ABSTRACT
The World Health Organization (WHO) proposed a set of criteria to be considered for the prioritization of COVID-19 candidate vaccines for further development of phase II/III clinical trials, thinking in a target audience that includes vaccine scientists, product developers, manufacturers, regulators, and funding agencies. In this paper, a knowledge-based or rational strategy is employed to perform a prioritization matrix of approved COVID-19 vaccines: BBIBP-CorV, JANSSEN, CORONAVAC, SPUTNIK V, MODERNA, PFIZER, and VAXZEVRIA, based on those proposed criteria by WHO, related to safety, efficacy, stability, implementation, and availability. We found that JANSSEN vaccine is the one with the highest score in the present study, but our analysis suggests that the WHO criteria could be more useful if they are considered separately, taking into account the social, demographic and economic characteristics of each country.
Introduction
Vaccines are a critical new tool in the battle against COVID-19 and it is hugely encouraging to see so many vaccines proving successful and going into development. At least seven different vaccines across three platforms have been rolled out in countries. At the same time, more than 200 additional vaccine candidates are in development, of which more than 60 are in clinical development, speeding up the development of safe and effective vaccines against COVID-19.Citation1
Vulnerable populations in all countries are the highest priority for vaccination, with this goal in mind, WHO led the development of a Fair Allocation Framework that aims to ensure that successful COVID-19 vaccines and treatments are shared equitably across all countries.Citation2 This framework a key part of the Access to COVID-19 Tools (ACT) Accelerator, a global collaboration to accelerate development, production, and equitable access to COVID-19 tests, treatments, and vaccines. The framework advises that as safe and effective COVID-19 vaccines are authorized for use, all countries should receive doses in proportion to their population size to immunize the highest-priority groups.
Thinking in a target audience includes vaccine scientists, product developers, manufacturers, regulators, and funding agencies, WHO proposed a set of attributes and criteria to be considered for the evaluation and prioritization of COVID-19 candidate vaccines for further development of phase II/III clinical trials.Citation3 This study aims to perform a comparative analysis of approved COVID-19 vaccines: BBIBP-CorV,Citation4 Ad26.COVS-S of JANSSEN,Citation5 CORONAVAC,Citation6 SPUTNIK V,Citation7,Citation8 MODERNA,Citation9 PFIZER,Citation10 and VAXZEVRIA,Citation11,Citation12 building a hierarchical model of them, based on those proposed criteria by WHO, related with safety, efficacy, stability, implementation, and availability. To meet the proposed objective, the Formal Concept Analysis (FCA) is used;Citation13 this is a method for knowledge representation and information management that is widely known among information scientists all around the world because of its broad range of applications outside mathematics like economics, industry, chemistry, linguistics and environmental sciences among others.Citation14–16
Briefly, Knowledge representation incorporates findings from psychologyCitation17 about how humans solve problems and represent knowledge to design formalisms that will make complex systems easier to design and build, it is grounded on an understanding of human thinking based on concepts, which according to the main philosophical tradition, are constituted by its extension, comprising all the objects, in this case, the set of WHO complemented criteria, which belong to the concept; and its intension, including all the approved COVID-19 vaccines, which applies to all objects of the extension.
From the application of those criteria developed by WHO and the use of a rational approach based on Formal Concept Analysis (FCA), we found that JANSSEN vaccine is the one with the highest score in the present study; but we consider that the WHO criteria could be more useful if they are considered separately, taking into account the socioeconomic characteristics of each country, ignoring, if possible, political considerations that are causing dangerous delays in vaccination campaigns in various parts of the world.
Theoretical framework
The conceptual analysis of COVID-19 vaccines in phase 3 clinical trials was performed using the theoretical framework of Formal Concept Analysis (FCA), a mathematical theory oriented, in particular, at applications in knowledge representation, knowledge acquisition, and data analysis. Recently, BurgosCitation18 applied these analytic tools to study COVID-19 vaccines in the phase III clinical-stage, where a detailed description of the theoretical framework is provided.
Criteria for COVID-19 approved vaccines
To apply our theoretical framework, we must first construct the Formal contextCitation17 for the WHO criteria for the vaccines considered (). The 5 criteria are safety, stability, efficacy, availability, and implementation, the last complemented with cost by dose and also, giving a nominal value to each of them, obtained from the literature.Citation19–23
Table 1. Context for the WHO prioritization criteria. For each criterion WHO proposed a numerical integer value, here placed under each criterion, which will be used to construct the prioritization table for the approved vaccines
Rational strategy by conceptual analysis
The rational strategy is based on a set-theoretical model for conceptual hierarchies. This model mathematizes the philosophical understanding of a concept, in this case, one of the WHO criteria, as a unit of thoughts consisting of two parts: the extension and the intension (comprehension). The extension covers all objects (criteria), while the intension comprises all the approved vaccines sharing all the criteria under consideration, which is denoted by a “1” in , defining a binary relation between the sets of criteria and vaccines, and creating the Formal Context for WHO prioritization criteria.
Once elaborated the Formal context, we proceed to construct the Concept lattices for each criterion (). Those vaccines who meet the criteria in the lattice were scored with the highest rate and using 5-point scales, values are assigned to vaccines that do not meet the criteria. Finally, a prioritization matrix () is constructed when all the values were consigned and an overall score can be assigned to each vaccine.
Table 2. Prioritization matrix. the qualification or punctuation for each criterion was assigned according to what was proposed by WHO. We give the highest score to those vaccines that meet the criteria in an optimal way and lower scores are given, according to the values for each criterion, on a scale of 5, thus for the implementation criterion, 15 points are assigned to JANSSEN, it is applied in a single dose, followed by CORONAVAC with 10 points, due to its dosage of 0 + 14 days. The other vaccines, which are applied under a dosage of 0 + 21 days, are scored with 5 points
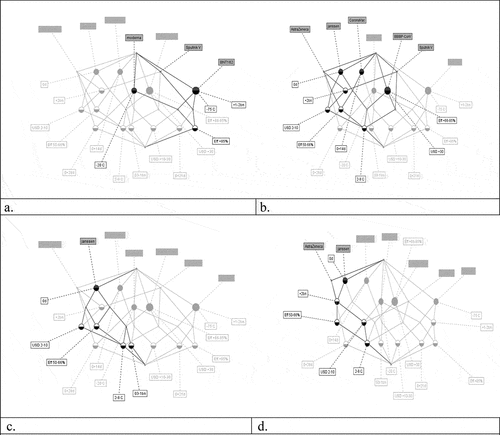
Figure 1. Concept lattices of WHO prioritization criteria for COVID-19 vaccines. Insert (a) corresponds to efficacy criterion highlighting MODERNA, PFIZER and SPUTNIK V vaccines. Insert (b) corresponds to stability criterion highlighting VAXZEVRIA of AstraZeneca, JANSSEN, CORONAVAC, BBIBP-CorV and SPUTNIK V. Insert (c) corresponds to implementation profile standing out JENSSEN vaccine. Finally, insert (d) corresponds to availability highlighting ASTRAZENECA and JANSSEN vaccines
Results and discussion
By WHO guide, the first criteria are safety. Taking into account the reported results of the 7 vaccines, we can conclude that all of them are safe, so we can assign the maximal punctuation, 25 points, to each vaccine. This is a very important issue because any of these vaccines has been rigorously evaluated in extensive clinical trials, whose results demonstrate a safety level where the incidence of adverse reactions was limited in general, to local and temporary symptoms.
However, recently some reports related to signals of blood clots in people vaccinated with VAXZEVRIA causes some concern about this vaccine, but EMACitation24 declares after an extraordinary committee held on 18 March 2021, that “the benefits of the vaccine in combating the still widespread threat of COVID-19 (which itself results in clotting problems and maybe fatal) continue to outweigh the risk of side effects” and that “the vaccine is not associated with an increase in the overall risk of blood clots (thromboembolic events) in those who receive it”. This case shows the need to implement a unified global surveillance system for careful monitoring of the adverse effects of COVID-19 vaccines around the world.
The efficacy concept ()), defined as evidence that the selected dose induces adequate immune responses in humans that might, at least, confer an 85% protection against SARS-CoV-2 infection in vaccinated individuals, contains three biologicals, MODERNA, PFIZER, and SPUTNIK V vaccines. MODERNA and PFIZER encompassing sub-concepts related to scalability levels ranging between 1 and 2 bn doses for 2021 and stability conditions of −20°C and −80° C, respectively. Following WHO’s criteria for prioritization of COVID-19 vaccine candidates, we assign 25 points to each of these three vaccines. But it is important to point out that all the considered vaccines induce protective neutralizing antibodies against SARS-CoV-2; however, seems to be that more reactogenic vaccinesCitation18 led to a strong immune status which could be useful to control the new variants of the coronavirus, how the PFIZER vaccine is showing. Based on our results, we suggest that the other two vaccines belonging to this concept of higher efficacy could be considered to be used in those countries where new variants of the virus are increasing.
Regarding stability ()), having defined as optimal storage temperature those between 2°C to 8°C, the FCA analysis shows a set of 5 vaccines belonging to this concept, VAXZEVRIA, JANSSEN, CORONAVAC, BBIBP-CorV, and SPUTNIK V; thus, following WHO guidelines, we assign 10 points to each of them. Taking into account the implementation criterion ()), FCA analysis demonstrated that of this set of 7 approved vaccines, just one, JANSSEN, meets the optimal value of 1 dose which enables us to assign it 15 points. This stability concept could be important for LMIC where usually there are no facilities devoted to freezing conditions of −20°C and even less −70°C, especially in remote regions.
Finally, regarding the availability criterion ()) or the capacity to scale up the production of the vaccines, VAXZEVRIA and SPUTNIK meet the optimal criterium of a production capacity higher than 2 billion doses for 2021, thus we assign 25 points to each of them. Currently, all pharmaceutical companies that produce the vaccines considered in this study have expressed their intention to expand their production capacity to meet the growing demand for SARS-CoV-2 vaccines around the world, so availability will be greater for all short-term approved vaccines.
As can be observed in the prioritization matrix (), the overall score for the seven approved COVID-19 vaccines, using the WHO criteria, led to a scale with JANSSEN vaccine in the first place, followed by VAXZEVRIA, PFIZER, and SPUTNIK V vaccines in second place, and in BBIBP-CorV, CORONAVAC and MODERNA in third place. Moreover, even when the overall score indicates that JANSSEN vaccine is the one with the highest value in the present study, we consider that the WHO criteria could be more useful if they are considered separately, taking into account the socioeconomic characteristics of each country, ignoring, if possible, political considerations that are causing dangerous delays in vaccination campaigns in various parts of the world.
Disclosure of potential conflicts of interest
The author declares no conflict of interests.
Additional information
Funding
References
- World Health Organization. Coronavirus disease (COVID-19) pandemic. https://covid19.who.int/
- World Health Organization. Coronavirus disease (COVID-19) pandemic. https://www.covid-19vaccinetracker.org/
- World Health Organization. Coronavirus disease (COVID-19) pandemic. https://www.who.int/publications/m/item/criteria-for-covid-19-vaccine-prioritization
- Xia S, Zhang Y, Wang Y, Wang H, Yang Y, Gao GF, Tan W, Wu G, Xu M, Lou Z. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2020;21:39–51. doi:10.1016/S1473-3099(20)30831-8.
- Sadoff J, Le Gars G, Shukarev D, Heerwegh D, Truyers C, De Groot AM, Stoop J, Tete S, Van Damme W, Leroux-Roels I. Interim Results of a Phase 1–2a Trial of Ad26.COV2. S Covid-19 Vaccine. NEJM. 2021. Downloaded from nejm.org on February 7, 2021. doi:10.1056/NEJMoa2034201.
- Zhang Y, Zeng G, Pan H, Li C, Hu Y, Chu K, Han W, Chen Z, Tang R, Yin W. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:181–92. doi:10.1016/S1473-3099(20)30843-4.
- Logunov DY, Dolzhikova IV, Zubkova OV, Tukhvatulin AI, Shcheblyakov DV, Dzharullaeva AS, Grousova DM, Erokhova AS, Kovyrshina AV, Botikov AG. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet. 2020;396:887–97. doi:10.1016/S0140-6736(20)31866-3.
- Logunov D, Dolzhikova I, Shcheblyakov D, Tukhvatulin AI, Zubkova OV, Dzharullaeva AS, Kovyrshina AV, Lubenets NL, Grousova DM, Erokhova AS. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021 February 2. doi:10.1016/S0140-6736(21)00234-8.
- Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, Coler RN, McCullough MP, Chappell JD, Denison MR, Stevens LJ. An mRNA vaccine against SARS-CoV-2 — preliminary report. N Engl J Med. 2020;383:1920–31. doi:10.1056/NEJMoa2022483.
- Mulligan MJ, Lyke KE, Kitchin N. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586:589–94. doi:10.1038/s41586-020-2639-4.
- Folegatti P, Katie J, Ewer K, Aley P, Becker S, Belij-Rammerstorfer S, Bellamy D, Bibi S, Bittaye M, Clutterbuck EA. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–78. doi:10.1016/S0140-6736(20)31604-4.
- Barrett J, Belij-Rammerstorfer S, Dold C, Ewer KJ, Folegatti PM, Gilbride C, Halkerston R, Hill J, Jenkin D, Stockdale L. Phase 1/2 trial of SARS-CoV-2 vaccine ChAdOx1 nCoV-19 with a booster dose induces multifunctional antibody responses. Nat Med. 2021;27:279–88. doi:10.1038/s41591-020-01179-4.
- Ganter B, Wille R. Formal concept analysis, mathematical foundations. Berlin Heidelberg (Germany): Springer-Verlag; 1999.
- Bourdon-García R, Burgos-Salcedo J. Formalization of an environmental model using formal concept analysis-FCA. J Physics Conf Series. 2016;738:1–7. doi:10.1088/1742-6596/738/1/012054.
- Wolff KE. Conceptual control of complex industrial production processes. Adv Knowledge Organ. 1994;4:294.
- Wolff KE. Conceptual quality control in chemical distillation columns. In: Janssen J, McClean S editors. Applied stochastic models and data analysis. UK: University of Ulster; 1995. p. 652–54.
- Schank R, Abelson R. Scripts, plans, goals, and understanding: an inquiry into human knowledge structures. NJ: Lawrence Erlbaum Associates, Inc; 1977. p. 246.
- Burgos-Salcedo J. A comparative analysis of clinical stage 3 COVID-19 vaccines using knowledge representation. medRxiv. Submitted to JMPH. doi:10.1101/2021.03.07.21253082.
- Klasse PJ, Nixon DF, Moore JP. Immunogenicity of clinically relevant SARS-CoV-2 vaccines in non-human primates and humans. Sci Adv. 2021;7:eabe8065. doi:10.1126/sciadv.abe8065.
- Amanat F, Krammer F. SARS-CoV-2 Vaccines: status Report. Immunity. 2020;52(4):583–89. doi:10.1016/j.immuni.2020.03.007.
- Poland GA, Ovsyannikova IG, Kennedy RB. SARS-CoV-2 immunity: review and applications to phase 3 vaccine candidates. Lancet. 2020;396(10262):1595–606. doi:10.1016/S0140-6736(20)32137-1.
- Kaur SP, Gupta V. COVID-19 Vaccine: a comprehensive status report. Virus Res. 2020;288:198114. doi:10.1016/j.virusres.2020.198114.
- Forni G, Mantovani A. COVID-19 vaccines: where we stand and challenges ahead. Cell Death Differ. 2021;28(2):626–39. doi:10.1038/s41418-020-00720-9.
- https://www.ema.europa.eu/en/news/covid-19-vaccine-astrazeneca-benefits-still-outweigh-risks-despite-possible-link-rare-blood-clots.
