ABSTRACT
In most countries of the Middle East and Northern African (MENA) region, a high hepatitis A virus (HAV) endemicity has been documented. Few others, such as Saudi Arabia and Turkey, are transitioning from high to intermediate endemicity. There is a paucity of recently published HAV disease burden that could be useful to inform or strengthen relevant national hepatitis A vaccination policy and other prevention strategies in the region. This review summarizes information on HAV epidemiology before and after the implementation of a childhood hepatitis A vaccination program in Saudi Arabia and Turkey. In both countries, a clear shift in the age of first HAV exposure has been documented, with more homogeneous trends across regions in Saudi Arabia compared to Turkey. Utilizing the experience of Saudi Arabia and Turkey with hepatitis A vaccination, countries in the region are encouraged to foster discussions on potential vaccination strategies suitable for their own setting.
Introduction
The hepatitis A virus (HAV) is a common infectious etiological agent of acute viral hepatitis.Citation1 It is frequently transmitted through the fecal-oral route and is acquired by mouth when a HAV-naive person ingests contaminated food or water or comes into close contact with an infectious person.Citation1,Citation2 It is challenging to diagnose hepatitis A from other types of viral hepatitis solely on the basis of clinical attributes. Therefore, serologic testing to confirm the presence of antibodies is required for diagnosis. While anti-HAV IgM antibodies indicate recent or current infection, IgG antibodies indicate past infection and usually persist throughout an individual’s lifespan after infection or vaccination.Citation1 HAV infection is confirmed in the acute or early convalescent phase of infection by the presence of anti-HAV immunoglobulin M (IgM) in serum, typically at detectable levels 5–10 days before the onset of symptoms and up to 6 months after infection with HAV. Anti-HAV immunoglobulin G (IgG) is also detected in the serum during the convalescent phase of infection, and remains in the serum throughout the lifetime of an individual, providing protection against disease during a future infection.Citation1 Together, these measures prove useful in providing important epidemiological insights and HAV seroprevalence estimates.Citation3
Infection with HAV is very common in the early years of life and is often asymptomatic among children below 6 years of age (YOA).Citation1 In older individuals, the disease is associated with an abrupt onset of symptoms: low-grade fever, malaise, anorexia, nausea, loss of appetite, abdominal discomfort or jaundice.Citation1 The majority of the clinical illnesses due to HAV tends to resolve itself within 2 months after infection, with approximately 10%–15% of individuals developing prolonged or relapsing symptoms lasting up to half a year.Citation1 When relapse occurs, hepatitis may cause severe complications such as fulminant hepatitis or acute liver failure and, in some cases, even death.Citation1 Importantly, the severity and clinical outcome ensuing HAV infection is determined mostly by the age of the infected individual, that is, HAV infection is associated with more severe disease, a higher risk of fulminant hepatitis and death with progressing age of the individual.Citation1
Worldwide, hepatitis A disease occurs sporadically, with a trend of cyclic recurrences.Citation2 HAV is estimated to be responsible for approximately 1.5 million clinical cases of viral hepatitis per year.Citation4 In high-income countries, hepatitis A accounts for 20–25% of the total viral hepatitis burden whereas this burden is expected to be higher in low- and middle-income countries.Citation5,Citation6 This is primarily due to improving socioeconomic indicators such as rising incomes and access to clean water and sanitation,Citation7–9 which has led to the evolution of HAV epidemiology over the last two decades. Four levels of HAV endemicity are defined according to the World Health Organization (WHO) on the basis of seroprevalence (anti-HAV IgG): high (≥90% by 10 YOA); intermediate (≥50% by 15 YOA, with <90% by 10 YOA); low (≥50% by 30 YOA, with <50% by 15 YOA); and very low (<50% by 30 YOA).Citation10 Paradoxically, a gradual transition from high to intermediate endemicity has been documented in several low- and middle-income countries, significantly increasing the incidence of clinical hepatitis A.Citation8,Citation9,Citation11,Citation12 Additionally, this shift in endemicity could lead to outbreaks in susceptible populations who have not been exposed to HAV in their life and do not have immunity.Citation2,Citation10 Consequently, this can be a source of burden on individuals due to impact on quality of life, and direct and indirect financial burden on families and health-care systems resulting from absence from work or school for several weeks or months.Citation13
Immunization against HAV is considered an effective intervention to prevent clinical hepatitis A. Both the inactivated and live attenuated hepatitis A vaccines are highly immunogenic, well-tolerated and safe in the target vaccine populace and generate long-lasting, possibly life-long, protection against hepatitis A.Citation14–16 The WHO recommends that vaccinations against HAV be integrated into the national immunization schedule for children ≥1 YOA if indicated on the basis of incidence of acute hepatitis A, change in the endemicity from high to intermediate, and consideration of cost-effectiveness.Citation10 Noting this recommendation, the WHO states that vaccination against hepatitis A should be part of a comprehensive plan for the prevention and control of viral hepatitis, including measures to improve hygiene and sanitation and measures for outbreak control. Countries that have seen rapid improvements in socioeconomic status may move from high to intermediate hepatitis A endemicity in a short span of time. In these countries, a relatively large proportion of the adult population would be susceptible to HAV and therefore large-scale hepatitis A vaccination is likely to be cost-effective.Citation10 To implement decision-making of an appropriate vaccination program in an individual country, robust epidemiological data is needed. In terms of assessing any shifts in HAV endemicity, age-specific HAV seroprevalence rates and age at the midpoint of population immunity (AMPI) are reliable indicators to ascertain the hepatitis A situation in a country.Citation17 Establishing HAV seroprevalence (measurement of anti-HAV IgG or combined anti-HAV IgG/IgM antibodies) by age facilitates the indirect measurement of age-specific incidence rates of infection by providing a measurement of the susceptibility of the age group to new infections. This is useful to understand the concept of transition and shifting of the risk to individuals of older ages that have not been infected in childhood.Citation3
Hepatitis A in the Middle East and North Africa (MENA) region
In the Middle East and Northern African (MENA) region, several countries, such as Iran, Jordan, Lebanon, Morocco, Tunisia, Egypt, Iraq, Palestine, Syria, and Yemen have high or very high levels of HAV endemicity.Citation18,Citation19 Among other countries in the region, such as Algeria, Saudi Arabia, Turkey, Kuwait, and the United Arab Emirates, substantial progress in the socioeconomic levels, sanitary conditions, access to clean water sources, and improvements in the quality of water has been documented, resulting in a transition in HAV endemicity levels.Citation20–22 A few of these countries have also implemented childhood hepatitis A vaccination programs.Citation23,Citation24 Given these developments in recent decades, a gradual shift in the age at infection with HAV has been observed in a few of these countries in the MENA region.Citation20–22 This phenomenon deserves urgent attention as it may lead to the occurrence of regular community-wide outbreaks, the consequences of which can be grave in the populations without immunity (such as adolescents and adults) in the region.Citation20–22
Published literature on HAV disease epidemiology and burden from the MENA region is often either outdated or missing. Adding to these issues is the limited experience of childhood hepatitis A vaccination programs from countries in the MENA region. These data are essential to help countries design appropriate interventional strategies to circumvent HAV disease burden. Moreover, if experience from vaccination programs from the region is available, it can be leveraged upon by other countries in the region without such programs to establish or strengthen relevant national hepatitis A vaccination policy.
Review objectives and methods
This review aims to summarize publicly available information to offer insights into the evolving pattern of HAV epidemiology in the MENA region before and after the implementation of a childhood vaccination program. Here, we used the examples of Saudi Arabia and Turkey, two countries which were among the first countries to introduce such a program into their national immunization calendars.
To collect information relevant to Saudi Arabia and Turkey, we identified published literature in PubMed (via Medline; search cutoff date: July 10, 2020) using the following search strategy: “hepatitis A” AND “Country” AND (“seroprevalence” OR “incidence”). Published estimates from the original research were supplemented with data from the Ministry of Health websites of Saudi Arabia (data available from the year 2005)Citation23 and Turkey (data available from the year 2007).Citation24 A gray literature source was consulted to obtain insights into the countries profiles.Citation25,Citation26 Articles published in English language and local languages were considered in this review. Outcomes of interest included estimates of epidemiology, such as new cases, incidence rate (per 100,000) and deaths due to HAV. When available, disease time trends and disease trends by age group, seasonality, region, gender, nationality, and other socioeconomic indicators (living standards [urban, rural], income level, migration patterns, etc.) have been extracted from the source publications and presented.
Hepatitis A in Saudi Arabia
Country profile
Saudi Arabia, officially known as the Kingdom of Saudi Arabia, is the second largest Arab, located in and making most of the Arabian Peninsula. The population of Saudi Arabia is estimated to be around 35 million (2020), and is reported to have between 3 and 5 million illegal immigrants from neighboring countries residing within its borders. Given its large area of 2.15 million square kilometers, the population density is approximately 16 individuals per square kilometer.Citation25
Individuals of 15–64 YOA constitute the majority of the total population (64.8%), whereas individuals of 0–14 YOA form nearly one-third (32.4%) and older adults (≥65 YOA) constitute 2.8% of the total population of Saudi Arabia. The median age in Saudi Arabia is 27.5 YOA with a life expectancy of 75.5 YOA (2020). This is likely influenced by improvements in provision of safe and clean drinking water, high performance of sanitation facility, and 4.7% of their Gross Domestic Product (GDP) expenditure on healthcare. A large proportion (83.3%) of Saudi Arabia’s population lives in urban areas, meaning that there are several large cities in the country. The capital, Riyadh, is the largest with a population of around 6.5 million, followed by Jeddah with roughly 4 million inhabitants and others, such as Mecca, Medina, Hofuf, and Ta’if that all have large populations ranging between 1 and 2 million.Citation25 Mecca and Medina also host the Hajj which leads to millions of pilgrims congregating from over 180 countries around the world every year.
Evolution of HAV epidemiology
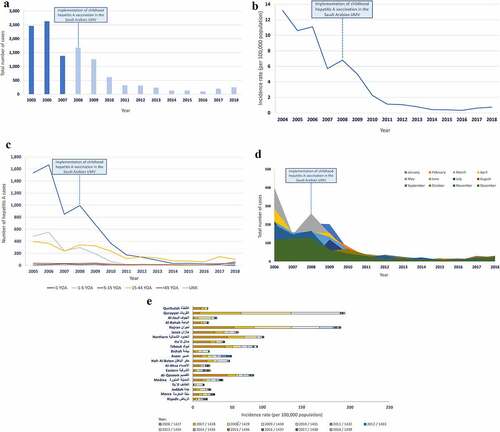
The epidemiology of hepatitis A in Saudi Arabia has undergone major changes, concurrent with major socioeconomic developments over the last two to three decades. We found little published evidence on the incidence of hepatitis A but several studies did provide data on HAV seroprevalence in Saudi Arabia ().Citation27–29
Table 1. HAV seroprevalence in Saudi Arabia
The incidence of hepatitis A cases was found in three studies.Citation27–29 One study reported a decline in incidence from 14.0 per 100,000 to 9.0 per 100,000 from 1992 to 2003.Citation28 After an outbreak in 2004, incidence resumed its decline with 8.0 per 100,000 in 2007, with the highest incidence reported in the Eastern region of Saudi Arabia compared to Central and Western regions; in males compared to females; in Saudi nationals compared to non-Saudi nationals; and in children <15 YOA compared to individuals ≥15 YOA.Citation29 A third study reported an incidence of 8.02 per 100,000 in 2008 (based on data from the Ministry of Health).Citation27
Evidence from studies investigating HAV seroprevalence reveal an overall decline in HAV seroprevalence regardless of age in the 1990s compared to those rates reported from studies conducted in the 1980s. Specifically, seroprevalence surveys in the 1980s usually found a prevalence of nearly 90% by 10 YOA but in the 1990s, more than 50% of children under 10 YOA; and more than 90% of teenagers had immunity to HAV, a serological heritage of past exposure.Citation31,Citation32 Into the early years of the twenty-first century, about one-third of the adolescent population and >50% of young adults (≥20 YOA) had immunity to HAV. The majority of studies provides evidence of differences in seroprevalence by age group (higher HAV seropositivity in older individuals compared to children), and regions/cities (higher HAV seropositivity in individuals from underdeveloped Central and Western regions of Saudi Arabia than individuals from other regions). Limited data was found to support differences in HAV seroprevalence by gender and nationality of the individual (). A few studies did report stark differences in HAV seroprevalence by urban or rural living conditions within regions or citiesCitation33,Citation34 and socioeconomic stratifications, most likely affected by the community’s socioeconomic development, in terms of education, housing conditions, water sanitation, GDP and improvements in healthcare infrastructure.
In terms of healthcare infrastructure, Saudi Arabia implemented childhood hepatitis A vaccination in its universal mass vaccination program in 2008. Since its implementation, hepatitis A vaccination is given as a two-dose schedule to children at 18 and 24 months of age. Three hepatitis A vaccines are licensed in Saudi Arabia, of which two vaccines are available through its universal mass vaccination program: Avaxim (Sanofi Pasteur) and Healive (Sinovac Biotech Ltd); both of which are inactivated hepatitis A vaccines. Publications describing the epidemiology and disease burden of HAV after the implementation of vaccination are available from the Ministry of Health of Saudi Arabia.Citation27 One publication that performed a cross-sectional analysis on data from the Ministry of Health show a much more pronounced decline in HAV incidence (from 8.02 per 100,000 in 2008 to 2.54 per 100,000 in 2010) after the implementation of the childhood hepatitis A immunization program.Citation27 Time trends of the number of total cases and incidence of HAV have also been made available by the Ministry of Health of Saudi Arabia ()). The data shows that since 2008, the total number of new hepatitis A cases has declined by about 90% ()). A corresponding decline in the incidence rate was observed, albeit with a small increase from 0.42 per 100,000 to 0.74 per 100,000 from 2016 to 2018 ()). Yet a significant increase (147%) in the total number of hepatitis A cases was documented from 2016 to 2018, with adults of 15–44 YOA and >45 YOA being the most impacted age group ()). Together, these data demonstrate a more distinct and sustained shift in HAV endemicity (i.e. the age at first infection with HAV) than that observed prior to the implementation of childhood vaccination in Saudi Arabia. In terms of trends in disease occurrence, a low number of hepatitis A cases has been reported in the summer season with a gradual shift in seasonality since 2014, with most hepatitis A cases being reported in the autumn seasons in the latter years ()). While the overall number of hepatitis A cases and incidence rate have declined in Saudi Arabia, there is significant heterogeneity reported across the different regions and cities of Saudi Arabia, with the highest incidence rate reported in Qurayyat followed by Najran ()). Qurayyat and Najran share their borders with Jordan and Yemen, respectively. While the hepatitis A burden in Jordan is comparable to this of Saudi Arabia, Yemen is suggested to have a high burden of hepatitis A.Citation19 Moreover, the vast majority of the populations in both cities are Bedouins who tend to live in rural areas and have to cope with inadequate water and sanitation facilities.
Figure 1. Evolution of hepatitis A in Saudi Arabia (a) Total number of cases (b) Incidence rate (c) Age-specific distribution of cases (d) Seasonal distribution of cases (e) Regional distribution of cases
Hepatitis A in Turkey
Country profile
Turkey, officially known as the Republic of Turkey, is a trans-continental country that includes Anatolia in West Asia and East Thrace in Southern Europe. The population of Turkey is estimated to be around 84 million (2020) with about 3,600,000 immigrants. Given its area of 769,630 square kilometers, the population density is approximately 110 individuals per square kilometer.Citation26
Individuals of 15–64 YOA constitute a majority of the total population (67.0%), whereas individuals of 0–14 YOA represent 27.0% of the total population. Older adults (≥65 YOA) account for 6.0% of the total population. The median age in Turkey is 30.9 years with a life expectancy of 75 YOA (2018).Citation26 This is likely influenced by socioeconomic and health-care improvements seen in recent decades. Notably, the entire population of Turkey has access to safe and clean drinking water and only 5% of the population struggles with access to improved sanitation facilities. A large proportion (70%) of Turkey’s population live in urban areas and the country harbors several large cities. The largest city, Istanbul, has a population of nearly 15 million, followed by the capital, Ankara, with 3.5 million inhabitants, and others such as Izmir, Bursa, Adana and Gaziantep that have large populations comprised between 1 and 2 million.Citation26
Evolution of HAV epidemiology
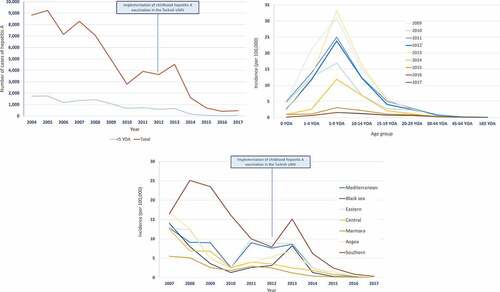
In the early 1990s, HAV disease was highly endemic in Turkey. Owing to gradual improvements in hygiene and sanitary conditions in Turkey, the burden of disease due to HAV saw a steady decline. We found one study that provided the incidence of hepatitis A cases; in 2004, 6.6 to 19.7 per 100,000 depending on the region with the highest incidence reported in 5- to 14 YOA followed by individuals >15 YOA.Citation28
Several studies have investigated HAV seroprevalence in Turkey (). While studies from the 1990s show high HAV seroprevalence among more than half of all young adolescents,Citation35,Citation42 studies performed in the 2000s show over half of the adolescent and young adult population with higher rates of HAV seropositivity, much higher than in children (). It is worth noting that the majority of data are reflective of a past infection with HAV (anti-HAV IgG) rather than a recent exposure (anti-HAV IgM); anti-HAV IgM levels when reported ranged from 0.0% to 22.7%, with children and adolescents ≤15 YOA being the most affected group, which can be explained by HAV infection occurring in schools.Citation43,Citation49 This evidence is reflective of a change in HAV endemicity in Turkey with a marked decrease in the HAV seroprevalence in the overall population, and an apparent shift in the pattern of HAV exposure toward older children, adolescents and young adults.
Table 2. HAV seroprevalence in Turkey
Noting these findings, it is important to consider the heterogeneity of findings in the different regions of Turkey. A strong West-East gradient is evident when observing HAV seroprevalence rates (). For example, one study shows that highly endemic regions in the Eastern and South-Eastern parts of the country have incidences of up to 23.6 per 100,000 whereas an average incidence rate of 10 per 100,000 has been documented in the intermediate endemic Western regions.Citation55
Besides age- and region-specific heterogeneities, the studies reveal important general trends. Notable differences in the hepatitis A endemicity profiles between urban and rural areas were documented in Turkey. In one study, it was found that less than one-third of children below 6 YOA living in urban areas had immunity.Citation56 Other studies conducted in Turkey demonstrated that living in crowded conditions, having a high number of siblings, and being part of a household with low income and education level (maternal and paternal) were risk factors for HAV exposure and transmission for children (). There is little evidence to support any true differences in HAV seroprevalence by gender, revealing that both males and females have nearly equivalent levels of HAV exposure, and no seroprevalence data were found by nationality. Two studies investigated HAV seroprevalence in adult patients with chronic viral hepatitis and chronic liver disease, respectively. In these patients, high HAV seropositivity was documented (>90%), with a higher HAV seroprevalence observed with increasing age.Citation57,Citation58 ().
An expanded government-funded program on immunization for children, including hepatitis A immunization, has been implemented in Turkey since October 2012. Hepatitis A vaccine is administered to children in two doses at 18 and 24 months of age.Citation59 Three inactivated hepatitis A vaccines have been licensed for use: Havrix (GSK), VAQTA (MSD), and Healive (Sinovac). The national hepatitis A immunization program of Turkey appears to have been highly effective in reducing further HAV cases ()). After the introduction of hepatitis A vaccination in Turkey, the incidence of hepatitis A saw a much more pronounced decline compared to the reduction documented before the implementation of childhood hepatitis A vaccination across individuals of all ages with suggestive herd protection effects.Citation50 However, since the implementation of vaccination in 2012, the trend in age-specific incidence of HAV reveals only a slight shift in the occurrence of HAV cases from very young children to older children, adolescents and young adults ()). Reductions in HAV incidence are seen shortly after vaccine implementation, and also in age groups not targeted by the vaccine ()). According to data from 2017, a downward trend in the incidence of hepatitis A was seen across all the regions of Turkey ()).
Figure 2. Burden of HAV disease in Turkey (a) Number of hepatitis A cases (b) Age-specific incidence of hepatitis A (c) Regional incidence of hepatitis A
Discussion
A large proportion of the population of the MENA region is living in low- and middle-income countries and is faced with viral hepatitis A as a major public health problem, especially those transitioning from high to intermediate HAV endemicity.Citation19,Citation20,Citation22 Most of the countries in this region have a high endemicity of HAV infection and several countries are stipulated to be transitioning from high to intermediate endemicity.Citation19,Citation20,Citation22 The epidemiological shift of infection burden from children to adults could lead to an increase in health-care costs. Given the recent and rapid socioeconomic developments in this region, determining the epidemiological pattern of HAV infection has become crucial for health-care policymakers to make informed decisions about implementing cost-effective disease management strategies. In this review, we describe the epidemiology and seroprevalence of HAV from two countries in the MENA region that have had several years of experience with a childhood hepatitis A vaccination program. In choosing these two countries as examples, we expect the findings to be largely applicable to the majority of the nations in the MENA region. Saudi Arabia can be considered representative of the transitioning countries in the MENA region, whereas Turkey can be considered representative of upper-middle-income nations in the MENA region, given its heterogeneity with common socioeconomic and health-care indicators observed across the different regions of Turkey (income levels, access to safe water and adequate sanitation facilities, urbanization, unemployment, inflation, the density of housing, living conditions and immunization services, migration patterns, among others).
In the last few decades, HAV epidemiology has changed in Saudi Arabia and Turkey, from a high to an intermediate endemicity pattern, whereby the average age of HAV infection has been increasing. This shift has been a well-known epidemiological feature worldwide in countries that have seen improvements in public health-care policies, sanitation, and education.Citation7–9,Citation11,Citation13 Furthermore, childhood vaccination programs were implemented in Saudi Arabia and Turkey in 2008 and 2012, respectively. Large declines in HAV disease burden have been seen in both countries with the shift in age groups becoming more pronounced, leaving adolescents and young adults susceptible to HAV. This is concerning because with increasing age, the risk of developing symptomatic disease and severe clinical manifestations also increases. Additionally, in these regions where a substantial proportion of adolescents and adults is susceptible, HAV may circulate, often through regular community-wide outbreaks. Thus, paradoxically, with the transition from high to intermediate endemicity, the incidence of clinically significant hepatitis A increases.
The changes in epidemiology and disease burden observed across the different regions of Saudi Arabia seem to be homogeneous as compared to those seen in Turkey. Regardless, important differences merit further discussion. In Saudi Arabia, although a general pronounced decrease in HAV incidence is seen after the implementation of childhood hepatitis A vaccination, a significant increase in the total number of hepatitis A cases was documented around 2018; specifically, an increase in hepatitis A cases among individuals of 15–44 YOA and ≥45 YOA. To avoid a further increase in cases and the occurrence of outbreaks, it is recommended to implement measures to ensure high vaccination coverage of hepatitis A vaccination is maintained among children. In addition, awareness programs about hepatitis A should be implemented for the general population. In contrast, the situation in Turkey depicts that older children, adolescents, and young adults remain susceptible to HAV infection. Turkey is characterized by marked regional differences in HAV endemicity – levels are high in Eastern regions, while Western Turkey shows only intermediate endemicity.Citation55 Moreover, the high level of HAV seroprevalence reported in marginalized populations, especially those living in the rural areas of Turkey, presents an important public health issue as a relatively large proportion of young adults are susceptible to infection. This can be explained by the depleting quality of water resources in the river basins of the Eastern region of Turkey (covering Ceyhan, Asi, and Southeast part of Firat-Dicle), due to the uncontrolled human-induced activities which have led to the deterioration of the environmental performance indicators representing environmental stressors.Citation60 Alternatively, internal and external migration patterns pertinent to Turkey have also been documented as a reason for such heterogeneous findings across the regions of Turkey. More people originating from highly endemic regions in Eastern Turkey are moving to the Western regions of the country, residing along susceptible second generations and who also travel back to the high-endemicity home regions during holidays.Citation55,Citation61 Migration of individuals from highly endemic conflict areas, such as Syria, Lebanon and Iraq could also be the reason for HAV transmission to susceptible individuals in Turkey and may have led to localized outbreaks in Turkish communities.Citation60,Citation62,Citation63 The burden of symptomatic disease must be addressed further to support specific programs of continued sanitation and education improvement and the possibility of vaccination in more susceptible regions. As a consequence, the general population of Turkey is at risk of HAV infection, mainly due to the high level of migration from the East to the West region of the country and migration into the rural areas from surrounding conflicted areas (Syria and Iraq) in the MENA region.
The childhood hepatitis A vaccination program in Turkey has been successful in reducing the burden of disease in children, with the potential to demonstrate herd immunity effects as indicated by reductions in HAV incidence seen in age groups not targeted by the vaccine. This finding could be relevant for the MENA region as it has been established that herd immunity effects more than doubled the savings from hepatitis A immunization during the first 10 years of the program.Citation64 Whether this will truly be the case for the Turkish program is still to be determined. In the context of herd immunity, high vaccination coverage in the target vaccination group in Turkey is the need of the hour. Moreover, a strengthened health surveillance system, nationwide serological surveys and tracking rate of childhood vaccination uptake will be valuable for monitoring the evolving HAV epidemiology in Turkey.
This narrative review has several limitations related to the methodology, such as the lack of a systematic approach to identify relevant literature and the inclusion of gray literature sources (i.e., literature not peer-reviewed prior to publication, such as government databases, websites, and reports) in the search. However, we found this methodology appropriate given the limited geographic scope of this review. Generalizability of the results from this review to other countries in the region may be hindered due to the different health systems in the region, the difference in reporting systems, and in case definitions. In addition, a lack of published impact of vaccination data from Saudi Arabia and Turkey limited comparison with data from before the childhood immunization program was implemented. Furthermore, while seroprevalence data and inferences about endemicity shifts are based on IgG measurements, some studies only report the total Ig (IgM+IgG). This is an important caveat in any interpretation on the endemicity shift from the available seroprevalence data. When reporting IgG levels, most studies do not distinguish between vaccine-induced IgG and infection-induced IgG. Techniques to differentiate the origin of acquired immunity have been developed,Citation79 but may not be widely used, yet if used in future studies, could be important for public health decisions and planning. Generalizability of the data from this review to the region might be limited due to a lack of data on outbreaks in the literature for both countries; possible reasons for this could be the review methodology or the fact that these data are generally lacking. Nonetheless, these data are essential to ascertain and confirm a HAV endemicity shift from high to intermediate in the region (). Finally, data on the economic burden attributable to the endemicity shift in the region albeit lacking would be helpful to make informed decisions about disease management strategies.
Conclusions
In Saudi Arabia and Turkey, the burden of HAV has decreased in the overall population and this is attributable to socioeconomic improvements and the implementation of a childhood hepatitis A vaccination program; nonetheless, older children continue to be affected in Turkey and an increasing number of adolescents and adults is being primarily affected by the disease in both countries. Published data on vaccination impact from both countries are lacking and therefore reliable epidemiological data from large cross-sectional observational studies are urgently needed to ensure that existing strategies to control HAV in the region are revisited and updated in time. Across regions that are still struggling with poor living conditions and access to clean and safe drinking water, efforts are urgently needed to ensure the supply of clean, safe water, and adequate sanitation facilities. Continuing the childhood hepatitis A vaccination program in both Saudi Arabia and Turkey remains beneficial considering the intermediate endemicity level, however, depending on the evolving epidemiology in the region, potential interim solutions, such as hepatitis A vaccination of adolescents and adults, including those with an underlying comorbidity, might be needed for the effective control and prevention of HAV exposure of these susceptible population groups. Building on the experience of Saudi Arabia and Turkey with childhood hepatitis A vaccination, countries in the MENA region can use these findings to support discussions on potential vaccination strategies suitable for their own countries.
Contributorship
Selim Badur, Serdar Öztürk, Mansour Khalaf, and Debasish Saha contributed to the study design. Selim Badur, Serdar Öztürk, Alev Ozakay and Mansour Khalaf performed the literature search. Selim Badur, Serdar Öztürk, Alev Ozakay, and Debasish Saha provided materials or tools for the analysis. Selim Badur performed the analysis. Selim Badur, Serdar Öztürk, Alev Ozakay, Debasish Saha, and Pierre Van Damme interpreted the results. All authors participated to the development of this manuscript. All authors had full access to the data and gave final approval before submission.
Trademark
Avaxim is a trademark owned by or licensed to Sanofi Pasteur. Havrix is a trademark owned by or licensed to the GSK group of companies. Healive is a trademark owned by or licensed to Sinovac Biotech Ltd. VAQTA is a trademark owned by or licensed to Merck & Co.
Disclosure of potential conflicts of interest
Selim Badur, Serdar Öztürk, Alev Ozakay, Mansour Khalafand Debasish Saha are employees and shareholders of the GSK group of companies. Pierre Van Damme declares no personal financial conflicts of interest, and reports grants to his institute, the University of Antwerp, from the GSK group of companies, Pfizer, Sanofi, Merck, Takeda, Baxter, CanSino China, Themis, Osivax, J&J and Abbott, and grants from The Bill & Melinda Gates Foundation, PATH, the Flemish government, and the European Union, outside the submitted work. Selim Badur, Serdar Öztürk, Alev Ozakay, Mansour Khalaf, Debasish Saha and Pierre Van Damme declare no other financial and non-financial relationships and activities.
Acknowledgments
The authors thank Business & Decision Life Sciences platform for editorial assistance and manuscript coordination on behalf of GSK. Amandine Radziejwoski coordinated the manuscript development and editorial support. Amrita Ostawal (Arete Communication UG, on behalf of GSK) provided medical writing support.
Additional information
Funding
References
- Centers for Disease Control and Prevention. Epidemiology and prevention of vaccine-preventable diseases. Washington (D.C.): Centers for Disease Control and Prevention; 2015.
- World Health Organization. Hepatitis A. Geneva (Switzerland): World Health Organization; 2019 [accessed 2020 Apr 15]. https://www.who.int/en/news-room/fact-sheets/detail/hepatitis-a.
- Kurkela S, Pebody R, Kafatos G, Andrews N, Barbara C, Bruzzone B, Butur D, Caplinskas S, Davidkin I, Hatzakis A, et al. Comparative hepatitis A seroepidemiology in 10 European countries. Epidemiol Infect. 2012;140(12):2172–81. doi:10.1017/s0950268812000015.
- Franco E, Meleleo C, Serino L, Sorbara D, Zaratti L. Hepatitis A: epidemiology and prevention in developing countries. World J Hepatol. 2012;4(3):68–73. doi:10.4254/wjh.v4.i3.68.
- Agrawal A, Singh S, Kolhapure S, Hoet B, Arankalle V, Mitra M. Increasing Burden of Hepatitis A in Adolescents and Adults and the Need for Long-Term Protection: a Review from the Indian Subcontinent. Infect Dis Ther. 2019;8(4):483–97. doi:10.1007/s40121-019-00270-9.
- John Hopkins Medicine. Viral hepatitis A and E. Internet. 2020 [accessed 2020 May 31]. https://www.hopkinsmedicine.org/health/conditions-and-diseases/hepatitis/viral-hepatitis-a-and-e.
- Jacobsen KH. Globalization and the changing epidemiology of hepatitis A virus. Cold Spring Harb Perspect Med. 2018;8(10):a031716. doi:10.1101/cshperspect.a031716.
- Jacobsen KH, Koopman JS. Declining hepatitis A seroprevalence: a global review and analysis. Epidemiol Infect. 2004;132(6):1005–22. doi:10.1017/s0950268804002857.
- Jacobsen KH, Koopman JS. The effects of socioeconomic development on worldwide hepatitis A virus seroprevalence patterns. Int J Epidemiol. 2005;34(3):600–09. doi:10.1093/ije/dyi062.
- World Health Organization. WHO position paper on hepatitis A vaccines – June 2012. Wkly Epidemiol Rec. 2012;87(28–29):261–276.
- Jacobsen KH, Wiersma ST. Hepatitis A virus seroprevalence by age and world region, 1990 and 2005. Vaccine. 2010;28(41):6653–57. doi:10.1016/j.vaccine.2010.08.037.
- Aggarwal R, Goel A. Hepatitis A: epidemiology in resource-poor countries. Curr Opin Infect Dis. 2015;28(5):488–96. doi:10.1097/qco.0000000000000188.
- Jacobsen K. The global prevalence of hepatitis A virus infection and susceptibility: a systematic review. https://apps.who.int/iris/handle/10665/70180,2009.WHO/IVB/10.01
- Lolekha S, Pratuangtham S, Punpanich W, Bowonkiratikachorn P, Chimabutra K, Weber F. Immunogenicity and safety of two doses of a paediatric hepatitis A vaccine in Thai children: comparison of three vaccination schedules. J Trop Pediatr. 2003;49(6):333–39. doi:10.1093/tropej/49.6.333.
- Raczniak GA, Bulkow LR, Bruce MG, Zanis CL, Baum RL, Snowball MM, Byrd KK, Sharapov UM, Hennessy TW, McMahon BJ. Long-term immunogenicity of hepatitis A virus vaccine in Alaska 17 years after initial childhood series. J Infect Dis. 2013;207(3):493–96. doi:10.1093/infdis/jis710.
- Sharapov UM, Bulkow LR, Negus SE, Spradling PR, Homan C, Drobeniuc J, Bruce M, Kamili S, Hu DJ, McMahon BJ. Persistence of hepatitis A vaccine induced seropositivity in infants and young children by maternal antibody status: 10-year follow-up. Hepatology. 2012;56(2):516–22. doi:10.1002/hep.25687.
- Mohd Hanafiah K, Jacobsen KH, Wiersma ST. Challenges to mapping the health risk of hepatitis A virus infection. Int J Health Geogr. 2011;10:57. doi:10.1186/1476-072x-10-57.
- Koroglu M, Jacobsen KH, Demiray T, Ozbek A, Erkorkmaz U, Altindis M. Socioeconomic indicators are strong predictors of hepatitis A seroprevalence rates in the Middle East and North Africa. J Infect Public Health. 2017;10(5):513–17. doi:10.1016/j.jiph.2016.09.020.
- Safiabadi M, Rezaee-Zavareh MS, Moayed Alavian S. Estimation of hepatitis A virus infection prevalence among Eastern Mediterranean and Middle Eastern Countries: a systematic review and pooled analysis. Hepat Mon. 2017;17(2):e44695. doi:10.5812/hepatmon.44695.
- Melhem NM, Talhouk R, Rachidi H, Ramia S. Hepatitis A virus in the Middle East and North Africa region: a new challenge. J Viral Hepat. 2014;21(9):605–15. doi:10.1111/jvh.12282.
- Itani T, Jacobsen KH, Nguyen T, Wiktor SZ. A new method for imputing country-level estimates of hepatitis A virus endemicity levels in the Eastern Mediterranean region. Vaccine. 2014;32(46):6067–74. doi:10.1016/j.vaccine.2014.09.006.
- Mahboobi N, Alavian S. Hepatitis A in the Eastern Mediterranean Region: a review on the prevalence. Scimetr. 2013;2:e14613. doi:10.5812/scimetr.14613.
- Ministry of Health Kingdom of Saudi Arabia. Statistical yearbook. 2018 [accessed 2020 July 10]. https://www.moh.gov.sa/en/Ministry/Statistics/book/Documents/book-Statistics.pdf.
- Turkish viral hepatitis prevention and control program (2018-2023), Ministry of health Ed. 1. Ankara; 2018 [accessed 2021 Mar 17]. https://hsgm.saglik.gov.tr/depo/birimler/Bulasici-hastaliklar-db/duyurular/Turkiye_Viral_Hepatit_Onleme_ve_Kontrol_Programi/Turkiye_Viral_Hepatit_Onleme_ve_Kontrol_Programi_TR.pdf.
- World Population Review. About Saudi Arabia. World Population Review; 2020 [accessed 2020 July 10]. https://worldpopulationreview.com/countries/saudi-arabia-population/.
- World Population Review. About Turkey. World Population Review; 2020 [accessed 2020 July 10]. https://worldpopulationreview.com/countries/turkey-population/.
- Alshabanat A, Albacker R, Basalamah A, Salamah A, Al-Frayh A. Profile of viral hepatitis in Saudi Arabia. Biomedical Res. 2013;24:396–99.
- Hendrickx G, Van Herck K, Vorsters A, Wiersma S, Shapiro C, Andrus JK, Ropero AM, Shouval D, Ward W, Van Damme P. Has the time come to control hepatitis A globally? Matching prevention to the changing epidemiology. J Viral Hepat. 2008;15(Suppl 2):1–15. doi:10.1111/j.1365-2893.2008.01022.x.
- Memish ZA, Knawy BA, El-Saed A. Incidence trends of viral hepatitis A, B, and C seropositivity over eight years of surveillance in Saudi Arabia. Int J Infect Dis. 2010;14(2):e115–120. doi:10.1016/j.ijid.2009.03.027.
- Babaeer MHS, Awfi MSHA. Prevalence Study of Hepatitis A Virus (HAV) on Jeddah Population. Biosci Biotech Res Asia. 2011;8(2).
- Ashraf SJ, Arya SC, Parande CM, Kristensen E. Hepatitis A virus among natives and expatriates in Saudi Arabia. J Med Virol. 1986;19(2):151–53. doi:10.1002/jmv.1890190207.
- Fathalla SE, Al-Jama AA, Al-Sheikh IH, Islam SI. Seroprevalence of hepatitis A virus markers in Eastern Saudi Arabia. Saudi Med J. 2000;21:945–49.
- Al-Faleh FZ, Al-Jeffri MH, Ramia ST, Al-Rashed RS, Arif MA, Mohammed OM, Bakhsh MH, Al-Freihi HM, Aljumah AA, Rezeig MA, et al. Hepatitis A in Saudi Arabia: a comparative sero-epidemiological study. Saudi Med J. 1999;20(9):678–81.
- El-Gilany A-H, Hammad S, Refaat K, Al-Enazi R. Seroprevalence of hepatitis A antibodies among children in a Saudi community. Asian Pac J Trop Med. 2010;3(4):278–82. doi:10.1016/S1995-7645(10)60068-5.
- Baki A, Aynaci M, Koksal I. Prevalence of antibody to hepatitis A virus among children in Trabzon, Turkey. Infection. 1993;21(2):132–33. doi:10.1007/bf01710752.
- Almuneef MA, Memish ZA, Balkhy HH, Qahtani M, Alotaibi B, Hajeer A, Qasim L, Al Knawy B. Epidemiologic shift in the prevalence of Hepatitis A virus in Saudi Arabia: a case for routine Hepatitis A vaccination. Vaccine. 2006;24(27–28):5599–5603. doi:10.1016/j.vaccine.2006.04.038
- Jaber SM, Prevalence of anti-hepatitis B and anti-hepatitis A antibodies among school aged children in Western Saudi Arabia. Saudi Med J. 2006;27(10):1515–1522.
- Almuneef MA, Memish ZA, Balkhy HH, Otaibi B, Helmi M. Seroprevalence survey of varicella, measles, rubella, and hepatitis A and B viruses in a Xhealthcare workforce in Saudi Arabia. Infect Control Hosp Epidemiol. 2006;27(11):1178–1183. doi:10.1086/508826
- Al-Tawfiq JA, Anani A. Profile of viral hepatitis A, B, and C in a Saudi Arabian hospital. Med Sci Monit. 2008;14(1):Cr52–56.
- Memish Z, Qasim L, Abed E, AlBasheer A, Aldraihim A, Knawy B, Hajeer AH. Pattern of viral hepatitis infection in a selected population from Saudi Arabia. Mil Med. 2003;168(7):565–568.
- Barrimah E, Salem KA, Gabal MS. An outbreak of hepatitis A associated with treated waste water used for irrigation. J Egypt Public Health Assoc. 1999;74(3–4):227–239.
- Ersoy B, Aydogan A, Dincoguz A, Sinan Meral M, Turul T. The seroprevalence of anti-HAV among 0-16-year-olds referred to pediatric outpatients clinics of a hospital. J Trop Pediatr. 1998;44(1):55–56. doi:10.1093/tropej/44.1.55.
- Sac RU, Bostanci I, Dallar Y, Cihan G, Atli O. Hepatitis A seroprevalence and demographics in Turkish children in Ankara. Pediatr Int. 2009;51(1):5–8. doi:10.1111/j.1442-200X.2008.02671.x.
- Arif M, Al-Faleh FZ, Al-Frayh AR, Ramia S. Reduction in the prevalence of antibody to hepatitis A virus among young Saudi adults: implications for hepatitis A vaccine. Saudi J Gastroenterol. 1995;1(2):93–96.
- Al-Knawy B, El-Mekki AA, Yarbough PO. The role of hepatitis E virus infection among patients with acute viral hepatitis in southern Saudi Arabia. Ann Saudi Med. 1997;17(1):32–34. doi:10.5144/0256-4947.1997.32
- Ghabrah TM, Stickland GT, Tsarev S, Yarbough P, Farci P, Engle R, Emerson S, Purcell R. Acute viral hepatitis in Saudi Arabia: seroepidemiological analysis, risk factors, clinical manifestations, and evidence for a sixth hepatitis agent. Clin Infect Dis. 1995;21(3):621–627. doi:10.1093/clinids/21.3.621
- Ramia S, Antibody against hepatitis A in Saudi Arabians and in expatriates from various parts of the world working in Saudi Arabia. J Infect. 1986;12(2):153–155. doi:10.1016/s0163-4453(86)93633-9
- Talukder MA, Waller DK, Nixon P, Al-Admawy AM. Prevalence of antibody to hepatitis A virus in a Saudi Arabian hospital population. J Infect Dis. 1983;148(6):1167. doi:10.1093/infdis/148.6.1167
- Nalbantoglu B, Donma MM, Ozdilek B, Karasu E, Nalbantoglu A. Shifting epidemiology of hepatitis a infection and vaccination status of children aged 6 months-12 years: time for mass vaccination. Iran J Pediatr. 2013;23:276–80.
- Yüksek SK, Tezer H, Parlakay A, Gülhan B, Kara A, Çiftçi E, Tapısız A, Çelik M, Özdemir H, Aykaç K, et al. Impact of the mandatory Hepatitis A immunization program: before and after the vaccine in Ankara, Central of Turkey. Turk J Pediatr. 2019;61(5):677–85. doi:10.24953/turkjped.2019.05.006.
- Kader Ç, Göçmen AY, Demir MI, Çolak NY, Gök SE, Arkan FI, Sara MY, Erbay A. Hepatitis A immunity in Yozgat, Turkey. Ann Saudi Med. 2019;39(1): 37–41. doi:10.5144/0256-4947.2019.37
- Karadeniz A, Akduman Alaşehir E. Seroepidemiology of hepatitis viruses, measles, mumps, rubella and varicella among healthcare workers and students: Should we screen before vaccination? J Infect Public Health. 2020;13(4):480–484. doi:10.1016/j.jiph.2020.01.309
- Köse Ş, Ödemiş I, Çelik D, Gireniz Tatar B, Akbulut I, Çiftdoğan DY. Hepatitis A, B, C and HIV seroprevalence among Syrian refugee children admitted to outpatient clinics. Infez Med. 2017;25(4):339–343.
- Yentür Doni N, Şimşek Z, Gürses G, Yıldız Zeyrek F, Akbaba M. The knowledge and high seroprevalence of hepatitis A in a high-risk group (agricultural reproductive-aged women) in the southeastern region of Turkey. Turk J Med Sci. 2017;47(4):1055–1060. doi:10.3906/sag-1505-22
- Viral Hepatitis Prevention Board. Epidemiology of viral hepatitis in Turkey. Antwerp (Belgium): VHPB; 2010, p. 26. https://www.vhpb.org/files/html/Meetings_and_publications/Viral_Hepatitis_Newsletters/vhv18n2.pdf.
- Atabek ME, Fyndyk D, Gulyuz A, Erkul I. Prevalence of anti-HAV and anti-HEV antibodies in Konya, Turkey. Health Policy (New York). 2004;67(3):265–69. doi:10.1016/s0168-8510(03)00123-4.
- Özden HT. Hepatitis A seroprevalence in patients with chronic viral hepatitis in Konya, Turkey. Eur J Gastroenterol Hepatol. 2016;28(3):333–37. doi:10.1097/meg.0000000000000547.
- Tulek N, Ozsoy M, Moroglu C, Cagla Sonmezer M, Temocin F, Tuncer Ertem G, Sebnem Erdinc F. Seroprevalence of Hepatitis A Virus Antibodies among the Patients with Chronic Hepatitis B in Turkey. Euroasian J Hepatogastroenterol. 2015;5(2):95–97. doi:10.5005/jp-journals-10018-1143.
- Saglik Bakanligi TC Hepatit A asi Uygulamasi Ust Yazisi. [accessed 2020 July 10]. https://dosyaism.saglik.gov.tr/Eklenti/12472,20121008-1509-hskdan-hepatit-a-asisinin-uygulanmasi-hakkinda-yazipdf.pdf?0.
- Dogru AO, David RM, Ulugtekin N, Goksel C, Seker DZ, Sozen S. GIS based spatial pattern analysis: children with Hepatitis A in Turkey. Environ Res. 2017;156:349–57. doi:10.1016/j.envres.2017.04.001.
- Ceyhan M, Yildirim I, Kurt N, Uysal G, Dikici B, Ecevit C, Aydogan A, Koc A, Yasa O, Koseoglu M, et al. Differences in hepatitis A seroprevalence among geographical regions in Turkey: a need for regional vaccination recommendations. J Viral Hepat. 2008;15(Suppl 2):69–72. doi:10.1111/j.1365-2893.2008.01034.x.
- Demiray T, Koroglu M, Jacobsen KH, Ozbek A, Terzi HA, Altindis M. Hepatitis A virus epidemiology in Turkey as universal childhood vaccination begins: seroprevalence and endemicity by region. Turk J Pediatr. 2016;58(5):480–91. doi:10.24953/turkjped.2016.05.004.
- Ergonul O, Tulek N, Kayi I, Irmak H, Erdem O, Dara M. Profiling infectious diseases in Turkey after the influx of 3.5 million Syrian refugees. Clin Microbiol Infect. 2020;26(3):307–12. doi:10.1016/j.cmi.2019.06.022.
- Armstrong GL, Billah K, Rein DB, Hicks KA, Wirth KE, Bell BP. The economics of routine childhood hepatitis A immunization in the United States: the impact of herd immunity. Pediatrics. 2007;119(1):e22–29. doi:10.1542/peds.2006-1572.
- Gözü Pirinççioğlu A, Adıgüzel S, Özekinci T. Seropositivity of Hepatitis A in Children Aged 7-14 Years in Diyarbakir Province Center. Med Sci Monit. 2018;24:936–943. doi:10.12659/msm.906861
- Karacaer Z, Tosun S, Batırel A, Şahin S, Altaş İ, Uysal S, Erol S, Ceran N, Albayrak A, Yıldız İE, et al. Changes in acute viral hepatitis epidemiology in the Turkish adult population: A multicenter study. Turk J Gastroenterol. 2018;29(2):177–182. doi:10.5152/tjg.2018.17431
- Ceran N, Yuksel Kocdogan F, Mert D, Erdem I, Dede B, Adaleti R, Ozyurek S, Karagul E, Goktas P. Hepatitis A seroprevalence in children and young adults in Istanbul, Turkey: seroprevalence change and associated factors. J Viral Hepat. 2012;19(1):72–76. doi:10.1111/j.1365-2893.2011.01454.x
- Karaman S, Karaman K, Kızılyıldız BS, Ceylan N, Kaba S, Parlak M, Beger B, Ceylan A. Seroprevalence of hepatitis a and associated factors among 1–15 year old children in Eastern Turkey. Int J Clin Exp Med. 2015;8(10):19394–19399.
- Alhan E, Kozanoglu B, Tumgor G, Celik U, Yaman A, Bozdemir N. Epidemiological shift of hepatitis A in central Adana, Turkey. Turk J Gastroenterol. 2014;25 Suppl 1:6–8. doi:10.5152/tjg.2014.4163
- Halicioglu O, Akman SA, Tatar B, Atesli R, Kose S. Hepatitis A seroprevalence in children and adolescents aged 1–18 years among a low socioeconomic population in Izmir, Turkey. Travel Med Infect Dis. 2012;10(1):43–47. doi:10.1016/j.tmaid.2012.01.001
- Kurugol Z, Aslan A, Turkoglu E, Koturoglu G. Changing epidemiology of hepatitis A infection in Izmir, Turkey. Vaccine. 2011;29(37):6259–6261. doi:10.1016/j.vaccine.2011.06.069
- Ince OT, Yalçin SS, Yurdakök K, Ozmert EN. Hepatitis A seroprevalence among infants aged 12 months in Ankara. Turk J Pediatr. 2011;53(1):114–116.
- Coskun O, Erdem H, Gul HC, Eyigun CP. Changes in hepatitis A prevalence rates between 1998 and 2007 in Eskisehir, Turkey. Int J Infect Dis. 2008;12(6):e141. doi:10.1016/j.ijid.2008.02.005
- Kaya D, Guler E, Ekerbicer HC, Dilber C, Karabiber H, Guler S, Davutoglu M, Ciragil P. Hepatitis A seroprevalence and its relationship with environmental factors in children of different age groups in Kahramanmaras, Eastern Mediterranean region of Turkey. J Viral Hepat. 2007;14(12):830–834. doi:10.1111/j.1365-2893.2007.00886.x
- Kaya AD, Ozturk CE, Yavuz T, Ozaydin C, Bahcebasi T. Changing patterns of hepatitis A and E sero-prevalences in children after the 1999 earthquakes in Duzce, Turkey. J Paediatr Child Health. 2008;44(4):205–207. doi:10.1111/j.1440-1754.2007.01248.x
- Tosun S, Ertan P, Kasirga E, Atman U. Changes in seroprevalence of hepatitis A in children and adolescents in Manisa, Turkey. Pediatr Int. 2004;46(6):669–672. doi:10.1111/j.1442-200x.2004.01969.x
- Kanra G, Tezcan S, Badur S, Turkish National Study Team. Hepatitis A seroprevalence in a random sample of the Turkish population by simultaneous EPI cluster and comparison with surveys in Turkey. Turk J Pediatr. 2002;44(3):204–210.
- Sıdal M, Unüvar E, Oğuz F, Cihan C, Önel D, Badur S. Age-specific seroepidemiology of hepatitis A, B, and E infections among children in Istanbul, Turkey. Eur J Epidemiol. 2001;17(2):141–144. doi:10.1023/A:1017524630372
- Lemon SM, Ott JJ, Van Damme P, Shouval D. Type A viral hepatitis: a summary and update on the molecular virology, epidemiology, pathogenesis and prevention. J Hepatol. 2018;68(1):167–84. doi:10.1016/j.jhep.2017.08.034.