use; ). We treated sex as binary variable (M/F) and constrained matching to an exact match between treated and untreated patients. We combined patients treated with ACEIs, ARBs, or both into one group (ACEIs/ARBs) because of small numbers. We evaluated the odds ratios (ORs) for 28-day hospital mortality in patients treated with statins alone, ACEIs/ARBs alone and combination treatment with statins and ACEIs/ARBs using conditional logistic regression with conditioning on matched sets. The use of PSM allowed us to minimize the potential effects of confounding variables and balance patient characteristics in treated and control groups. The Logit Propensity Score summarized the collective influence of all covariates on treatment assignment for each treated group (statins alone, ACEIs/ARBs alone and statin + ACEI/ARB combinations) and their corresponding untreated control groups. The Standardized Mean Differences (SMDs) between patients in treated and untreated control groups were also calculated.
Table 1. Patient baseline characteristics
To account for competing risks (i.e., the chance of hospital discharge competing with the risk of death), we also calculated cumulative incidence functions (CIFs) and the associated Gray’s tests to compare treated and untreated control groups.Citation60–62
Results
Among all 959 patients (mean age 69.2 years, 54.5% men) (), 388 (40.6%) were treated as inpatients with one or more of these drugs (statins and/or ACEIs/ARBs). In the treated group, 49.4% had underlying cardiovascular diseases or hypertension. Among all patients, 707 were ≥ 60 years of age (). In this older age group, 357 (50.5%) were treated with these one or more of drugs, accounting for 92.0% of all 388 patients who received these treatments.
Among all patients, 150 (15.6%) died in hospital within 28 days (). All but three deaths occurred among patients ≥ 60 years of age. Unadjusted mortality rates in this age group were lower in patients who had been treated with one or more of these drugs compared with those not treated.
Table 2. Unadjusted in-hospital 28-day mortality by age and treatment group
illustrates the Standardized Mean Differences (SMDs) after the PSM procedure. The logit propensity score (“Logit Prop Score”) summarizes the collective influence of all observed covariates on treatment assignment. In addition to the SMDs for all observations and the matched observations, also shows the SMDs for “region observations”. This is a selection of observations whose propensity scores (or equivalently, logits of propensity scores) lie in the region of common support for the treated and control groups. This region is the largest interval that contains propensity scores (or logits of propensity scores) for subjects in both groups.
Figure 1. Standardized Mean Differences (SMDs) before and after propensity score matching (PSM) for treatment with statins alone (a); ACEIs/ARBs alone (b); and combination treatment with statins + ACEIs/ARBs (c) versus the control group (no treatment with statins or ACEIs/ARBs) for each treated group. The shaded areas indicate SMDs <0.25. SMDs were < 0.10 for 9/13 variables for statins alone (a), 12/13 for ACEI/ARB alone (b) and 6/13 for combination treatment (c). x = all observations before matching, ◊ = region observations (see text for definition), o = propensity score observations after matching. See text for definition of Logit Prop Score. Abbreviations for co-morbidities are defined in the legend for Table 1
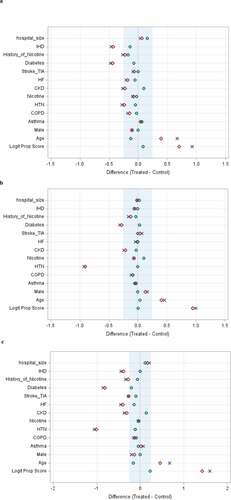
Using propensity score matching (PSM) in all matched sets, the SMDs between treated and untreated control groups were considered acceptable if <0.25, although many individual SMDs were <0.10 (). For combination treatment with statins + ACEIs/ARBs (117 matched sets), the adjusted (conditional logistic regression) odds ratio (OR) for 28-day hospital mortality was 0.33 (95% CI 0.17–0.69; p = .002, SMD = 0.22) (). For treatment with statins alone (177 matched sets) and ACEIs/ARBs alone (90 matched sets), the adjusted ORs were 0.56 (95% CI 0.39–0.93, p = .024, SMD = 0.08) and 0.52 (95% CI 0.23–1.17, p = .11, SMD = 0.006), respectively ().
Table 3. Observational studies of 28-day hospital mortality following in-hospital treatment of COVID-19
Using Gray’s test, the CIF, which accounted for the difference in mortality between the statins + ACEIs/ARBs vs no statins and no ACEIs/ARBs, was highly significant; p = .0040 ()). The CIFs for mortality did not reach significance for statins alone vs no statins (p = .0670; )) and for ACEIs/ARBs alone vs no ACEIs/ARBs (p = .1928; )).
Figure 2. Cumulative Incidence Functions (CIFs) for days to death after PSM for treatment with statins alone (a), ACEIs/ARBs alone (b) and combination treatment with statins + ACEIs/ARBs (c). Because the number of PSM-matched patients in each treated group was different, the corresponding number of patients in each PSM-matched untreated control group was different
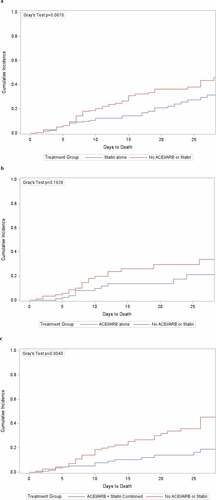
In all treatment groups, the beneficial effects of treatment were evident beginning 7 to 8 days after hospital admission ().
Discussion
Newly developed vaccines are currently being used in a limited number of high-income countries. These countries account for most of the advanced purchase orders for COVID-19 vaccines.Citation63 However, in low- and middle-income countries people will have little access to COVID-19 vaccines until well after most of them have been infected. Similarly, most of the studies of new treatments involve patented agents that will be expensive and in short supply. These agents are unlikely to be widely available in resource-poor countries.
The results of our study compare favorably with those of other observational studies of inpatient treatment of COVID-19 patients with statins ().Citation64–67 Similar to our study, these reports show that in-hospital treatment with statins alone improved survival. Whether the same is true for ACEIs and ARBs is uncertain.Citation68,Citation69 In addition, we observed that combination treatment with statins and ACEIs/ARBs was associated with a 3-fold reduction in the odds of 28-day hospital mortality (OR = 0.33; ).
We did not have information on whether our treated COVID-19 inpatients had also been treated as outpatients, although it is likely that many who had been treated as outpatients continued their treatment after hospital admission. However, several individual studies (for example, refs.Citation70-72) and meta-analysesCitation73,Citation74 of statin treatment have yielded conflicting results. In these studies, treatment was ascertained largely on the basis of outpatient (not inpatient) information. For example, in a study of 247 statin-treated COVID-19 patients, 46% of treatments were initiated after hospital admission and yet 29% of all statin treatments were discontinued because of elevated liver function or creatine kinase tests.Citation75 Another study showed that outpatient statin treatment was associated with decreased COVID-19 mortality, although only 77% of outpatient-treated patients continued statin treatment after hospital admission.Citation76 If statins are withdrawn at the time of hospital admission, their beneficial effects are likely to be reduced.Citation77,Citation78 Thus, statin treatment should be continued (or started) after hospital admission.Citation79 Clinical guidelines now recommend that outpatient statin and ACEI/ARB treatments should be continued in hospitalized COVID-19 patients.Citation80,Citation81
The endothelial dysfunction that characterizes COVID-19 is associated with microvascular immunothrombosis and venous thrombosis, which are the result of widespread disturbances in coagulation.Citation16–21 Many patients with severe COVID-19 also have a variety of functional autoantibodies that cause thrombosisCitation82,Citation83 and compromise interferon activity.Citation84 Drugs such as statins, ACEIs and ARBs were initially developed to treat cardiovascular diseases. Because of their pleiotropic activities,Citation49 some investigators believe they could be repurposed to treat the many immunometabolic derangements that characterize COVID-19.Citation4,Citation50–52 For example, randomized controlled trials have been initiated in ICU-admitted and non-ICU-admitted COVID-19 patients to evaluate whether therapeutic anticoagulation is more beneficial than prophylactic anticoagulation. Recently, enrollment of ICU (but not non-ICU) patients in these studies was “paused” for reasons of futility and safety.Citation85 It is not yet known which of these patients might have been receiving other treatments. Statins have demonstrated anticoagulant effectsCitation86,Citation87 and counteract the effects of autoantibodies on endothelial cells.Citation88 There is little information on the anticoagulant effects of ACEIs and ARBs, although ARBs have beneficial effects on many of the signaling pathways involved in coagulation (see in ref.Citation4).
Treatment with statins, ACEIs/ARBs, or a combination of both might be considered for persons who test positive for COVID-19 but have yet to develop symptomatic disease or be admitted to hospital. Preventing the development of symptomatic disease might even prevent “long COVID”.Citation89 In a small study of 154 nursing home residents in Belgium who were screened and found to be COVID-19-positive, those who were being treated with statins had significantly fewer episodes of symptomatic COVID-19.Citation90 Although residents taking ACEIs and ARBs tended to have fewer episodes of symptomatic disease, the results were not statistically significant. Similarly, those taking statins, ACEIs and ARBs had fewer episodes of serious COVID-19 requiring hospitalization, but again the results failed to reach statistical significance.
Most investigators who have reported observational studies showing that statin and/or ACEI/ARB treatment reduces COVID-19 mortality have called for randomized controlled trials (RCTs) to confirm their findings.Citation64,Citation66,Citation67,Citation70,Citation71 Some investigators insist that RCTs are essential.Citation91,Citation92 Most of these drugs are widely available, inexpensive generics that could be repurposed for COVID-19 treatment. Unfortunately, only 7% of the studies registered on Clinicaltrials.gov are focused on cardiovascular treatments and most of them are single center studies of ACEIs and ARBs.Citation93 Recently, a proposal for an RCT that included combination treatment (NCTTO4343001) was withdrawn because funding could not be obtained. To our knowledge, no RCT of combination treatment of COVID-19 patients is currently underway.
Our observational study has several limitations. The sample size (959 patients) was small; our findings will need to be replicated in larger studies. We could not evaluate separately the effects of ACEI and ARB treatment, although investigators have reported greater effectiveness for ACEI compared with ARB treatment.Citation94 We did not correct for immortal time bias. We did not have access to information on outpatient treatments and thus could not evaluate whether outpatient statin treatment was continued in the hospital or initiated only after hospital admission. We could not evaluate deaths that occurred after 28 days or that might have occurred after hospital discharge. We also did not evaluate the effects of treatment on length of stay, ICU admissions or mechanical ventilation.
We are currently expanding our study to include the second pandemic wave (1 August to 31 December 2020) and additional outcomes (e.g., ICU admissions, mechanical ventilation). We will also evaluate separately the contributions of ACEI and ARB treatments alone or in combination with statins to reducing in-hospital COVID-19 mortality and their effectiveness in treating asymptomatic (but PCR test-positive) patients.
Observational studies and RCTs both have their places in the hierarchy of research designs.Citation95 However, if there are no RCTs,Citation96 physicians and health officials will be forced to consider the trade-off between pandemic learning and doing.Citation97 In this circumstance, they might have to rely on well-conducted observational studies alone to determine whether combination treatment of the host response in COVID-19 patients is effective.Citation98
Conclusion
Our observational study in Belgium has shown that COVID-19 patients treated in-hospital with statins in combination with ACEIs/ARBs experienced a 3-fold reduction in the odds of 28-day hospital mortality. Given the absence of RCTs of combination statin + ACEI and statin + ARB treatment, investigators should undertake additional observational studies (as well as RCTs) of these treatments in hospitalized COVID-19 patients. In light of the recent increase in COVID-19 cases and hospitalizations and the strong signal of potential effectiveness shown in our study, they should be able to do so quickly. Moreover, regardless of underlying co-morbidities and unless contraindicated, physicians might also consider combination treatment with these drugs for all hospitalized COVID-19 patients.
Disclosure of potential conflicts of interest
The authors of this report did not receive specific grants for this research from funding agencies in the public, commercial, or not-for-profit sectors. They have no conflicts of interest to declare.
Acknowledgments
The authors thank the four Belgian hospitals for their support: AZ-Delta, Roeselare, AZ-Vesalius, Tongeren, AZ-Sint-Jan, Brugge, and RZ-Heilig Hart,Tienen and Zorgi for facilitating the extraction of medication data.
References
- Guan W-J, Ni Z-Y, Hu Y, Liang W-H, Ou C-Q, He J-X, Liu L, Shan H, Lei C-L, Hui DSC, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–20. doi:10.1056/NEJMoa2002032.
- Libby P, Luscher T. COVID-19 is, in the end, an endothelial disease. Eur Heart J. 2020;41(32):3038–44. doi:10.1093/eurheartj/ehaa623.
- Fosse JH, Haraldsen G, Falk K, Edelmann R. Endothelial cells in emerging viral infections. Front Cardiovasc Med. 2021 Feb 24. doi:10.3389/fcvm.2021.619690.
- Fedson DS. Treating the host response to emerging virus diseases: lessons learned from sepsis, pneumonia, influenza and Ebola. Ann Transl Med. 2016;4:421. doi:10.21037/atm.2016.11.03.
- Zhou T, Su TT, Mudianto T, Wang J. Immune asynchrony in COVID-19 pathogenesis and potential immunotherapies. J Exp Med. 2020 Oct 5;217:e20200674. doi:10.1084/jem.20200674.
- Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LF. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363–74. doi:10.1038/s41577-020-0311-8.
- Grasselli G, Tonetti T, Protti A, Langer T, Girardis M, Bellani G, Laffey J, Carrafiello G, Carsana L, Rizzuto C, et al. Pathophysiology of COVID-19-associated acute respiratory distress syndrome: a multicentre prospective observational study. Lancet Respir Med. 2020;8:1201–08. doi:10.1016/S2213-2600(20)30370-2.
- Lee S, Channappanavar R, Kanneganti T-D. Coronaviruses: innate immunity, inflammasome activation, inflammatory cell death, and cytokines. Trends Immunol. 2020;41:1083–99. doi:10.1016/j.it.2020.10.005.
- El Baba R, Herbein G. Management of epigenomic networks entailed in coronavirus infections and COVID-19. Clin Epigenetics. 2020;12:118. doi:10.1186/s13148-020-00912-7.
- Sparks MA, South AM, Badley AD, Baker-Smith CM, Batlle D, Bozkurt B, Cattaneo R, Crowley SD, Dell’Italia LJ, Ford AL, et al. Severe acute respiratory syndrome coronavirus 2, COVID-19, and the renin-angiotensin system: pressing needs and best research practices. Hypertension. 2020;76(5):1350–67. doi:10.1161/HYPERTENSIONAHA.120.15948.
- Stein RA, Young LM. From ACE2 to COVID-19: a multiorgan endothelial disease. Int J Infect Dis. 2020;100:425–30. doi:10.1016/j.ijid.2020.08.083.
- Teuwen L-A, Geldhof V, Pasut A, Carmeliet P. COVID-19: the vasculature unleashed. Nat Rev Immunol. 2020;20(7):389–91. doi:10.1038/s41577-020-0343-0.
- Rovas A, Osiaevi I, Buscher K, Sackarnd J, Tepasse P-R, Fobker M, Kuhn J, Braune S, Gobel U, Tholking G, et al. Microvascular dysfunction in COVID-19: the MYSTIC study. Angiogenesis. 2021;24(1):135–47. doi:10.1007/s10456-020-09753-7.
- Smadja DM, Guerin CL, Chocron R, Yatim N, Boussier J, Gendron N, Khider L, Hadjadj J, Goudot G, Debuc B, et al. Angiopoietin-2 as a marker of endothelial activation is a good predictor factor for intensive care unit admission of COVID-19 patients. Angiogenesis. 2020;23(4):611–20. doi:10.1007/s10456-020-09730-0.
- Yamaoka-Tojo M. Endothelial glycocalyx damage as a systemic inflammatory microvascular endotheliopathy in COVID-19. Biomed J. 2020;43(5):399–413. doi:10.1016/j.bj.2020.08.007.
- Evans PC, Rainger GE, Mason JC, Guzik TJ, Osto E, Stamataki Z. Endothelial dysfunction in COVID-19: a position paper of the ESC working group for atherosclerosis and vascular biology, and the ESC council of basic cardiovascular science. Cardiovasc Res. 2020;116(14):2177–84. doi:10.1093/cvr/cvaa230.
- Merrill JT, Erkan D, Winakur J, James JA. Emerging evidence of a COVID-19 thrombotic syndrome has treatment implications. Nat Rev Rheumatol. 2020;16(10):581–89. doi:10.1038/s41584-020-0474-5.
- Iba T, Levy JH, Levi M, Connors JM, Thachil J. Coagulopathy of coronavirus disease 2019. Crit Care Med. 2020;48(9):1358–64. doi:10.1097/CCM.0000000000004458.
- Lu Y-F, Pan L-Y, Zhang -W-W, Cheng F, Hu S-S -S-S, Zhang X, Jiang H-Y. A meta-analysis of the incidence of venous thromboembolic events and impact of anticoagulation on mortality in patients with COVID-19. Int J Infect Dis. 2020;100:34–41. doi:10.1016/j.ijid.2020.08.023.
- Skendros P, Mitsios A, Chrysanthopoulou A, Mastellos DC, Metallidis S, Rafailidis P, Ntinopoulou M, Sertaridou E, Tsironidou V, Tsigalou C, et al. Complement and tissue factor-enriched neutrophil extracellular traps are key drivers in COVID-19 Complement and tissue factor-enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis. J Clin Invest. 2020;130(11):6151–57. doi:10.1172/JCI141374.
- Godoy LC, Goligher EC, Lawler PR, Slutsky AS, Zarychanski R. Anticipating and managing coagulopathy and thrombotic manifestations of severe COVID-19. CMAJ. 2020;192(40):E1156–E1161. doi:10.1503/cmaj.201240.
- Kvernland A, Kumar A, Yaghi S, Raz E, Frontera J, Lewis A, Czeisler B, Kahn DE, Zhou T, Ishida K et al. Anticoagulation use and hemorrhagic stroke in SARS-CoV-2 patients treated at a New York healthcare system. Neurocrit Care. 2020 Aug 24;(9):1–12. doi:10.1007/s12028-020-01077-0.
- Horie S, McNicholas B, Rezoagli E, Pham T, Curley G, McAuley D, O’Kane C, Nichol A, Dos Santos C, Rocco PRM, et al. Emerging pharmacological therapies for ARDS: COVID-19 and beyond. Intensive Care Medicine. 2020;46(12):2265–83. doi:10.1007/s00134-020-06141-z.
- Singh TU, Parida S, Lingaraju MC, Kesavan M, Kumar D, Singh RK. Drug repurposing approach to fight COVID-19. Pharmacol Rep. 2020;72:1479–508. doi:10.1007/s43440-020-00155-6.
- Riva L, Yuan S, Yin X, Martin-Sancho LM, Matsunaga N, Pache L, Burgstaller-Muehlbacher S, De Jesus PD, Teriete P, Hull MV, et al. Discovery of SARS-CoV-2 antiviral drugs through large-scale compound repurposing. Nature. 2020;586:113–19. doi:10.1038/s41586-020-2577-1.
- Farne H, Kumar K, Ricthie A, Finney LJ, Johnston SL, Singanayagam A. Repurposing existing drugs for the treatment of COVID-19. Ann Am Thorac Soc. 2020;17:1186–94. doi:10.1513/AnnalsATS.202005-566FR.
- Gordon DE, Hiatt J, Bouhaddou M, Rezelj VV, Ulferts S, Braberg H, Jureka AS, Obernier K, Guo JZ, Batra J, et al. Comparative host-coronavirus protein interaction networks reveal pan-viral disease mechanisms. Science. 2020 Dec 4;370:eabe9403. doi:10.1126/science.abe9403.
- Fajnzylber J, Regan J, Coxen K, Corry H, Wong C, Rosenthal A, Worrall D, Giguel F, Piechocka-Trocha A, Atyeo C, et al. SARS-CoV-2 viral load is associated with increased disease severity and mortality. Nat Commun. 2020;11:5493. doi:10.1038/s41467-020-19057-5.
- Bermejo-Martin JF, Gonzales-Rivera M, Almansa R, Micheloud D, Tedim AP, Dominguez-Gil M, Resino S, Martin-Fernandez M, Mura PR, Perez-Garcia F, et al. Viral RNA load in plasma is associated with critical illness and a dysregulated host response in COVID-19. Crit Care. 2020;24:691. doi:10.1186/s13054-020-03398-0.
- Wofel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, Niemeyer D, Jones TC, Vollmar P, Rothe C, et al. Virological assessment of hospitalized patients with COVID-19. Nature. 2020;581:465–69. doi:10.1038/s41586-020-2196-x.
- Walsh KA, Jordan K, Clyne B, Rohde D, Drummond L, Byrne P, Ahern S, Carty PG, O’Brien KK, O’Murchu E, et al. SARS-CoV-2 detection, viral load and infectivity over the course of an infection. J Infect. 2020;81:357–71. doi:10.1016/j.jinf.2020.06.067.
- WHO Solidarity Trial Consortium, Pan H, Peto R, Henao-Restrepo A-M, Preziosi M-P, Sathiyamoorthy V, Karim Q, Alejandra MM, Garcia C, Kieny M-P, et al. Repurposed antiviral drugs for Covid-19 - interim WHO solidarity trial results. N Engl J Med. 2021;384:497–511. doi:10.1056/NEJMoa2023184.
- Simonovich VA, Pratx LDB, Scibona P, Beruto MV, Vallone MG, Vazquez C, Savoy N, Giunta DH, Perez LG, Sanchez MDL, et al. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med. 2021;384:619–29. doi:10.1056/NEJMoa2031304.
- ACTIV-3/TICO LY-CoV555 Study Group, Lundgren JD, Grund B, Barkauskas C, Holland T, Gottlieb R, Sandkovsky U, Brown S, Knowlton K, Self W, et al. A neutralizing monoclonal antibody for hospitalized patients with Covid-19. N Engl J Med. 2021;384:905–14. doi:10.1056/NEJMoa2033130.
- Thomson EC, Rosen LE, Shepherd JG, Spreafico R, Da Silva Filipe A, Wojcechowskyj JA, Davis C, Piccoli L, Pascall DJ, Dillen J, et al. Circulating SARS-CoV-2 spike N439K variants maintain fitness while evading antibody-mediated immunity. Cell. 2021;184:1171–87.e20. doi:10.1016/jcell.2021.01.037.
- Cele S, Gazy I, Jackson L, Hwa S-H, Tegally H, Lustig G, Giandhari J, Pillay S, Wilkinson E, Naidoo Y, et al. Escape of SARS-CoV-2 501Y.V2 variants from neutralization by convalescent plasma. Nature. 2021;593:142–46. doi:10.1038/s41586-021-03471-w.
- Wang P, Nair MS, Liu L, Iketani S, Luo Y, Guo Y, Wang M, Yu J, Zhang B, Kwong PD, et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature. 2021;593:130–35. doi:10.1038/s41586-021-03398-2.
- Pirofski L-A, Casadevall A. Pathogenesis of COVID-19 from the perspective of the damage-response framework. mBio. 2020;11:e01175–20. doi:10.1128/mBio.01175-20.
- RECOVERY Collaborative Group, Horby P, Lim W, Emberson JR, Mafham M, Bell J, Linsell L, Staplin N, Brightling C, Ustianowski A, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi:10.1056/NEJMoa2021436.
- WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group, Jac S, Murthy S, Diaz J, Slutsky A, Villar J, Angus D, Annane D, Azvedo L, Berwanger O, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324:330–41. doi:10.1001/jama.2020.17023.
- Matthay MA, Thompson BT. Dexamethasone in hospitalised patients with COVID-19: addressing uncertainties. Lancet Respir Med. 2020;8:1170–72. doi:10.1016/S2213-2600(20)30503-8.
- RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397:1637–45. doi:10.1016/S0140-6736(21)00676-0.
- REMAP-CAP Investigators, Gordon AC, Mouncey PR, Al-Beidh F, Rowan KM, Nichol AD, Arabi YM, Annane D, Beane A, van Bentum-puijk W, et al. Interleukin-6 receptor antagonists in critically ill patients with Covid-19.N Engl J Med. 2021;384:1491–1502. doi:10.1056/NEJMoa2100433.
- Salvarani C, Dolci G, Massari M, Merlo DF, Cavuto S, Savoldi L, Bruzzi P, Boni F, Braglia L, Turra C, et al. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181:24–31. doi:10.1001/jamainternmed.2020.6615.
- Hermine O, Mariette X, Tharaux P-L, Resche-Rigon M, Porcher R, Ravaud P; CORIMUNO-19 Collaborative Group. Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;18:32–40. doi:10.1001/jamainternmed.2020.6820.
- Rosas IO, Brau N, Waters M, Go RC, Hunter BD, Bhagani S, Skiest D, Aziz MS, Cooper N, Douglas IS, et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med. 2021 Feb 25. doi:10.1056/NEJMoa2028700.
- Rubin EJ, Longo DL, Baden LR. Interleukin-6 receptor inhibition in Covid-19 - cooling the inflammatory soup. N Engl J Med. 2021;384:1564–65. doi:10.1056/NEJMe2103108.
- Fedson DS. Treating influenza with statins and other immunomodulatory agents. Antiviral Res. 2013;99:417–35. doi:10.1016/j.antiviral.2013.06.018.
- Oesterle A, Laufs U, Liao JK. Pleiotropic effects of statins on the cardiovascular system. Circ Res. 2017;120:229–43. doi:10.1161/CIRCRESAHA.116.308537.
- Parihar SP, Guler R, Brombacher F. Statins: a viable candidate for host-directed therapy against infectious diseases. Nat Rev Immunol. 2019;19:104–17. doi:10.1038/s41577-018-0094-3.
- Gomes Silva IV, de Figueiredo RC, Alves Rios DR. Effect of different classes of antihypertensive drugs on endothelial function and inflammation. Int J Mol Sci. 2019;20:3458. doi:10.3390/ijms20143458.
- Nagele MP, Haubner B, Tanner FC, Ruchitzka F, Flammer AJ. Endothelial dysfunction in COVID-19: current findings and therapeutic implications. Atherosclerosis. 2020;314:58–62. doi:10.1016/j.atherosclerosis.2020.10.014.
- Kruger P, Bailey M, Bellomo R, Cooper DJ, Harward M, Higgins A, Howe B, Jones D, Joyce C, Kostner K, et al. A multicenter randomized trial of atorvastatin therapy in intensive care patients with severe sepsis. Am J Respir Crit Care Med. 2013;187:743–50. doi:10.1164/rccm.201209-1718OC.
- De Roquetaillade C, Jamme M, Charpentier J, Chiche JD, Cariou A, Mira JP, Pene F, Litjos J-F. Hemodynamic impact of cardiovascular antihypertensive medications in patients with sepsis-related acute circulatory failure. Shock. 2020;54:315–20. doi:10.1097/SHK.0000000000001524.
- Koh KK, Sakuma I, Shimada K, Hayashi T, Quon MJ. Combining potent statin therapy with other drugs to optimize simultaneous cardiovascular and metabolic benefits while minimizing adverse events. Korean Circ J. 2017;47:432–39. doi:10.4070/kcj.2016.0406.
- Fedson DS. Pandemic influenza: a potential role for statins in treatment and prophylaxis. Clin Infect Dis. 2006;43:199–205. doi:10.1086/505116.
- Fedson DS, Rordam OM. Treating Ebola patients: a ‘bottom up’ approach using generic statins and angiotensin receptor blockers. Int J Infect Dis. 2015;36:80–84. doi:10.1016/j.ijid.2015.04.019.
- Fedson DS, Opal SM, Rordam OM. Hiding in plain sight; an approach to treating patients with severe COVID-19 infection. mBio. 2020;11:e00398–20. doi:10.1128/mBio.00398-20.
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–62. doi:10.1016/S0140-6736(20)30566-3.
- Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol. 2009;170:244–56. doi:10.1093/aje/kwp107.
- Stuart EA. Matching methods for causal inference: a review and a look forward. Stat Sci. 2010;25:1–21. doi:10.1214/09-STS313.
- Austin PC, Fine JP. Propensity-score matching with competing risks in survival analysis. Stat Med. 2019;38:751–77. doi:10.1002/sim.8008.
- So AD, Woo J. Reserving coronavirus disease 2019 vaccines for global access: cross sectional analysis. BMJ. 2020;371:m4750. doi:10.1136/bmj.m4750.
- Zhang XJ, Qin JJ, Cheng X, Shen L, Zhao YC, Yuan Y, Lei F, Chen -M-M, Yang H, Bai L, et al. In-hospital use of statins is associated with a reduced risk of mortality among individuals with COVID-19. Cell Metab. 2020;32:176–87. doi:10.1016/j.cmet.2020.06.015.
- Mallow PJ, Belk KW, Topmiller M, Hooker EA. Outcomes of hospitalized COVID-19 patients by risk factors: results from a United States hospital claims database. J Health Econ Outcomes Res. 2020;7:165–74. doi:10.36469/jheor.2020.17331.
- Fan Y, Guo T, Yan F, Gong M, Zhang XA, Li C, He T, Luo H, Zhang L, Chen M, et al. Association of statin use with the in-hospital outcomes of 2019-coronavirus disease patients: a retrospective study. Front Med (Lausanne). 2020;7:584870. doi:10.3389/fmed.2020.584870.
- Masana L, Correig E, Rodriguez-Borjabad C, Anoro E, Arroyo JA, Jerico C, Pedragosa A, la Miret M, Naf S, Pardo A, et al. Effect of statin therapy on SARS-CoV-2 -related mortality in hospitalized patients. Eur Heart J Cardiovasc Pharmacother. 2020 Nov 2; pvaa128. doi:10.1093/ehjcvp/pvaa128.
- Lam KW, Chow KW, Vo J, Hou W, Li H, Richman PS, Mallipattu S, Skopicki HA, Singer AJ, Duong TQ. Continued in-hospital angiotensin-converting enzyme inhibitor and angiotensin II receptor blocker use in hypertensive COVID-19 patients is associated with positive clinical outcomes. J Infect Dis. 2020;222:1256–64. doi:10.1093/infdis/jiaa447.
- Cohen JB, Hanff TC, William P, Sweitzer N, Rosado-Santander NR, Medina C, Rodriguez-Mori JE, Renna N, Chang TI, Corrales-Medina V, et al. Continuation versus discontinuation of renin-angiotensin system inhibitors in patients admitted to hospital with COVID-19: a prospective, randomised, open-label trial. Lancet Respir Med. 2021;9:275–84. doi:10.1016/S2213-2600(20)30558-0.
- McCarthy CP, Murphy S, Jones-O’Connor M, Olshan DS, Khambhati JR, Rehman S, Cadigan JB, Cui J, Meyerowitz E, Phillippides G, et al. Early clinical and sociodemographic experience with patients hospitalized with COVID-19 at a large American healthcare system. EClinicalMedicine. 2020;26:100504. doi:10.1016/j.eclinm.2020.100504.
- Daniels LB, Sitapati AM, Zhang J, Zou J, Bui QM, Ren J, Longhurst CA, Criqui MH, Messer K. Relation of statin use prior to admission to severity and recovery among COVID-19 inpatients. Am J Cardiol. 2020;136:149–55. doi:10.1016/j.amjcard.2020.09.012.
- Rosenthal N, Cao Z, Gundrum J, Sianis J, Safo S. Risk factors associated with in-hospital mortality in a US national sample of patients with COVID-19. JAMA Netw Open. 2020 Dec 1;3:e2029058. doi:10.1001/jamanetworkopen.2020.29058.
- Kow CS, Hasan SS. Meta-analysis of effectiveness of statins in patients with severe COVID-19. Am J Cardiol. 2020;134:153–55. doi:10.1016/j.amjcard.2020.08.004.
- Scheen AJ. Statins and clinical outcomes with COVID-19: meta-analyses of observational studies. Diabetes Metab. 2020 Dec 23;47(6):101220. doi:10.1016/j.diabet.2020.101220.
- Nicholson CJ, Wooster L, Sigurslid HH, Li RF, Jiang W, Tian W, Cardenas CL, Malhotra R. Estimating risk of mechanical ventilation and mortality among adult COVID-19 patients admitted to mass general brigham: the VICE and DICE scores. EClinicalMedicine. 2021 Mar;33:100765. doi:10.1016/j.eclinm.2021.100765.
- Gupta A, Madhavan MV, Poterucha TJ, DeFilippis EM, Hennessey JA, Redfors B, Eckhardt C, Bikdeli B, Platt J, Nalbandian A, et al. Association between antecedent statin use and decreased mortality in hospitalized patients with COVID-19. Nat Commun. 2021;12:1325. doi:10.1038/s41467-021-21553-1.
- Fonarow GC, Wright RS, Spencer FA, Fredrick PD, Dong W, Every N, French WJ; National Registry of Myocardial infarction 4 Investigators. Effect of statins use within the first 24 hours of admission for acute myocardial infarction on early morbidity and mortality. Am J Cardiol. 2005;96:611–16. doi:10.1016/j.amjcard.2005.04.029.
- Cubeddu LX, Seamon MJ. Statin withdrawal: clinical implications and molecular mechanisms. Pharmacotherapy. 2006;26:1288–96. doi:10.1592/phco.26.9.1288.
- Fedson DS. Statin treatment of COVID-19. Am J Cardiol. 2020;136:171–73. doi:10.1016/j.amjcard.2020.09.050.
- Iqbal Z, Ho JH, Adam S, France M, Syed A, Neely D, Rees A, Khatib R, Cegla J, Byrne C, et al. Managing hyperlipidaemia in patients with COVID-19 and during its pandemic: an expert panel position statement from HEART UK. Atherosclerosis. 2020;313:126–36. doi:10.1016/j.atherosclerosis.2020.09.008.
- ESC Council on Hypertension. Position statement of the ESC Council on hypertension on ACE-inhibitors and angiotensin receptor blockers. 2020 [accessed 29 Dec 2020]. https://www.escardio.org/Councils/Council-on-Hypertension-(CHT)/News/position-statement-of-the- esc-council-on-hypertension-on-ace-inhibitors-and-ang .
- Zuo Y, Estes SK, Ali RA, Gandhi AA, Yalavarthi S, Shi H, Sule G, Gockman K, Madison JA, Zuo M, et al. Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19. Sci Transl Med. 2020;12:eabd3876. doi:10.1126/scitranslmed.abd3876.
- Wang EY, Mao T, Klein J, Dai Y, Huck JD, Jaycox JR, Liu F, Zhou T, Israelow B, Wong P, et al. Diverse functional autoantibodies in patients with COVID-19. Nature. 2021 May 19. doi:10.1038/s41586-021-03631-y.
- Bastard P, Rosen LB, Zhang Q, Michailidis E, Hoffmann HH, Zhang Y, Dorgham K, Phillippot Q, Rosain J, Beziat V, et al. Auto-antibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020 Oct 23;370(6515):eabd4585. doi:10.1126/science.abd4585.
- Hughes S. COVID-19 anticoagulation trials ‘paused’ for futility, safety. Medscape Internal Med. 2020 Dec 22 [accessed 15 Jan 2021]. medscape.com .
- Bianconi V, Sahebkar A, Banach M, Pirro M. Statins, hemostatic factors and thrombotic risk. Curr Opin Cardiol. 2017;32:460–66. doi:10.1097/HCO.0000000000000397.
- Kunutsor SK, Seidu S, Khunti K. Statins and primary prevention of venous thromboembolism: a systematic review and meta-analysis. Lancet Haematol. 2017;4:e83–e93. doi:10.1016/S2352-3026(16)30184-3.
- Meroni PL, Raschi E, Testoni C. Endothelium as a target for anti-phospholipid antibodies and for therapeutical intervention. Autoimmun Rev. 2002;1:55–60. doi:10.1016/s1568-9972(01)00014-3.
- Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, Kang L, Guo L, Liu M, Zhou X, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–32. doi:10.1016/S0140-6736(20)32656-8.
- De Spiegeleer A, Bronselaer A, Teo JT, Byttebier G, De Tre G, Belmans L, Dobson R, Wynendaele E, Van De Wiele C, Vandaele F, et al. The effects of ARBs, ACEis, and statins on clinical outcomes of COVID-19 infection among nursing home residents. J Am Med Dir Assoc. 2020;21:909–14.e2. doi:10.1016/j.jamda.2020.06.018.
- Kalil AC. Treating COVID-19-off-label drug use, compassionate use, and randomized clinical trials during pandemics. JAMA. 2020;323:1897–98. doi:10.1001/jama.2020.4742.
- Fanaroff AC, Califf RM, Harrington RA, Granger CB, McMurray JJV, Patel MR, Bhatt DL, Windecker S, Hernandez AF, Gibson SM, et al. Randomized trials versus common sense and clinical observation: JACC review topic of the week. J Am Coll Cardiol. 2020;76:580–89. doi:10.1016/j.jacc.2020.05.069.
- Trump S, Lukassen S, Anker MS, Chua RL, Liebig J, Thurmann L, Corman VM, Binder M, Loske J, Klasa C, et al. Hypertension delays viral clearance and exacerbates airway hyperinflammation in patients with COVID-19. Nat Biotechnol. 2020 Dec 24. doi:10.1038/s41587-020-00796-1.
- Varshney AS, Wang DE, Bhatt AS, Blood A, Sharkawi MA, Siddiqi HK, Vaduganathan M, Monteleone PP, Patel MR, Jones WS, et al. Characteristics of clinical trials evaluating cardiovascular therapies for coronavirus disease 2019 registered on ClinicalTrials.gov: a cross-sectional analysis. Am Heart J. 2021;232:105–15. doi:10.1016/j.ahj.2020.10.065.
- Concato J, Shah N, Horwitz R. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med. 2000;342:1887–92. doi:10.1056/NEJM200006223422507.
- Fedson DS. COVID-19, host response treatment, and the need for political leadership. J Public Health Policy. 2021;42:6–14. doi:10.1057/s41271-020-00266-7.
- Angus DC. Optimizing the trade-off between learning and doing in a pandemic. JAMA. 2020;323:1895–96. doi:10.1001/jama.2020.4984.
- Fedson DS. Clinician-initiated research on treating the host response to pandemic influenza. Hum Vaccin Immunother. 2018;14:790–95. doi:10.1080/21645515.2017.1378292.
