ABSTRACT
Continued outbreaks of Ebola virus disease, including recent outbreaks in the Democratic Republic of the Congo (DRC), highlight the need for effective vaccine programs to combat future outbreaks. Given the population flow between DRC and Rwanda, the Rwanda Ministry of Health initiated a preventive vaccination campaign supported by a vaccination monitoring platform (VMP). The campaign aimed to vaccinate approximately 200,000 people from Rwanda’s Rubavu and Rusizi districts with the two-dose vaccine regimen Ad26.ZEBOV, MVA-BN-Filo. The VMP encompassed: biometric identification (iris scanning), mobile messaging, and an interactive reporting dashboard. The VMP collected data used to register and identify participants at subsequent visits. Mobile message reminders supported compliance. To 13 November 2020, the campaign was half complete with Ad26.ZEBOV administered to 116,974 participants and MVA-BN-Filo to 76,464. MVA-BN-Filo should be given to participants approximately 8 weeks after the Ad26.ZEBOV with a compliance window of −14 and +28 days. Of the 83,850 participants who were eligible per this dosing window for the subsequent MVA-BN-Filo vaccine, 91.2% (76,453/83,850) received it and 82.9% (69,505/83,850) received it within the compliance window defined for this campaign. Utilization of the VMP was instrumental to the success of the campaign, using biometric technology, dashboard reporting of near real-time data analysis and mobile phone communication technology to support vaccine administration and monitoring. A comprehensive VMP is feasible in large-scale health-care campaigns, beneficial for public health surveillance, and can allow effective response to an infectious disease outbreak.
Introduction
Even when effective vaccines are available, effective education and vaccination campaigns need to be implemented quickly and effectively to reduce disease and death.Citation1–3 Nonetheless, irrespective of the disease, successful vaccination campaigns rely on communication, community engagement and technology.Citation4–6
Recently, biometric and mobile messaging technologies have been increasingly used in public health interventions to identify individuals and communicate with the public, respectively, in order to overcome logistical challenges.Citation7–10 Biometric identification is one reliable identification method that authenticate individuals’ identity; it also provides the opportunity to collect data and metrics.Citation11 Methods of biometric identification include fingerprinting scanning, facial recognition, iris scanning, and voice recognition.Citation12–15 While fingerprint identification is affordable and easy to use, it is suboptimal in children due to fingerprint changes during childhood.Citation10,Citation16 Challenges are also faced with voice and facial recognition, including disturbances in noise or lighting as well as variation in facial expression or speech.Citation8 Iris patterns, however, remain consistent throughout childhood into adulthood; therefore, iris scanning identification is a useful tool for identifying populations of all ages, with the additional benefit that it does not require any physical contact between individuals, reducing the potential for transmission.Citation17–20 Mobile messaging is also increasingly being used successfully in public health interventions (e.g., diabetes self-management, weight loss, and medication adherence for antiretroviral therapy).Citation21
Following the August 2018 outbreak of Ebola virus disease in the Democratic Republic of the Congo (DRC), the World Health Organization declared the outbreak a Public Health Emergency of International Concern, stating ‘optimal vaccine strategies that have maximum impact on curtailing the outbreak as recommended by the World Health Organization’s Strategic Advisory Group of Experts (SAGE), should be implemented rapidly’.Citation22,Citation23 As part of the response to the epidemic, in July 2019, SAGE recommended the administration of Janssen’s investigational vaccine regimen consisting of two components, Ad26.ZEBOV and MVA-BN-Filo,Citation24,Citation25 to individuals at risk of Ebola infection living in areas close to the outbreak zone, with the goal of preventing further spread of the virus to neighboring countries.Citation26,Citation27
The ongoing development program of the Ad26.ZEBOV, MVA-BN-Filo two-dose vaccine regimen has involved clinical studies in many African countries (including Sierra Leone, Guinea, and DRC), the USA, and Europe, with more than 6,500 volunteers. Results have demonstrated that the two-dose vaccine is well tolerated and induces robust and durable immune responses against the Ebola virus for at least 360 days in healthy African adult volunteers.Citation28–30 These data contributed to the Rwanda Food and Drug Authority granting conditional approval on 27 September 2019 under exceptional emergency, of Ad26.ZEBOV, MVA-BN-Filo.Citation31 In May 2020, the European Medicines Agency’s Committee for Medicinal Products for Human Use also granted Ad26.ZEBOV, MVA-BN-Filo a positive opinion for marketing authorizations under exceptional circumstances (under the trade names Zabdeno and Mvabea), followed by European commission approval on 1 July 2020.Citation32 The Rwanda mass vaccination program aimed to reach approximately 200,000 people near the DRC border. The program is called ‘Umurinzi’, which means ‘guardian’ in Kinyarwanda, an official language of Rwanda.
Here, approximately half-way through the vaccination program (having successfully administered more than 100,000 people with at least one dose of the vaccine), we describe the implementation of a digital health technology platform, a vaccination monitoring platform (VMP) to support the Umurinzi vaccine campaign, and the associated challenges and learnings. Lessons learnt could facilitate future like-minded campaigns potentially applicable to any communicable disease setting.
Methods
Setting
The Innovative Medicines Initiative Ebola+ program, launched in response to the 2014 West Africa Ebola virus disease outbreak, includes a number of initiatives, such as the Ebola vaccine (EBOVAC) and Ebola Vaccine Deployment and the Acceptance and Compliance (EBODAC) projects (EBODAC partners: Grameen Foundation, Janssen, London School of Hygiene & Tropical Medicine and World Vision).Citation33
The Rwandan Ministry of Health appointed Project San Francisco/Center for Family Health Research, a clinical research organization, to implement the large-scale Umurinzi vaccine campaign through the training and set-up of vaccination administration field teams, and the overall oversight of the campaign on behalf of the Rwandan Ministry of Health. EBODAC partners (Janssen and Grameen Foundation) developed and helped to implement the VMP components (biometric identification and reporting and mobile messaging, respectively) and Johnson & Johnson provided the investigational Ad26.ZEBOV, MVA-BN-Filo Ebola vaccines. The Johnson and Johnson Health and Wellness Solutions (JJHWS) team provided content update of the mobile messaging and automated calls platform based on evidenced-based behavior science principles. Vaccination teams were composed of Project San Francisco/Center for Family Health Research staff and local health center staff.
Ad26.ZEBOV, MVA-BN-Filo vaccine
The heterologous two-dose vaccination regimen comprises the Ad26.ZEBOV vaccine, administered first, with the MVA-BN-Filo vaccination given approximately 8 weeks later.Citation24,Citation25,Citation28,Citation29 A dosing window of 56 days minus 14 and plus 28 days (day 42–day 84) was defined in this program for administration of the second vaccination. This dosing interval has previously demonstrated a tendency to elicit a higher antibody response compared with shorter intervals, while immune responses observed with longer intervals are at least similar.Citation28
Vaccination strategy
In a strategy consistent with WHO recommendations for the use of an investigational vaccine to help combat the Ebola outbreak,Citation26 the Rwanda Ministry of Health opted for a prophylactic, large-scale vaccination campaign that targeted individuals who were at greatest risk of infection with Ebola. People living in the border districts of Rubavu and Rusizi who transit across the border into the DRC for work or trading purposes were considered high risk for transmitting the virus into Rwanda due to the active outbreak of Ebola in DRC. After consultations with the Rwandan Government, Johnson & Johnson donated sufficient quantities of the two-dose Ebola vaccine regimen to vaccinate up to 200,000 individuals. This number was based on government records, which document the number of people frequently in transit across the border; approximately 50,000 people are estimated to cross the border daily in the Rubavu and Rusizi districts.Citation34
The vaccination campaign commenced in Rwanda in December 2019. Vaccination sites were set up in existing health-care centers as well as pop-up vaccination clinics in tents at the border in order to increase accessibility to the campaign. All participants were provided with a fact sheet detailing the vaccination campaign; documented informed consent was not required.
Vaccination monitoring platform (VMP)
The VMP comprises biometric-based identification tools, a telephone messaging-based engagement tool and a reporting dashboard. The VMP collected participant registration data at the first dose and provided data to identify patients at subsequent visits; data was also used to track participant adherence to both vaccine doses. The platform provided data regarding the operational, behavior science, and management teams of the Umurinzi campaign to enable informed operational decisions, facilitate campaign progress monitoring, and make real-time modifications to the campaign in order to maximize vaccine coverage and compliance as the campaign progressed.
Aim of the platform
The aim of the VMP was to ensure that correct vaccine administration with the highest possible compliance. In addition, the platform was designed to allow close to real-time insights into how the vaccination campaign through the reporting dashboard (illustrated in the results). Accordingly, the platform allowed operational improvements to be made throughout the campaign.
Equipment required for the platform
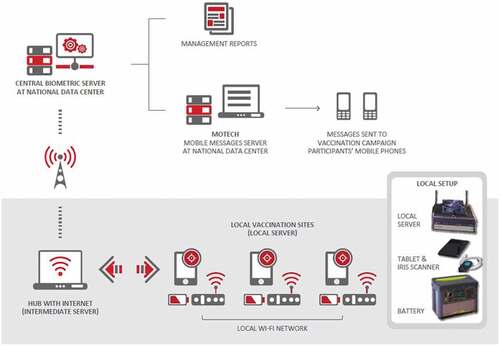
All equipment was transported to each health-care center in ruggedized cases, specifically designed to protect against harsh usage conditions. Equipment kits comprised a local server, tablet, iris scanning camera, and battery pack (). Each health-care center was provided with multiple tablets and iris scanners to allow parallel registrations to be carried out simultaneously. The operational set-up in the clinical trial vaccination programs was adapted and improved as detailed.Citation35,Citation36 The tablet was used to identify vaccination campaign participants, record participant demographics, and capture participant passport photos. The user interface on the tablet also supported scanning a Quick Response (QR) code on participant campaign cards and visual inspection of participant records on file through iris recognition via the camera. Vaccination vial labels were also scanned. Data from each tablet were transferred using local Wi-Fi to a local server and stored until Wi-Fi connectivity was sufficient to synchronize the data to a national data center in Kigali ().
Figure 1. Umurinizi Ebola vaccination monitoring program equipment and data flow schematic The uninterruptible power supply battery pack allowed field staff to work off-grid for up to 8 hours, if required, in cases where electricity was unavailable or intermittent. The Cincoze DX-1100 rugged server with external fan could withstand a wide range of DC power inputs (9 ~ 48 volts DC) and provided a local Wi-Fi network at each health-care center. The contactless iris scanning procedure was performed using a Samsung Galaxy Tab S2 8” 32 GB BT tablet or Samsung Active Pro tablet and an IriShield™ MK 2120 U monocular camera (Iritech, Inc.)
Components of the platform: biometric participant registration and identification
Biometric identification was used to uniquely identify each participant. During the registration process, participants’ irises were scanned and a digital template thereof were captured; at the follow-up vaccination appointment, the iris scan was the primary method of participant verification. In addition, all participants were given a campaign card (Supplementary Figure S1) at the first visit featuring a unique participant identification number and QR code; this was scanned at the subsequent appointment, along with the participant’s national ID card if available. A passport photo and demographic profile (e.g. age and gender) were also recorded and visualized on the tablet interface. As appropriate, a participant’s mobile phone number was recorded on the tablet interface, and the participant received campaign updates such as reminders for the follow-up vaccination appointment. For participants who did not have or wish to register a mobile phone number, the campaign card contained the date of the next appointment.
Components of the platform: mobile messaging service
Continued engagement with campaign participants via mobile phone voice messaging and texts following their initial health center visit was used to enhance their compliance with the vaccine regimen. Mobile phone technology (MOTECH communication platform; automated phone messaging and data collection system)Citation37 was used to communicate key information to participants. The Grameen Foundation were responsible for the set-up of the messaging platform. Voice messages recorded in Kinyarwanda were sent to participants via voice call (see Supplementary Table S1 for examples); if the participant did not receive the call, the messaging platform called up to three times. Furthermore, an automated SMS text in Kinyarwanda was sent to the participant containing the same information as the previous voice recordings (see Supplementary Table S1 for examples). The message and voice call content were informed based on empirical evidence from the literature that identified the determinants of vaccine acceptance, adherence, and hesitancy in sub-Saharan Africa. Evidence also informed effective behavior change techniques that influence these determinants – using such an approach to guide and inform content/components of an mHealth population-based intervention is well supported.Citation23,Citation26,Citation38
Message content addressed recalling participants for their visit appointments, engagement messages, and also ad hoc practical information pertaining to Health Center opening hours. Initial messages (both voice messages and texts in local language) were standardized around attending the clinic (reminders to re-attend the health-care center). However, in addition, during the campaign, phone messages (both voice messages and texts in local language) were customized (e.g. regarding closure of vaccination sites due to the COVID-19 outbreak). The mobile messaging system was also able to capture information on individual attendance to clinical appointments and determine if follow-up was needed. Reports on call completions and listening patterns were also generated to help the clinical team understand if additional community engagement was needed.
Components of the platform
Reporting dashboard
The Johnson & Johnson team utilized Microsoft Power BI software to automatically generate a series of dashboard reports in near real time – operational data reports for the field staff as well as campaign management reports of anonymized aggregated data. The daily reports provided an up-to-date overview of the number of individuals vaccinated, the metrics for each participating health-care center, and compliance rates, which depicted the number of vaccines administered within the preferred dosing window for all participants. Reports were also available to relevant stakeholders on mobile devices and could be viewed at any time. Operational reports were accessible to the operations team and contained more detailed participant identification listings. Field staff were able to access these reports on a real-time basis as required; management reports were distributed twice weekly, but could also be consulted in real time.
Metrics collected through the reporting dashboard
Data were/are being collected throughout the campaign (initiation 8 December 2019) through to completion of the campaign. The data presented here represent all data collected from initiation up to the successful vaccination of more than 100,000 people with at least one dose of the vaccine (the approximate half-way point of the vaccination program [reached on 13 November 2020] aiming to vaccinate approximately 200,000 people). Metrics for the second dose are calculated based on the population eligible for the second dose that are still within the defined compliance window (day 56 [−14 days, +28 days] for their second dose); i.e. excluding those participants who have not yet received the second dose and that have not yet reached preferred upper date for dosing (day 85 threshold).
The principal metric collected by the VMP was the overall uptake of the first and second doses of the vaccines. Data were collected in near real time during the campaign, such as the number of vaccines administered by age group, gender, and location. Data were collected with the permission and cooperation of all program participants and the Rwanda Ministry of Health.
A preliminary analysis to explore acceptance and adherence was conducted in a sub-sample of participants who had received the first dose early enough such that they were in the vaccine window to receive the second dose prior to the COVID-19 interruption cutoff date of 20 March 2020. Results of this analysis and compliance data around the behavior change aspects of the different campaign strategies will be published elsewhere.
Ethical considerations and confidentiality of participants
Participants consented to provide their contact details, including mobile phone numbers. Confidentiality of the participants was/is maintained throughout, with all personal data being hosted at the Rwanda National Data Center in Kigali that is equipped with both physical and logical access protection. All biometric data were/are totally separated from any clinical data, with no-one beyond the biometric administrator in Rwanda (part of Project San Francisco clinical team) having any access to the biometric data. In addition, all biometric templates are encrypted during the transition from the tablet to the national data center storage center and also at rest. To continue to work in the context of COVID-19 all vaccination centers followed national health guidelines and also took additional steps to ensure the prevention of virus transmission to either the clinical staff or the campaign participants. Staff were provided with personal protective equipment, and visit scheduling was organized with consideration for social distancing. Handwashing and temperature controls were already in place.
Results
Campaign metrics
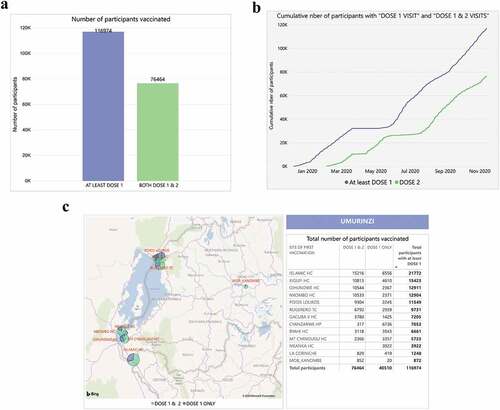
As of 13 November 2020, the campaign has reached approximately half of the targeted population with at least one dose of vaccine (). A total of 13 health-care centers in the Rubavu and Rusizi districts were involved in the vaccination program ()). Overall, during the program, a total of 1,602 community health-care workers were recruited and trained. Typically, throughout the campaign, project staff included two district coordinators (one each in Rubavu and Rusizi districts), two physicians (one in each district) and >120 field staff (split equally across the two districts, including receptionists, nurses, and data entry clerks). The field-based staff were supported at the central level by the principal physician, two study physicians, one program manager, and three data managers.
Figure 2. Dashboard reports from the vaccination monitoring platform (VMP) illustrating the number of participants vaccinated (a) overall, (b) cumulatively over time and (c) by vaccination site
VMP metrics
During the timeframe of the campaign (8 December 2019 to 13 November 2020), Ad26.ZEBOV vaccine was administered to 116,974 participants; all were registered in the Biometrics Iris scan database. Characteristics of the vaccinated population are detailed in .
Table 1. Baseline characteristics of Umurinzi vaccination campaign participants
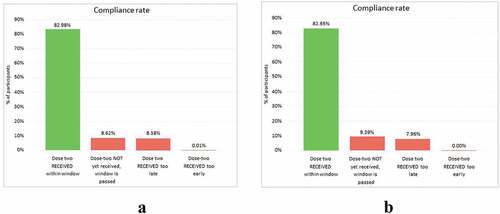
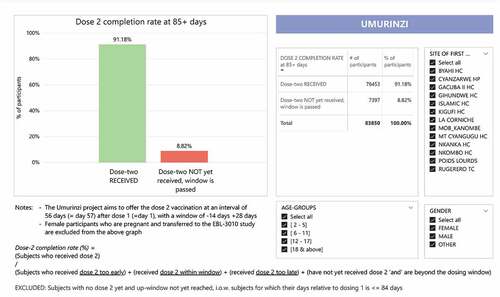
Of these 116,974 participants, 83,850 reached the end of the ideal dosing two window (85 days post dosing one date) within our timeframe of observation. Of these 83,850 participants who were expected for the second dose, 76,453 participants received the consecutive MVA-BN-Filo vaccination – a completion rate of 91.2% (76,453/83,850) (). Further, of the 83,850 participants, 82.9% (69,505/83,850) received the second dose within the defined 56-day (−14 days, +28 days) compliance window. Participants continued to receive reminders, given the possibility that they needed to be vaccinated beyond the defined compliance window to optimize the second dose coverage and potential protection.
Figure 3. Dashboard report from the vaccination monitoring platform (VMP) illustrating compliance rates of participants receiving the two-dose vaccine
Mobile messaging
Of the 116,974 participants involved, as of 13 November 2020, 84,140 (72%) successfully registered their phone numbers with the MOTECH system. Of the 458,253 SMS sent to these 84,140 participants during their participation, 451,662 (99%) SMS were successfully received. Of the 458,253 voice recorded messages sent (simultaneous with SMS), 283,559 (62%) calls were answered. On average, participants who answered the phone listened to 95% of voice recorded messages. In addition, in 93% of cases, participants listened to 90% or more of the message.
Challenges and learnings
Identifying participants and reporting data during this vaccination campaign posed several challenges to be overcome ().
Table 2. Challenges of implementation of the Umurinzi vaccination monitoring platform
Implementing the VMP
Initially, it was anticipated that the VMP would be implemented in more remote areas, requiring teams to work off-grid at mobile vaccination sites. Thus, the platform was designed to operate with minimal infrastructure and lacking electricity and internet connectivity. Equipment were fully mobile and transportable in protective, ruggedized cases by off-road vehicles and motorbikes. In addition, the back-up battery packs provided up to 8 hours of power. However, within Rubavu and Rusizi districts the campaign was able to be implemented in fixed health-care centers, which reduced the need for fully mobile equipment.
Training on the VMP
A ‘train-the-trainer’ model was adopted, whereby initial training provided selected operators with sufficient information to facilitate the subsequent training of fellow field staff across all participating health-care centers, at any point of the campaign. Training involved visual slide presentation of the following core elements: set-up of the system; how to capture an iris scan; process at registration and Dose 1 visit (vaccination card; iris scan; photographing, capturing biographical data [e.g. age and gender]); process at Dose 2 visit (participant recognition; vaccination card, iris scanning, identity check); process around exceptions; and understanding the operational reports.
The easy-to-use equipment and tablet interface enabled capturing large volumes of biometric data accurately along with demographics. Servers were dimensioned to process and store large volumes of data within relatively short periods of time.
Overcoming challenges with identification
Multiple identification approaches were foreseen with iris scanning the primary mode of identification. Iris recognition is one of the most accurate biometrics currently available. Previous technical issues with iris scanning had been resolved through operator training and ensuring controlled environmental conditions.Citation31 For example, in the health-care centers, any environmental conditions that may impede the success and quality of iris capture were controlled (e.g. curtains to block out sunlight and improve the light conditions). Importantly, iris scanning was well received by all participants with no reported concerns over its use.
Back-up methods were also used to ensure accurate identification in all cases. All participants had their photograph taken, were provided with a campaign appointment card and had their national identification card scanned (or if damaged their national identity number was entered manually).
Overcoming challenges around collecting high quality data
Learnings during the initial stages of implementation of the VMP led to adjustments to reduce the likelihood of operational errors being made. For example, operators could initially inadvertently generate data errors when inputting participant information – it was possible to record a date of birth beyond the current year. Such errors were detected through the reports, showing registrations unallocated to an age category. The user interface was subsequently adapted to include additional edit checkpoint prompts, for example, confirming the date entered is correct and preventing date of birth that occur in the future to be recorded.
Additionally, errors in the mobile telephone numbers recorded were observed. To ensure the correct telephone number was recorded, the process was integrated with the MOTECH mobile messaging platform. Participants would be sent an automated welcome text within 15 minutes of registration and prior to the leaving the initial consultation, and field staff would confirm the message receipt. If no message had been received, details were reviewed and amended, to ensure participants would successfully receive mobile messaging, including details of the next appointment.
Finally, as the tablets were able to capture Global Positioning System (GPS) details during registration; this feature could automatically provide geographical insights relating to vaccine coverage and compliance, however, it has not yet been fully exploited.
Overcoming challenges with data reporting
The monitoring platform was set up to provide dashboard reports in near real-time for daily distribution of metrics throughout the campaign. Initially, report templates were rudimentary but evolved as the operations team required additional data. Close collaboration between on-site personnel and the platform design team provided valuable input to the campaign design, and allowed for reactive customization of the campaign reports. Field staff were able to successfully access and utilize the dashboard reports generated. The monitoring platform was regularly used by the supply chain team, allowing identification of expected demand at each of the health-care centers and ensure stock, delivery, and storage capacities were aligned with local demand. However, as the software used to manage the data reporting is technical, improvements could be made to ensure easier set-up and maintenance.
Overcoming challenges with software updates
Another unanticipated issue during the campaign design stage related to software updates. Due to the use of hand-held tablets to record data, all software updates had to be made manually on each tablet, a process that required trained staff in carrying out the updates. Additionally, the optimal timing of software updates on all tablets needed to be identified so that all tablets were synchronized to latest version, without impacting daily operations. Deploying software updates required a reliable internet connection. A central hub with internet connectivity could provide a potential solution, whereby tablets are taken to be updated overnight. Also major software changes may require additional training of field staff operating the tablets.
Overcoming challenges when participants did not have a mobile phone
Although uptake of mobile phones is high within Rwanda, it was critical to ensure that complementary systems were established for all participants. Multiple touchpoints were used to encourage return for a second vaccine dosage – such as community engagement procedures, signage at border crossings, and the possession of a campaign card. Since participants with a phone had similar dosing window compliance rates for the second vaccine compared with those without a phone (83.0% [51,638 of 62,232] versus 82.6% [17,867 of 21,618), respectively) (), this suggests that these back-up procedures were successful.
Discussion
To our knowledge, these are the first data collected on implementation of a preventative Ebola vaccination campaign, focusing on how digital technologies support the campaign’s operational process. This campaign in Rwanda, launched in December 2019, aimed to vaccinate 200,000 participants with a regimen requiring administration in two injections separated by approximately 8 weeks. The implementation was very successful, with 82.9% (69,505/83,850) of the participants eligible for the second dose per dosing window receiving both vaccines, within the defined 56-day (−14 days, +28 days) compliance window. It should also be noted that a later dosing does not negatively impact the induced immune response and is possible in order to maximize second dose coverage.Citation23 Consequently, we consider the VMP used to be an example of best practice for future vaccination campaigns, while reporting the many challenges associated with implementing a campaign of this nature and communicating the ways in which they can be addressed. Cases of Ebola virus disease remained confined within the DRC and the outbreak was declared over on 25 June 2020.Citation39
Although the overall two-dose compliance rate was 82.9%, any second doses of the vaccine received outside of the vaccination window were not considered for the calculation of compliance (overall completion rate for the second dose was 91.2% [76,453/83,850]). We consider that the high dosing window compliance and high return visit rates for the second dose with the Ebola vaccine in Rwanda (despite some COVID-19 interruption) is in part due to the implementation of some of the best practice learnings from the Ebola vaccine clinical trial in Sierra Leone. The success to date of this preventive vaccination campaign in Rwanda demonstrates the feasibility of a preventive vaccination program with a two-dose vaccination regimen in Africa.
This compliance rate compares favorably with previous two-dose vaccine campaigns, albeit in other vaccine-preventable diseases, where participants’ reasons to be vaccinated may be very different.Citation40–42 A mass vaccination campaign with a two-dose vaccine to prevent cholera in 43,485 refugees in a long-standing refugee camp in Thailand had an uptake of 81% of refugees receiving at least one dose and 64% receiving two doses.Citation40 A similar campaign that administered a two-dose oral cholera vaccine in a refugee camp among 44,000 South Sudanese refugees in Uganda showed a drop-out rate of 22.5% between the two doses of vaccine.Citation41 Furthermore, a similar two-dose oral cholera vaccination campaign in Mozambique reported an overall vaccination coverage with at least one dose of 69.5%, with a two-dose uptake of 51.2%.Citation42 The low uptake rates, particularly for the follow-up vaccine, were due to the lack of awareness of the campaign; participants also reported they were unaware the second vaccine was due to be administered.Citation42
We consider the low drop-out rate in our campaign is due to ensuring multiple touchpoints with each participant, primarily through the use of mobile phone technology linked to the data monitoring system. Registration and identification using the biometric technology allowed reminders to be sent to each participant, reinforcing the critical importance of appropriately timed attendance for the second vaccination to coincide with the defined 56-day schedule. Automated voice messages and text messages were sent in local language ahead of the scheduled second visit – a convenient and non-stigmatizing reminder; the latter also being important since earlier observations highlighted that people may not want to be associated with Ebola virus disease.Citation43 In addition, use of participant campaign cards highlighting the date of the next vaccination visit provided a reminder to participants without a mobile telephone. During our campaign, compliance rates were similar between participants with or without a mobile phone (83.0% versus 82.6%, respectively) suggesting that this combined approach was important in realizing the high attendance rates at scheduled vaccination visits.
Despite the low drop-out rate, a notable decline in compliance rates was observed corresponding with the COVID-19 pandemic. The World Health Organization declared the SARS-CoV-2 outbreak a global pandemic on 11 March, 2020;Citation44 the decrease in Ebola vaccine compliance in our campaign is consistent with this declaration and the subsequent impact of restrictions on travel and activity. In addition to declines in compliance rates, as previously highlighted,Citation45 we expect that the COVID-19 pandemic will have also have impacted the overall coverage rate of this campaign – some potential participants may either have not attended for the initial vaccination dose or delayed their participation in the campaign until later.
Registration and identification of the participants using the biometric interface were critical to the success of this Ebola vaccine campaign. Biometric identification offers a secure, accurate, and responsible method of uniquely identifying and authenticating health-care users,Citation36 which is crucial for the efficient and effective delivery of health services and public health management. Identification and authentication of participants is not only applicable to large-scale vaccination programs or Ebola-specific containment campaigns, but in the wider context of communicable diseases.
In many countries where national ID cards are not available/misplaced/illegible, novel techniques are required for patient identification. Biometric identification can be a practical option to support health-care campaigns worldwide; this method of identification was well received, with no participants declining this identification step.
Iris scanning provides accurate identification for returning participants; as iris scans are unique to every individual, clinical teams are able to correctly identify each participant at the follow-up vaccination, regardless of location. Where iris scanning is less acceptable campaign cards can be used for identification. However, cards can be lost, especially during longer running vaccination campaigns. Biometric identification advantages were: easy utilization alongside a strong ‘train-the trainer’ training framework, being able to fully function with limited internet connectivity.
The data reporting tool provided near real-time data, supporting management of operations such as stock control and swift resolution of errors in the registration process. The daily reporting dashboard was always accessible to support the campaign and estimate coverage (vaccination compliance rates and metrics for each participating site were available). A minor limitation is the technical set-up of the software could benefit from easier set-up and maintenance. Nevertheless, the reporting dashboard could be adapted for any large-scale vaccination campaign or clinical study.
Of broader significance, biometric systems have also been identified as a key technology for early detection, patient screening, and public safety monitoring, and, as such, may be beneficial in the effort to contain the spread of COVID-19.Citation46 Effective infection control and immunization campaigns depend on reliable and robust patient data, including patient identification.Citation3 Contactless technologies, such as iris scanning, offer a highly accurate method of patient identification while reducing physical contact with potentially contaminated surfaces.Citation8,Citation36
Conclusion
The Umurinzi campaign was successfully supported by the use of the VMP: biometric identification, a reporting dashboard, and mobile messaging functionality, demonstrating the feasibility of a preventive two-dose Ebola vaccination program. The campaign also provided insights into the ways in which community education strategies and mobile communication can maintain engagement and support participant compliance. Similar VMP approaches are feasible to help future vaccine campaigns leapfrog into a new era that is data driven and more insightful in terms of impact.
Authors’ contributions
Romain Rutten, Serge Masyn, Annik Willems, Anne De Paepe, Paula Mc Kenna contributed to the conception, design & implementation of the campaign. Jean Baptiste Mazarati, Felix Sayinzoga, Etienne Karita, Jean Nepo Nduwamungu, Julien Nyombayire, Rosine Ingabire, Amelia Mazzei, Monica Amponsah and Seth Gogo Egoeh supported implementation of the campaign and were responsible for acquisition of the data. Romain Rutten, Serge Masyn, Annik Willems, Anne De Paepe, Paula Mc Kenna, Monica Amponsah, Seth Gogo Egoeh and Nnamdi Ezeanochie contributed to the analysis and interpretation of data. All authors contributed to drafting the manuscript. All authors read and approved the final manuscript.
Competing interests
Romain Rutten, Serge Masyn, Annik Willems, Anne De Paepe, Paula Mc Kenna and Nnamdi Ezeanochie are Janssen employees and may be stock owners of Johnson and Johnson. Jean Baptiste Mazarati, Felix Sayingoza, Etienne Karita, Jean Nepo Nduwamungu, Rosine Ingabire, Amelia Mazzei, Monica Amponsah and Seth Gogo Egoeh have no conflicts of interest to declare.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Ethics approval and consent to participate
The Rwanda Food and Drug Authority granted conditional approval under exceptional emergency for use of the Ad26.ZEBOV, MVA-BN-Filo Ebola vaccine regimen. Written information was provided to all participants ahead of them being vaccinated.
Supplemental Material
Download ()Acknowledgments
We thank the participants, community health-care workers and support staff for their participation and efforts during the campaign and also the Ministry of Health of Rwanda. We are also very grateful to the Rinda Ubuzima organization for their work around community engagement for this campaign. We are thankful to Johnson and Johnson Health and Wellness Solutions team for their behavioral science work around mobile messaging.
Data availability statement
The datasets used and/or analyzed during the current campaign are the property of the Government of Rwanda.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2021.1920872.
Additional information
Funding
References
- World Health Organization. Immunization, vaccines and biologicals. Global Vaccine Action Plan 2011–2020. 2020 [accessed 2021 Jan 19]. https://www.who.int/immunization/global_vaccine_action_plan/GVAP_doc_2011_2020/en/ .
- World Health Organization. Ebola virus disease in African countries, Version 4. 2020 Jan 30 [accessed 2021 Jan 19]. https://www.who.int/medicines/news/JJEVD_VxRoadmap2020.pdf?ua=1 .
- Hardt K, Bonanni P, King S, Santos JI, El-Hodhod M, Zimet GD, Preiss S. Vaccine strategies: optimising outcomes. Vaccine. 2016;34:6691–99. doi:10.1016/j.vaccine.2016.10.078.
- Usman S, Bologna L, Stamidis KV. The CORE Group Partners Project in North East Nigeria: community engagement strategies to combat skepticism and build trust for vaccine acceptance. Am J Trop Med Hyg. 2019;101(4_Suppl):68–73. doi:10.4269/ajtmh.19-0143.
- Frederiksen LSF, Zhang Y, Foged C, Thakur A. The long road toward COVID-19 herd immunity: vaccine platform technologies and mass immunization strategies. Front Immunol. 2020;11:1817. doi:10.3389/fimmu.2020.01817.
- Dumit EM, Novillo-Ortiz D, Contreras M, Velandia M, Danovaro-Holliday MC. The use of eHealth with immunizations: an overview of systematic reviews. Vaccine. 2018;36(52):7923–28. doi:10.1016/j.vaccine.2018.06.076.
- Ross A, Jain A, Reisman J. A hybrid fingerprint matcher. Pattern Recognit. 2003;36:1661–73. doi:10.1016/S0031-3203(02)00349-7.
- Saini R, Rana N. Comparison of various biometric methods. Int J Adv Sci Eng Technol. 2014;2:24–30.
- Cresswell KM, Sheik A. Information technology–based approaches to reducing repeat drug exposure in patients with known drug allergies. J Allergy Clin Immunol. 2008;121:1112–17. doi:10.1016/j.jaci.2007.12.1180.
- Odei-Lartey E, Boateng D, Danso S, Kwarteng A, Abokyi L, Amenga-Etego S, Gyaase S, Asante KP, Owusu-Agyei S. The application of a biometric identification technique for linking community and hospital data in rural Ghana. Glob Health Action. 2016;9:29854. doi:10.3402/gha.v9.29854.
- UNICEF. Data for children: faces, fingerprints & feet. Guidance on assessing the value of including biometric technologies in UNICEF-supported programs. 2019 Jul.
- Jain AK, Ross A, Prabhakar S. An introduction to biometric recognition. IEEE Trans Circuits Syst Video Technol. 2004;14(1):4–20. doi:10.1109/TCSVT.2003.818349.
- Grother P, Matey JR, Tabassi E, Quinn GW, Chumakov M. Temporal stability of iris recognition accuracy, IREX VI K National Institute of Standards and Technology (NIST) Interagency Report 7948. 2013 [accessed 2021 Jan 19]. http://nvlpubs.nist.gov/nistpubs/ir/2013/NIST.IR.7948.pdf .
- Jain AK, Arora SS, Best-Rowden L, Cao K. Biometrics for child vaccination and welfare: persistence of fingerprint recognition for infants and toddlers. [accessed 2020 Oct]. http://biometrics.cse.msu.edu/Publications/Fingerprint/Jainetal_BiometricsChildVaccinationWelfare_MSUTechRepMSU-CSE-15-7.pdf ..
- Yoon S, Jain AK. Longitudinal study of fingerprint recognition. Proc Natl Acad Sci USA. 2015;112(28):8555–60. doi:10.1073/pnas.1410272112.
- European Commission Joint Research Centre. Fingerprint recognition for children. Final report. Institute for the Protection and Security of the Citizen and Digital Citizen Security Unit; 2013 [accessed 2021 Jan 19]. http://publications.jrc.ec.europa.eu/repository/bitstream/JRC85145/fingerprint%20re%20for%20children%20final%20report%20%28pdf%29.pdf .
- Daugman JG. High confidence visual recognition of persons by a test of statistical independence. IEEE Trans Pattern Anal Mach Intell. 1993;15(11):1148–61. doi:10.1109/34.244676.
- Giusti M ‘Golden Age’ for iris recognition? SecureID news. AVISIAN Publications. [accessed 2021 Jan 19]. https://www.secureidnews.com/news-item/golden-age-for-iris-recognition/.
- World Health Organization. Ebola virus disease fact sheet. 2018 [accessed 2020 Oct]. http://www.who.int/mediacentre/factsheets/fs103/en/ .
- Jacobs JA, Van Ranst M. Biometric fingerprinting for visa application: device and procedure are risk factors for infection transmission. J Travel Med. 2008;15(5):335–43. doi:10.1111/j.1708-8305.2008.00232.x.
- Hall AK, Cole-Lewis H, Bernhardt JM. Mobile Text Messaging for Health: a Systematic Review of Reviews. Annu Rev Public Health. 2015;18(36):393–415. doi:10.1146/annurev-publhealth-031914-122855.
- World Health Organization. Ebola health update – DRC. 2019 [accessed 2021 Jan 19]. https://www.who.int/emergencies/diseases/ebola/drc-2019 .
- World Health Organization. Ebola Virus Disease, Democratic Republic of the Congo, External Situation Report 45. 2019 [accessed 2021 Jan 19]. https://apps.who.int/iris/bitstream/handle/10665/325242/SITREP_EVD_DRC_UGA_20190612-eng.pdf?ua=1 .
- Pollard AJ, Launay O, Lelievre JD, Lacabaratz C, Grande S, Goldstein N, Robinson C, Gaddah A, Bockstal V, Wiedemann A, et al. EBOVAC2 EBL2001 study group. Safety and immunogenicity of a two-dose heterologous Ad26.ZEBOV and MVA-BN-Filo Ebola vaccine regimen in adults in Europe (EBOVAC2): a randomised, observer-blind, participant-blind, placebo-controlled, phase 2 trial. Lancet Infect Dis. 2020 Nov 17;S1473-3099(20)30476-X. [ Epub ahead of print. PMID: 33217361]. doi:10.1016/S1473-3099(20)30476-X.
- Ebovac projects. The vaccines. [accessed 2021 Mar 17]. https://www.ebovac.org/the-vaccines/.
- World Health Organization. Strategic Advisory Group of Experts (SAGE) on Immunization Interim Recommendations on Vaccination against Ebola Virus Disease (EVD). 2019c May 7 [accessed 2021 Jan 19]. https://www.who.int/immunization/policy/position_papers/interim_ebola_recommendations_may_2019.pdf?ua=1&ua=1.
- Inungu J, Iheduru-Anderson K, Odio OJ. Recurrent Ebolavirus disease in the Democratic Republic of Congo: update and challenges. AIMS Public Health. 2019;6:502–13. doi:10.3934/publichealth.2019.4.502.
- Anywaine Z, Whitworth H, Kaleebu P, Praygod G, Shukarev G, Manno D, Kapiga S, Grosskurth H, Kalluvya S, Bockstal V, et al. Safety and Immunogenicity of a 2-dose heterologous vaccination regimen with Ad26.ZEBOV and MVA-BN-Filo Ebola Vaccines: 12-month data from a Phase 1 randomized clinical trial in Uganda and Tanzania. J Infect Dis. 2019;220:46–56. doi:10.1093/infdis/jiz070.
- Mutua G, Anzala O, Luhn K, Robinson C, Bockstal V, Anumendem D, Douoguih M. Safety and Immunogenicity of a 2-dose heterologous vaccine regimen with Ad26.ZEBOV and MVA-BN-Filo Ebola Vaccines: 12-month data from a Phase 1 randomized clinical trial in Nairobi, Kenya. J Infect Dis. 2019;220:57–67. doi:10.1093/infdis/jiz071.
- Wang Y, Li J, Hu Y, Liang Q, Wei M, Zhu F. Ebola vaccines in clinical trial: the promising candidates. Hum Vacc Immuno. 2017;13:153–68. doi:10.1080/21645515.2016.1225637.
- Rwanda Ministry of Health. The Ministry of Health launched UMURINZI Ebola Vaccine Program Campaign. 2019 Sept 12 [accessed 2021 Jan 19]. https://moh.gov.rw/index.php?id=19&tx_news_pi1%5Bnews%5D=99&tx_news_pi1%5Bday%5D=9&tx_news_pi1%5Bmonth%5D=12&tx_news_pi1%5Byear%5D=2019&cHash=3ba2f0147e07d852c32a087d336276a8 .
- European Medicines Agency. New vaccine for prevention of Ebola virus disease recommended for approval in the European Union. [accessed 2021 Jan 19]. https://www.ema.europa.eu/en/news/new-vaccine-prevention-ebola-virus-disease-recommended-approval-european-union. https://ec.europa.eu/commission/presscorner/detail/en/ip_20_1248 .
- Innovative Medicines Initiative. Ebola+. 2020 [accessed 2021 Jan 19]. https://www.imi.europa.eu/projects-results/project-factsheets/ebola .
- Social Science in Humanitarian Action. Rwanda – DRC cross border dynamics. 2019 Apr [accessed 2021 Jan 19]. https://reliefweb.int/sites/reliefweb.int/files/resources/SSHAP%20-%20Cross-border%20dynamics%2C%20Rwanda-DRC.PDF .
- Smout B, Schulz W, Larson H, Willems A, Mc Kenna P. A guidebook on community engagement, communications, and technology for clinical trials in outbreak settings. [accessed 2021 Jan 19]. https://static1.squarespace.com/static/5d4d746d648a4e0001186e38/t/5da9a8b0da5d5c5fdd6d6f30/1571399935098/EBODAC+Guidebook-2018-06-07_v04_final.pdf .
- Masyn S, Vuchelen A, Santermans E, Rasschaert F, Bangura A, Parys W, Rutten R. Overcoming the Challenges of Iris Scanning to Identify Minors (1-4 Years) in the Real-World Setting. BMC Res Notes. 2019;12:448. doi:10.1186/s13104-019-4485-8.
- Rukanda G, Amponsah M, Banura E, Kanwagi R, Gogo Egoeh S, Ngoka E, Babughirana G. Mobile phone automated messaging service’s and the fulfilment of planned clinical appointments: lessons from an Ebola clinical study in Kambia district, Sierra Leone. Int J Inform Commun Sci. 2021;6:1–10.
- Bull S, Ezeanochie N. From Foucault to Freire through Facebook: toward an integrated theory of mHealth. Health Educ Behav. 2016;43:399–411. doi:10.1177/1090198115605310.
- World Health Organization. Ebola Virus Disease, Democratic Republic of the Congo, External Situation Report 98. 2020c [accessed 2021 Jan 19]. https://www.who.int/publications/i/item/10665-332654 .
- Phares CR, Date K, Travers P, Déglise C, Wongjindanon N, Ortega L, Bhuket PR. Mass vaccination with a two-dose oral cholera vaccine in a long-standing refugee camp, Thailand. Vaccine. 2016;34:128–33. doi:10.1016/j.vaccine.2015.10.112.
- Legros D, Paquet C, Perea W, Marty I, Mugisha NK, Royer H, Neira M, Ivanoff B. Mass vaccination with a two-dose oral cholera vaccine in a refugee camp. Bull World Health Organ. 1999;77:837–42.
- Semá Baltazar C, Rafael F, Langa JPM, Chicumbe S, Cavailler P, Gessner BD, Pezzoli L, Barata A, Zaina D, Inguane DL, et al. Oral cholera vaccine coverage during a preventive door-to-door mass vaccination campaign in Nampula, Mozambique. PLoS One. 2018;13:e0198592. doi:10.1371/journal.pone.0198592.
- Dada S, McKay G, Mateus A, Lees S. Lessons learned from engaging communities for Ebola vaccine trials in Sierra Leone: reciprocity, relatability, relationships and respect (the four R’s). BMC Public Health. 2019;19:1665. doi:10.1186/s12889-019-7978-4.
- World Health Organization. COVID-19 media briefing. Mar 2020b [accessed 2021 Jan 19]. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19—11-march-2020 .
- Nelson R. COVID-19 disrupts vaccine delivery. Lancet Infect Dis. 2020;20:546. doi:10.1016/S1473-3099(20)30304-2.
- Carlaw S. Impact on biometrics of Covid-19. Biometric Technol Today. 2020;2020:8–9. doi:10.1016/S0969-4765(20)30050-3.