ABSTRACT
Classical Hodgkin lymphoma is a neoplastic hematological disease. Standard first-line therapy, including chemotherapy and radiotherapy, is curative in >85% of early-stage patients, with a 5-year survival rate of >95%. However, approximately 15% of patients have hard-to-treat lymphoma with poor outcomes, and new treatment strategies are needed for these young adults. There are several well-documented cases in the medical literature on hematologic cancer remission following natural human viral infections. Therefore, hoping to reproduce these spontaneous tumor regressions, researchers have been investigating various viruses with oncolytic properties. There is a high rationale for using virotherapy in the treatment of Hodgkin lymphoma, in which tumor cells are often infected with the Epstein-Barr virus. Modern viral technologies and current knowledge about the relationship between viruses and cancer could accelerate the discovery of effective viral oncolytic therapies. This article reviews the use of oncolytic viruses as innovative therapies for treating Hodgkin lymphoma.
Introduction
Classical Hodgkin lymphoma (cHL) is a B-cell lymphoid neoplasm that comprises a rich microenvironment with a minority of tumor cells, called Hodgkin Reed-Sternberg cells.Citation1–3
On their surface, Hodgkin Reed-Sternberg cells express CD30, a cell membrane protein and part of the tumor necrosis factor receptor superfamily, and CD15 in approximately 70% of cases.Citation4,Citation5 Programmed death-ligand 1, which blocks cytotoxic T lymphocytes, is overexpressed in virtually all cases of cHL.Citation6,Citation7
Hodgkin Reed-Sternberg cells are infected by Epstein-Barr virus (EBV) in approximately 40% of cases.Citation8 The EBV plays an important role in the pathogenesis of cHL (mainly the mixed cellularity subtype) and exhibits a type II form of virus latency with the Epstein-Barr nuclear antigen-1, latent membrane protein (LMP) 1, and LMP 2 A.Citation9 Epstein-Barr nuclear antigen-1 is a transcription factor that regulates both viral and cellular gene expression and recruits regulatory T-cells. LMP 1 acts as a mimic of CD40 and may constitutively activate the NF-κB and JAK/STAT pathways. LMP 2A involves B-cell receptor (BCR) signaling, leading to B-cell activation in the absence of normal BCR signaling.Citation10
From a therapeutic perspective, polychemotherapy combined with radiotherapy in early stage cHL is a common first-line therapy for cHL, with 85% curability.Citation11 The 15% of patients who do not achieve complete response after first-line therapy represent a hard-to-treat population.Citation12,Citation13
In patients with cHL relapsing or refractory (R/R cHL) to first-line therapy, second-line polychemotherapy followed by consolidation with autologous stem cell transplantation (ASCT) is curative in approximately 50% of cases.Citation14,Citation15 Of note, ASCT may not be an option for some elderly patients with comorbidities or in cases of chemorefractory disease at the time of salvage.Citation16
As a new, modern, and vectorized chemotherapy, brentuximab vedotin is a targeted antibody-drug conjugate that is active against CD30-positive cancer cells. The drug was approved by the Food and Drug Administration (FDA) in 2011 for R/R cHL patients who were post-ASCT, or for those patients not eligible for ASCT after at least two lines of therapies.Citation17,Citation18
More recently, monoclonal antibodies targeting the programmed death receptor 1 (PD-1) have dramatically improved the prognosis of patients with R/R cHL by attaining up to 68% antitumoral response.Citation19 Two anti-PD1 therapies (nivolumab and pembrolizumab) were approved in the last 5 years by the FDACitation20 for R/R cHL patients, showing impressive efficacy, even in heavily pre-treated patients.Citation21,Citation22 The median progression free survival with anti-PD1 was approximately 12 months, and most patients achieved a partial response,Citation19,Citation23–26 indicating that anti-PD1 does not cure most patients. To overcome resistance to anti-PD1 and improve response quality, combinations with other immunotherapies are in various stages of development;Citation27,Citation28 however, to date, no anti-PD1 combination has demonstrated significant efficacy in overcoming anti-PD1 resistance in Hodgkin lymphoma.
In fact, new treatment methods that show accurate tumor targeting, powerful tumor-killing properties, and low toxic side effects need to be proposed.Citation29 Virotherapy could be a good candidate, especially in the treatment of Hodgkin lymphomas, in which tumor cells are often infected with the EBV.
Oncolytic viruses (OVs) are natural viruses or genetically modified viruses that exhibit antitumor and antiviral activity with tumor tropism that target and kill cancer cells without harming normal cells.Citation30 Oncolytic viruses have the ability to induce immunogenic cell death as part of its replication cycle, and they also have powerful immunomodulatory effects that induce both innate and adaptive tumor responses.Citation31 In this review, we discuss approaches with OVs to treat patients with R/R cHL, and their ability to eventually enhance the antitumor effect of other therapies such as anti-PD1 immunotherapies.
General properties of oncolytic viruses
Oncolytic viruses consist of an envelope containing the viral capsid, which in turn surrounds the viral genome, a single- or double-stranded RNA or DNA.Citation32 The selection of oncolytic viruses for therapeutic drug development is based on numerous virus criteria, such as the viral genome, size, pathogenicity, immunogenicity, transgene capacity, blood-brain penetration, mechanism of cell entry, and stability of the virus ().
Table 1. Overview of oncolytic viruses characteristics in ongoing drug development in oncology.Citation31
Clinical experience with DNA viruses such as herpes simplex virus (HSV) and adenovirus (Ad) is more extensive, and they are generally easier to engineer genetically than RNA viruses because they can encode large transgenes for increasing therapeutic activity or immune modulation. They induce a strong immune response with strong anti-tumor immunity, but they can also be cleared more rapidly by the immune system.Citation32 Therefore, DNA viruses may be more suitable for intratumoral administration. In contrast, RNA viruses such as the measles virus (MV; paramyxovirus) and vesicular stomatitis virus (VSV; rhabdovirus) are not as conducive to genetic manipulation because of their small size, but can penetrate tumors with efficacy and are more likely to cross the blood-brain barrier. The reduced likelihood of preexisting immunity to RNA viruses could make intravenous administration more feasible.Citation33
There are two types of OV. On one hand, there are naturally occurring viruses that are nonpathogenic to humans, such as the myxoma virus (MYXV; poxvirus) and Newcastle disease virus (paramyxovirus). On the other hand, there are viruses that are genetically manipulated for use as vaccine vectors, including MV, which has an excellent safety record after decades of use in humans,Citation34 poliovirus (picornavirus), and vaccinia virus (VV; poxvirus), and those viruses genetically engineered with mutations or deletions in genes such as Ad. Although Ad encodes protein E1A, to promote safety and prevent replication in normal cells, the E1A gene was deleted.Citation35 The HSV has strong lytic properties, and many variants have been engineered via deletion of ICP34.5 neurovirulence and ICP6. VSV is an engineered variant that overexpresses interferon β to protect normal cells from infection and causes tumor cell–specific destruction and antitumor immune responses.Citation36
Insertion of foreign sequences can provide selectivity for cancer cells and safety, as well as alter virus tropism by targeting translation, transcription, or transduction.
Oncolytic viral activity toward cHL
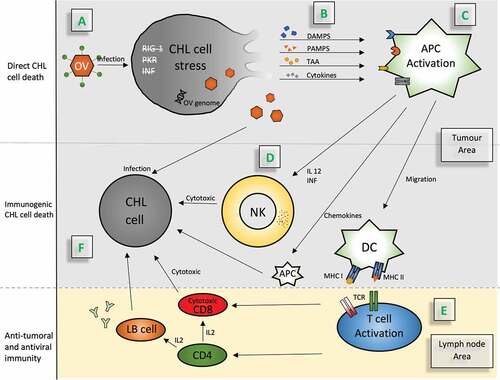
Oncolytic viruses can target and kill cHL cells via the natural interactions between viruses, tumor cells, and the immune systemCitation37 (). They can be engineered to selectively target Hodgkin Reed-Sternberg cells without harming normal cells, as described in a study by Hanauer et al. studyCitation38 where MV-CD30 and VSV-CD30 were generated by replacing envelope genes in the genome of MV and VSV with those encoding CD30-targeting glycoproteins.
Figure 1. Mechanisms of action of oncolytic viruses as indicators of anti-tumoral activity in Hodgkin lymphoma
Importantly, in the context of EBV-positive Hodgkin lymphoma,Citation39,Citation40 an adenoviral vector expressing a transgene (either LMP2 alone or both LMP2 and LMP1) can act as a vaccine to treat R/R cHL, as shown by Bollard et al.Citation41
Oncolytic adenovirus can also accommodate genetic insertion of therapeutic and immunomodulatory transgenes, such as the immune target anti-PD1, leading to enhanced efficacy in R/R cHL.Citation42–44
Some researchers suggest that intratumoral or intravenous OV administration can selectively access tumor cells by fusion with the plasma membrane, endocytosis, or by binding to tumor cell surface receptors. The oncolytic virus then replicates in the cHL cells and induces direct cell deathCitation32,Citation45 through an abnormal response to stress and dysfunctional virus detection and clearance by protein kinase R, retinoic acid inducible gene 1, and interferon type 1 responses. The tumor tropism of the OV is used as a key property to maximize direct killing effects on the tumor, while minimizing damage to surrounding normal tissues.Citation46
The direct killing of cHL cells by OVs result in the release of danger signals such as damage-associated molecular patterns, pathogen-associated molecular patterns, and tumor-associated antigens, thus inducing innate immunity.Citation47 These danger signals are picked up by antigen-presenting cells (APCs), such as macrophages and dendritic cells, which express antigens coupled with the major histocompatibility complex classes II and I. In addition, APCs promote the release of cytokines (such as tumor necrosis factor-α, interferon, interleukin [IL] 12, and IL-6), resulting in the recruitment and activation of innate immune cells.Citation46 The innate cells recruited are natural killer cells, natural killer T-cells, and γδ T-cells.Citation32,Citation48 Natural killer cells have the potential to induce cHL death through a cytotoxic immune response boosted by cytokine secretion. This cell death mechanism is also known as immunogenic cell death.Citation49
Mature dendritic cells, in the presence of IL-12, migrate to regional lymph nodes where they present T-cells with danger signals that are coupled to major histocompatibility complex classes I and II. This mechanism could lead to tumor regression at distant tumor sites not exposed to the OV via increased T-cell recruitment and immune cell activation.Citation50 Local administration of OV is globally well tolerated. The most common adverse effects of OV are local reactions at the injection site and flu-like symptoms, which may be more severe after systemic administration and which do not allow the inclusion of immunocompromised patients or those with active viral infections.Citation51
Many viruses are also pathogens and cause human disease, which could limit their clinical use, although deletion of virulence genes may render these viruses safe for human administration. In addition, some viruses remain sensitive to oral antiviral drugs.Citation52, Citation53
Oncolytic viruses as an innovative approach to target tumor cells
Oncolytic viruses and solid tumors
In 2015, the FDA approved the first OV for melanoma, talimogene laherparepvec,Citation53,Citation54 an oncolytic herpes virus (HSV1) engineered to produce granulocyte macrophage colony-stimulating factor (GM-CSF). Talimogene laherparepvec demonstrated a superior and durable response rate and overall survival in patients.Citation54 Since then, many trials have been conducted to assess the safety and efficacy of multiple OVs in patients with various types of cancers.Citation55
Oncolytic viruses and the general approach to treat Hodgkin lymphoma
The oncolytic potential of viruses in Hodgkin lymphoma was first recognized in the 1970s, with a case series showing regression of Hodgkin’s disease in patients with concurrent measles infections.Citation56,Citation57 In the context of R/R cHL, some recent clinical studies have engineered OVs to target LMPI, LMP2A, and dominant negative receptor II TGF β (DNRII), and demonstrated cancer remission without significant toxicity.Citation41,Citation58 Recent studies have demonstrated the safety and activity of in vivo expansion of OVs to treat patients with R/R cHL or to eventually prevent relapse ().
Table 2. Summary of safety and efficacy results for oncolytic viral approaches to treat relapsed or refractory Hodgkin lymphoma in clinical trials
Oncolytic viruses in Hodgkin lymphoma, targeting LMP 1 and LMP 2
Bollard et al. engineered genetically modified APCs transduced with an adenoviral vector expressing either LMP2 alone or associated with LMP1, for the purpose of expanding autologous cytotoxic T-lymphocytes (CTLs) to target EBV-associated lymphomas, including R/R cHL. Twenty-eight of 29 (96%) high-risk or multiple-relapse patients receiving LMP-CTLs as adjuvant therapy remained in remission at a median of 3.1 years after CTL infusion. Sustained remission without toxicity related to virotherapy were observed.Citation41 In comparison, in a study evaluating the efficacy of anti-PD1 re-treatment in R/R cHL after anti-PD1 discontinuation in seven patients, the disease was in complete remission in six patients and partial remission in one patient. The median time to relapse after anti-PD1 was 12.1 months (range: 5.3–26.7 months).Citation59
Oncolytic viruses in Hodgkin lymphoma, targeting TGF β
Another study was conducted in patients with R/R cHL by Bollard et al. and consisted of CTLs that expressed DNRII transduced by an Ad expressing LMP1 and LMP2 transgenes. In this study, DNRII-T cells expanded and persisted for up to 4 years after infusion in patients with EBV-positive R/R cHL. Four out of seven patients with active disease were clinically responsive, and of these four, two patients had ongoing clinical response at 4 years.Citation58
Oncolytic viruses in Hodgkin lymphoma, targeting LAG-3
Lymphocyte activation gene-3 (LAG-3) is a marker of regulatory T-cells and is strongly expressed on infiltrating lymphocytes present in proximity to Hodgkin Reed-Sternberg cells.Citation60 EBV-positive cHL contains regulatory T-cells in its microenvironment, which generally highly expresses LAG-3. Lymphocyte activation gene-3 expression on tumor-infiltrating lymphocytes increases impairment of T-cell activity against LMP1 and LMP2.Citation60 These data suggest the potential capacity of engineered OVs like Ad to block LAG-3, a critical immune checkpoint in EBV-positive Hodgkin tumors.
Oncolytic viruses in Hodgkin lymphoma, targeting CD30
Hanauer et al. generated CD30-targeted MV (MV-CD30) and VSV (VSV-CD30) in order to selectively target CD30-positive cHL-cells in a xenograft mouse model.Citation38 CD30 targeting relies on engineered MV envelope glycoproteins created by replacing envelope genes in the genomes of MV and VSV with single-chain variable fragments encoding the specific stability-engineered anti-CD30. Each virus exhibited limited infection and significant spreading within the CD30-positive cHL cells. On comparing their oncolytic activities, VSV-CD30 turned out to be more efficient than MV-CD30 and resulted in a more rapid and efficient killing of cultivated cHL-derived cell lines. In the clinical study, the OVs induced no side effects (including neurotoxic effects), regardless of intratumoral or systemic administration, and it was shown that the CD30-targeted viruses selectively infected CD30-positive cells. Furthermore, CD30-targeted viruses can be transported via CD30-positive extracellular vesicles in the vicinity of cHL tumor cells,Citation61 leading to infection and killing of bystander cells in the tumor microenvironment.Citation38
Rationale for oncolytic viruses in combination with traditional therapies for Hodgkin lymphoma
Oncolytic viruses in combination with chemotherapy and radiotherapy
Some studies suggest that the combination of chemotherapy and radiotherapy with OV treatment could have synergic antitumor activity.Citation62 Chemotherapy induces tumor apoptosis through endoplasmic reticulum stress, and combination with ONYX-015, an Ad with a deleted E1B gene, could enhance direct cell death.Citation63 In addition, some OVs like HSV1 sequester DNA damage-response proteins and enhance radiation sensitizers.Citation64 In addition, chemotherapy and radiotherapy could increase OV antitumor activity by releasing danger signals and cytokines.Citation31,Citation65
Oncolytic viruses combined with autologous stem cell transplantation
Some researchers suggest that oncolytic viruses, such as adenoviral vectors expressing the thymidine kinase gene and HSV, have the potential to prevent relapse after autologous stem cell transplantation by treating residual tumor cells before the transplant procedure.Citation66 Despite improvements in outcomes after ASCT, nearly 50% of patients relapse after transplant. Some studies have proposed that graft contamination by tumor cells can be the origin of relapses.Citation67 CHL cells also release extracellular vesicles containing CD30 cleaved by metalloproteinases and detected in the plasma of cHL patients.Citation61(p30) Uccini et al. suggested that EBV-infected cHL cells may release LMP1 through exosomes and contribute to the generation of the immunosuppressive microenvironment of cHL by producing immunomodulatory cytokines such as IL10.Citation68 Ex vivo manipulations of the ASCT to purge cancer cells using chemotherapies and toxins have been attempted, but these strategies often impair normal hematopoietic stem cell survival in the graft.Citation67
Oncolytic viruses such as MYXV may be used as purging agents that selectively target cancer cells and exosomes while sparing normal stem cells within the collection product; this approach can be applied without any additional toxicity for the graft recipient.Citation69,Citation70 Preclinical in vitro and in vivo studies have proposed the use of different OVs, such as MV, VV, parvovirus, VSV, and coxsackie virus, to purge cancer cells.Citation71–73 These data suggest that OVs targeting CD30 and/or LMP1-purging strategies could be explored in further studies in the context of R/R cHL patients undergoing ASCT.
Oncolytic viruses combined with immune checkpoint inhibitors (ICIs)
Oncolytic viruses can eventually enhance the antitumor effect of ICIs in R/R cHL, as demonstrated in multiple ongoing clinical trials to treat patients with solid tumors, and have shown encouraging preliminary results.Citation74,Citation75 Oncolytic HSV1 may improve clinical benefits of ICIs without overlapping toxicity profiles.Citation76 It can modulate tumor-specific CD8-positive T-cell responses and release TAA, danger signals, and pro-inflammatory cytokines. When administered intratumorally, this heightened inflammatory response can induce a systemic or abscopal effect and render distant tumors increasingly susceptible to ICI therapy through increased T-cell recruitment and immune cell activation.Citation50,Citation77,Citation78
A phase II trial combining intratumoral oncolytic HSV1 engineered to produce GM-CSF and intravenous ipilimumab (talimogene laherparepvec plus anti-CTLA-4 antibody) in patients with advanced melanoma demonstrated abscopal responses at tumor sites distant to the injected lesions, and decreases in visceral lesions were observed in 52% of patients in the combination arm versus 23% of patients in the anti-CTLA-4 antibody alone arm.Citation79,Citation80
Oncolytic adenoviruses also increase the expression of PD1 and other checkpoint molecules in immune cells. Approximately 30% of infused DNRII-T cells express PD1.Citation58 Some studies, such as the ongoing Masterkey-265 phase Ib/III trial, have proposed assessment of intralesional talimogene laherparepvec in combination with intravenous pembrolizumab.Citation80
Moreover, OVs can be engineered to express ICI genes to increase their antitumor activity without additional toxicity. Dias et al. reported an oncolytic Ad that included a transgene expressing a specific CTLA-4 antibody. In peripheral blood mononuclear cells of patients with advanced tumors, OVs can demonstrate prolonged and localized delivery of CTLA-4 antibodies at a high level.Citation81
Oncolytic viruses in the context of allogenic stem cell transplant: A rationale for the prevention and treatment of graft-versus-host disease
In patients with cHL who relapse after anti-PD1 treatment, allogenic stem cell transplant (Allo SCT) can be a therapeutic option for those who achieve a complete response with salvage therapies and have a donor available. However, acute graft-versus-host disease (GvHD) and transplant-related mortality remain important issues with Allo SCT.Citation82 The onset of GvHD is mediated by allogenic donor-derived T lymphocytes, which upon transplantation, are activated by mismatched major and/or minor histocompatibility complex tissue-specific antigens of the recipient. As a result, the pro-inflammatory cytokines released by these activated T-cells ultimately target and damage healthy organs and tissues such as the digestive tract, liver, and skin.Citation83 A pre-clinical study reported that MYXV can prevent GvHD in xenografted mice without impairing graft-versus-tumor (GvT) effects against residual cancer cells.Citation84 Several preclinical data have suggested that ex vivo virotherapy of human HSCT samples with MYXV can delay the onset of GvHD by interfering with donor T-cells. Furthermore, histological analyses of the recipient organs revealed very low infiltration by donor lymphocytes.Citation85
In a subsequent study,Citation86 MYXV confirmed its potential to inhibit the development of GvHD after xeno-SCT. The virus efficiently binds to resting human CD3-positive T lymphocytes, becoming “carrier cells,” following T-cell activation. The virus replication cycle simultaneously inhibits T-cell proliferation and decreases the expression of GvHD-promoting cytokines. Furthermore, MYXV-infected activated T-cells were able to deliver viruses to cancer cell lines, resulting in their infection and further killing of tumor cells, as well as an enhanced GvT effect. Therefore, in this study, MYXV demonstrated an interesting dual role in not only inhibiting GvHD, but also enhancing GvT effects against residual cancer cells.
Conclusion
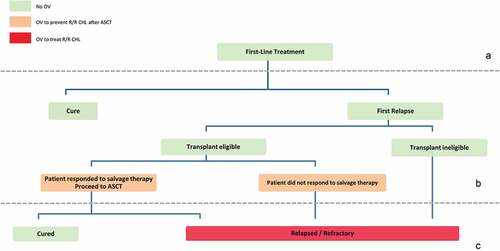
Among the various virotherapeutic perspectives for treating patients with R/R Hodgkin lymphoma, the main therapeutic approaches are targeted toward the viral proteins LMP1 and LMP2. Virotherapeutic approaches can also target the tumor itself, such as via CD30, and the surrounding suppressive tumor microenvironment via TGF-β and LAG3. Virotherapy could thus have multiple applications in therapeutic sequences by providing consolidation treatment after ASCT relapse and boosting the efficacy of anti-PD1 (). Further studies are warranted to assess the potential role of virotherapy in the treatment of Hodgkin’s lymphoma.
Figure 2. Choosing virotherapy for the treatment of relapsed and refractory classical Hodgkin lymphoma
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Molin D, Edström A, Glimelius I, Glimelius B, Nilsson G, Sundström C, Enblad G. Mast cell infiltration correlates with poor prognosis in Hodgkin’s lymphoma. Br J Haematol. 2002;119(1):122–24. doi:10.1046/j.1365-2141.2002.03768.x.
- Enblad G, Sundstrom C, Glimelius B. Infiltration of eosinophils in Hodgkin’s disease involved lymph nodes predicts prognosis. Hematol Oncol. 1993;11(4):187–93. doi:10.1002/hon.2900110404.
- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th rev ed. IARC; 2017 Sep 18.
- Anagnostopoulos I, Hansmann M-L, Franssila K, Harris M, Harris NL, Jaffe ES, Han J, Van Krieken JM, Poppema S, Marafioti T, et al. European Task Force on Lymphoma project on lymphocyte predominance Hodgkin disease: histologic and immunohistologic analysis of submitted cases reveals 2 types of Hodgkin disease with a nodular growth pattern and abundant lymphocytes. Blood. 2000;96(5):1889–99. doi:10.1182/blood.V96.5.1889.
- Lanzkowsky’s manual of pediatric hematology and oncology. 6th ed. [ accessed 2021 Mar 13]. https://www.elsevier.com/books/lanzkowskys-manual-of-pediatric-hematology-and-oncology/fish/978-0-12-801368-7.
- Pileri SA, Ascani S, Leoncini L, Sabattini E, Zinzani PL, Piccaluga PP, Pileri A, Giunti M, Falini B, Bolis GB, et al. Hodgkin’s lymphoma: the pathologist’s viewpoint. J Clin Pathol. 2002;55(3):162–76. doi:10.1136/jcp.55.3.162.
- Carbone A, Gloghini A, Carlo-Stella C. Are EBV-related and EBV-unrelated Hodgkin lymphomas different with regard to susceptibility to checkpoint blockade? Blood. 2018;132(1):17–22. doi:10.1182/blood-2018-02-833806.
- Massini G, Siemer D, Hohaus S. EBV in Hodgkin Lymphoma. Mediterr J Hematol Infect Dis. 2009;1(2). doi:10.4084/MJHID.2009.013.
- Murray PG, Young LS, Rowe M, Crocker J. Immunohistochemical demonstration of the Epstein-Barr virus-encoded latent membrane protein in paraffin sections of Hodgkin’s disease. J Pathol. 1992;166(1):1–5. doi:10.1002/path.1711660102.
- Murray PG, Young LS. An etiological role for the Epstein-Barr virus in the pathogenesis of classical Hodgkin lymphoma. Blood. 2019;134(7):591–96. doi:10.1182/blood.2019000568.
- Engert A, Schiller P, Josting A, Herrmann R, Koch P, Sieber M, Boissevain F, De Wit M, Mezger J, Duhmke E, et al. Involved-field radiotherapy is equally effective and less toxic compared with extended-field radiotherapy after four cycles of chemotherapy in patients with early-stage unfavorable Hodgkin’s lymphoma: results of the HD8 trial of the German Hodgkin’s Lymphoma Study Group. J Clin Oncol Off J Am Soc Clin Oncol. 2003;21(19):3601–08. doi:10.1200/JCO.2003.03.023.
- Engert A, Plütschow A, Eich HT, Lohri A, Dörken B, Borchmann P, Berger B, Greil R, Willborn KC, Wilhelm M, et al. Reduced treatment intensity in patients with early-stage Hodgkin’s lymphoma. N Engl J Med. 2010;363(7):640–52. doi:10.1056/NEJMoa1000067.
- Skoetz N, Will A, Monsef I, Brillant C, Engert A, Von Tresckow B. Comparison of first-line chemotherapy including escalated BEACOPP versus chemotherapy including ABVD for people with early unfavourable or advanced stage Hodgkin lymphoma. Cochrane Database Syst Rev. 2017;5:CD007941. doi:10.1002/14651858.CD007941.pub3.
- André M, Henry-Amar M, Pico JL, Brice P, Blaise D, Kuentz M, Coiffier B, Colombat P, Cahn JY, Attal M, et al. Comparison of high-dose therapy and autologous stem-cell transplantation with conventional therapy for Hodgkin’s disease induction failure: a case-control study. Société Francaise de Greffe de Moelle. J Clin Oncol Off J Am Soc Clin Oncol. 1999;17(1):222–29. doi:10.1200/JCO.1999.17.1.222.
- Lazarus HM, Rowlings PA, Zhang MJ, Vose JM, Armitage JO, Bierman PJ, Gajewski JL, Gale RP, Keating A, Klein JP, et al. Autotransplants for Hodgkin’s disease in patients never achieving remission: a report from the autologous blood and marrow transplant registry. J Clin Oncol Off J Am Soc Clin Oncol. 1999;17(2):534–45. doi:10.1200/JCO.1999.17.2.534.
- Akpek G, Ambinder RF, Piantadosi S, Abrams RA, Brodsky RA, Vogelsang GB, Zahurak ML, Fuller D, Miller CB, Noga SJ, et al. Long-term results of blood and marrow transplantation for Hodgkin’s lymphoma. J Clin Oncol Off J Am Soc Clin Oncol. 2001;19(23):4314–21. doi:10.1200/JCO.2001.19.23.4314.
- NICE. Brentuximab vedotin for treating CD30-positive Hodgkin lymphoma | guidance | NICE. [ accessed 2019 May 9]. https://www.nice.org.uk/guidance/ta446.
- NICE. Brentuximab vedotin for treating cd30positive hodgkin lymphoma. Published online 2018.
- Amraee A, Evazi MR, Shakeri M, Roozbeh N, Ghazanfarpour M, Ghorbani M, Ansari J, Darvish L. Efficacy of nivolumab as checkpoint inhibitor drug on survival rate of patients with relapsed/refractory classical Hodgkin lymphoma: a meta-analysis of prospective clinical study. Clin Transl Oncol Off Publ Fed Span Oncol Soc Natl Cancer Inst Mex. Published online 2019 Feb 9. doi:10.1007/s12094-018-02032-4.
- Kasamon YL, De Claro RA, Wang Y, Shen YL, Farrell AT, Pazdur R. FDA approval summary: nivolumab for the treatment of relapsed or progressive classical Hodgkin lymphoma. Oncologist. 2017;22(5):585–91. doi:10.1634/theoncologist.2017-0004.
- NICE. Pembrolizumab for treating relapsed or refractory classical Hodgkin’s lymphoma [ID1062]. Published online 2017.
- Younes A, Gopal AK, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, Ramchandren R, Bartlett NL, Cheson BD, De Vos S, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J Clin Oncol Off J Am Soc Clin Oncol. 2012;30(18):2183–89. doi:10.1200/JCO.2011.38.0410.
- Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry D, Freeman GJ, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372(4):311–19. doi:10.1056/NEJMoa1411087.
- Maruyama D, Hatake K, Kinoshita T, Fukuhara N, Choi I, Taniwaki M, Ando K, Terui Y, Higuchi Y, Onishi Y, et al. Multicenter phase II study of nivolumab in Japanese patients with relapsed or refractory classical Hodgkin lymphoma. Cancer Sci. 2017;108(5):1007–12. doi:10.1111/cas.13230.
- Herbaux C, Gauthier J, Brice P, Drumez E, Ysebaert L, Doyen H, Fornecker L, Bouabdallah K, Manson G, Ghesquières H, et al. Efficacy and tolerability of nivolumab after allogeneic transplantation for relapsed Hodgkin lymphoma. Blood. 2017;129(18):2471–78. doi:10.1182/blood-2016-11-749556.
- Armand P, Engert A, Younes A, Fanale M, Santoro A, Zinzani PL, Timmerman JM, Collins GP, Ramchandren R, Cohen JB, et al. Nivolumab for relapsed/refractory classic Hodgkin lymphoma after failure of autologous hematopoietic cell transplantation: extended follow-up of the multicohort single-arm phase II CheckMate 205 trial. J Clin Oncol Off J Am Soc Clin Oncol. 2018;36(14):1428–39. doi:10.1200/JCO.2017.76.0793.
- Jenkins RW, Barbie DA, Flaherty KT. Mechanisms of resistance to immune checkpoint inhibitors. Br J Cancer. 2018;118(1):9–16. doi:10.1038/bjc.2017.434.
- Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168(4):707–23. doi:10.1016/j.cell.2017.01.017.
- Cao G, He X, Sun Q, Chen S, Wan K, Xu X, Feng X, Li P, Chen B, Xiong M. The oncolytic virus in cancer diagnosis and treatment. Front Oncol. 2020:10. doi:10.3389/fonc.2020.01786.
- Chan WM, Rahman MM, McFadden G. Oncolytic myxoma virus: the path to clinic. Vaccine. 2013;31(39):4252–58. doi:10.1016/j.vaccine.2013.05.056.
- Bommareddy PK, Shettigar M, Kaufman HL. Integrating oncolytic viruses in combination cancer immunotherapy. Nat Rev Immunol. 2018;18(8):498–513. doi:10.1038/s41577-018-0014-6.
- Kaufman HL, Kohlhapp FJ, Zloza A. Oncolytic viruses: a new class of immunotherapy drugs. Nat Rev Drug Discov. 2015;14(9):642–62. doi:10.1038/nrd4663.
- Haseley A, Alvarez-Breckenridge C, Chaudhury AR, Kaur B. Advances in oncolytic virus therapy for glioma. Recent Patents CNS Drug Discov. 2009;4(1):1–13. doi:10.2174/157488909787002573.
- Russell SJ, Peng KW. Measles virus for cancer therapy. Curr Top Microbiol Immunol. 2009;330:213–41. doi:10.1007/978-3-540-70617-5_11.
- Yu W, Fang H. Clinical trials with oncolytic adenovirus in China. Curr Cancer Drug Targets. 2007;7(2):141–48. doi:10.2174/156800907780058817.
- Russell SJ, Peng K-W, Bell JC. Oncolytic virotherapy. Nat Biotechnol. 2012;30(7):658–70. doi:10.1038/nbt.2287.
- Rühl J, Citterio C, Engelmann C, Haigh T, Dzionek A, Dreyer J, Khanna R, Taylor GS, Wilson JB, Leung CS, et al. Heterologous prime-boost vaccination protects against EBV antigen–expressing lymphomas. J Clin Invest. 2019;129(5):2071–87. doi:10.1172/JCI125364.
- Hanauer JDS, Rengstl B, Kleinlützum D, Reul J, Pfeiffer A, Friedel T, Schneider IC, Newrzela S, Hansmann M-L, Buchholz CJ, et al. CD30-targeted oncolytic viruses as novel therapeutic approach against classical Hodgkin lymphoma. Oncotarget. 2018;9(16):12971–81. doi:10.18632/oncotarget.24191.
- Kapatai G, Murray P. Contribution of the Epstein Barr virus to the molecular pathogenesis of Hodgkin lymphoma. J Clin Pathol. 2006;60(12):1342–49. doi:10.1136/jcp.2007.050146.
- Verheije MH, Rottier PJM. Retargeting of viruses to generate oncolytic agents. Adv Virol. 2012:2012. doi:10.1155/2012/798526.
- Bollard CM, Gottschalk S, Torrano V, Diouf O, Ku S, Hazrat Y, Carrum G, Ramos C, Fayad L, Shpall EJ, et al. Sustained complete responses in patients with lymphoma receiving autologous cytotoxic T lymphocytes targeting epstein-barr virus latent membrane proteins. J Clin Oncol. 2014;32(8):798–808. doi:10.1200/JCO.2013.51.5304.
- Hermiston T. Gene delivery from replication-selective viruses: arming guided missiles in the war against cancer. J Clin Invest. 2000;105(9):1169–72. doi:10.1172/JCI9973.
- Bauzon M, Hermiston T. Armed therapeutic viruses - a disruptive therapy on the horizon of cancer immunotherapy. Front Immunol. 2014;5:74. doi:10.3389/fimmu.2014.00074.
- Zhang Q, Liu F. Advances and potential pitfalls of oncolytic viruses expressing immunomodulatory transgene therapy for malignant gliomas. Cell Death Dis. 2020;11(6):1–11. doi:10.1038/s41419-020-2696-5.
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. doi:10.1016/j.cell.2011.02.013.
- Lichty BD, Breitbach CJ, Stojdl DF, Bell JC. Going viral with cancer immunotherapy. Nat Rev Cancer. 2014;14(8):559–67. doi:10.1038/nrc3770.
- Davola ME, Mossman KL. Oncolytic viruses: how “lytic” must they be for therapeutic efficacy? OncoImmunology. 2019;8(6):e1581528. doi:10.1080/2162402X.2019.1596006.
- Yin J, Markert JM, Leavenworth JW. Modulation of the intratumoral immune landscape by oncolytic herpes simplex virus virotherapy. Front Oncol. 2017:7. doi:10.3389/fonc.2017.00136.
- Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9(5):503–10. doi:10.1038/ni1582.
- Zamarin D, Holmgaard RB, Subudhi SK, Park JS, Mansour M, Palese P, Merghoub T, Wolchok JD, Allison JP. Localized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy. Sci Transl Med. 2014;6(226):226ra32. doi:10.1126/scitranslmed.3008095.
- Cripe TP, Ngo MC, Geller JI, Louis CU, Currier MA, Racadio JM, Towbin AJ, Rooney CM, Pelusio A, Moon A, et al. Phase 1 study of intratumoral Pexa-Vec (JX-594), an oncolytic and immunotherapeutic vaccinia virus, in pediatric cancer patients. Mol Ther J Am Soc Gene Ther. 2015;23(3):602–08. doi:10.1038/mt.2014.243.
- Lawler SE, Speranza M-C, Cho C-F, Chiocca EA. Oncolytic viruses in cancer treatment: a review. JAMA Oncol. 2017;3(6):841–49. doi:10.1001/jamaoncol.2016.2064.
- Pol J, Kroemer G, Galluzzi L. First oncolytic virus approved for melanoma immunotherapy. Oncoimmunology. 2015;5(1). doi:10.1080/2162402X.2015.1115641.
- Andtbacka RHI, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, Delman KA, Spitler LE, Puzanov I, Agarwala SS, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol Off J Am Soc Clin Oncol. 2015;33(25):2780–88. doi:10.1200/JCO.2014.58.3377.
- Breitbach CJ, Lichty BD, Bell JC. Oncolytic viruses: therapeutics with an identity crisis. EBioMedicine. 2016;9:31–36. doi:10.1016/j.ebiom.2016.06.046.
- Zygiert Z. Hodgkin’s disease: remissions after measles. The Lancet. 1971;297(7699):593. doi:10.1016/S0140-6736(71)91186-X.
- Taqi A. Regression of Hodgkin’s disease after measles. The Lancet. 1981;317(8229):1112. doi:10.1016/S0140-6736(81)92286-8.
- Bollard CM, Tripic T, Cruz CR, Dotti G, Gottschalk S, Torrano V, Dakhova O, Carrum G, Ramos CA, Liu H, et al. Tumor-specific T-cells engineered to overcome tumor immune evasion induce clinical responses in patients with relapsed Hodgkin lymphoma. J Clin Oncol. 2018;36(11):1128–39. doi:10.1200/JCO.2017.74.3179.
- Manson G, Brice P, Herbaux C, Bouabdallah K, Antier C, Poizeau F, Dercle L, Houot R. Efficacy of anti-PD1 re-treatment in patients with Hodgkin lymphoma who relapsed after anti-PD1 discontinuation. Haematologica. 2020;105(11):2664–66. doi:10.3324/haematol.2019.242529.
- Gandhi MK, Lambley E, Duraiswamy J, Dua U, Smith C, Elliott S, Gill D, Marlton P, Seymour J, Khanna R. Expression of LAG-3 by tumor-infiltrating lymphocytes is coincident with the suppression of latent membrane antigen–specific CD8+ T-cell function in Hodgkin lymphoma patients. Blood. 2006;108(7):2280–89. doi:10.1182/blood-2006-04-015164.
- Hansen HP, Trad A, Dams M, Zigrino P, Moss M, Tator M, Schön G, Grenzi PC, Bachurski D, Aquino B, et al. CD30 on extracellular vesicles from malignant Hodgkin cells supports damaging of CD30 ligand-expressing bystander cells with Brentuximab-Vedotin, in vitro. Oncotarget. 2016;7(21):30523–35. doi:10.18632/oncotarget.8864.
- Ottolino-Perry K, Diallo J-S, Lichty BD, Bell JC, McCart JA. Intelligent design: combination therapy with oncolytic viruses. Mol Ther J Am Soc Gene Ther. 2010;18(2):251–63. doi:10.1038/mt.2009.283.
- Khuri FR, Nemunaitis J, Ganly I, Arseneau J, Tannock IF, Romel L, Gore M, Ironside J, MacDougall RH, Heise C, et al. A controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat Med. 2000;6(8):879–85. doi:10.1038/78638.
- Harrington KJ, Hingorani M, Tanay MA, Hickey J, Bhide SA, Clarke PM, Renouf LC, Thway K, Sibtain A, McNeish IA, et al. Phase I/II study of oncolytic HSV GM-CSF in combination with radiotherapy and cisplatin in untreated stage III/IV squamous cell cancer of the head and neck. Clin Cancer Res Off J Am Assoc Cancer Res. 2010;16(15):4005–15. doi:10.1158/1078-0432.CCR-10-0196.
- Kon T, Zhang X, Huang Q, Yang Z, Liu S, Yan B, Li F, Wang H, Li C-Y. Oncolytic virus-mediated tumor radiosensitization in mice through DNA-PKcs-specific shRNA. Transl Cancer Res. 2012;1(2):4–14. doi:10.3978/j..2218-676X.2012.05.02.
- Alain T, Thirukkumaran C, Morris DG, Urbanski SJ, Janowska-Wieczorek A, Lee PWK, Kossakowska AE. Lymphomas and oncolytic virus therapy. Clin Lymphoma. 2003;4(2):104–11. doi:10.3816/clm.2003.n.019.
- Billett AL, Sallan SE. Autologous bone marrow transplantation in childhood acute lymphoid leukemia with use of purging. Am J Pediatr Hematol Oncol. 1993;15(2):162–68. doi:10.1097/00043426-199305000-00003.
- Uccini S, Al-Jadiry MF, Pepe G, Pasquini A, Alsaadawi AR, Al-Hadad SA, Di Napoli A, Tripodo C, Ruco L. Follicular dendritic cells display microvesicle-associated LMP1 in reactive germinal centers of EBV+ classic Hodgkin lymphoma. Virchows Arch. 2019;475(2):175–80. doi:10.1007/s00428-019-02605-w.
- Kim M, Madlambayan GJ, Rahman MM, Smallwood SE, Meacham AM, Hosaka K, Scott EW, Cogle CR, McFadden G. Myxoma virus targets primary human leukemic stem and progenitor cells while sparing normal hematopoietic stem and progenitor cells. Leukemia. 2009;23(12):2313–17. doi:10.1038/leu.2009.219.
- Au GG, Lincz LF, Enno A, Shafren DR. Oncolytic Coxsackievirus A21 as a novel therapy for multiple myeloma. Br J Haematol. 2007;137(2):133–41. doi:10.1111/j.1365-2141.2007.06550.x.
- Tsang JJ, Atkins HL. The ex vivo purge of cancer cells using oncolytic viruses: recent advances and clinical implications. Oncolytic Virotherapy. 2015;4:13–23. doi:10.2147/OV.S45525.
- Adachi Y, Reynolds PN, Yamamoto M, Wang M, Takayama K, Matsubara S, Muramatsu T, Curiel DT. A midkine promoter-based conditionally replicative adenovirus for treatment of pediatric solid tumors and bone marrow tumor purging. Cancer Res. 2001;61:7882–88.
- Ong HT, Timm MM, Greipp PR, Witzig TE, Dispenzieri A, Russell SJ, Peng K-W. Oncolytic measles virus targets high CD46 expression on multiple myeloma cells. Exp Hematol. 2006;34(6):713–20. doi:10.1016/j.exphem.2006.03.002.
- Chen C-Y, Hutzen B, Wedekind MF, Cripe TP. Oncolytic virus and PD-1/PD-L1 blockade combination therapy. Oncolytic Virotherapy. 2018;7:65–77. doi:10.2147/OV.S145532.
- Chen C-Y, Wang P-Y, Hutzen B, Sprague L, Swain HM, Love JK, Stanek JR, Boon L, Conner J, Cripe TP. Cooperation of oncolytic herpes virotherapy and PD-1 blockade in murine rhabdomyosarcoma models. Sci Rep. 2017;7(1):2396. doi:10.1038/s41598-017-02503-8.
- Puzanov I, Milhem MM, Minor D, Hamid O, Li A, Chen L, Chastain M, Gorski KS, Anderson A, Chou J, et al. Talimogene laherparepvec in combination with ipilimumab in previously untreated, unresectable stage IIIB-IV melanoma. J Clin Oncol Off J Am Soc Clin Oncol. 2016;34(22):2619–26. doi:10.1200/JCO.2016.67.1529.
- Hardcastle J, Mills L, Malo CS, Jin F, Kurokawa C, Geekiyanage H, Schroeder M, Sarkaria J, Johnson AJ, Galanis E. Immunovirotherapy with measles virus strains in combination with anti-PD-1 antibody blockade enhances antitumor activity in glioblastoma treatment. Neuro-Oncol. 2017;19(4):493–502. doi:10.1093/neuonc/now179.
- Woller N, Gürlevik E, Fleischmann-Mundt B, Schumacher A, Knocke S, Kloos AM, Saborowski M, Geffers R, Manns MP, Wirth TC, et al. Viral infection of tumors overcomes resistance to PD-1-immunotherapy by broadening neoantigenome-directed T-cell responses. Mol Ther J Am Soc Gene Ther. 2015;23(10):1630–40. doi:10.1038/mt.2015.115.
- Chesney J, Puzanov I, Collichio F, Singh P, Milhem MM, Glaspy J, Hamid O, Ross M, Friedlander P, Garbe C, et al. Randomized, open-label phase II study evaluating the efficacy and safety of talimogene laherparepvec in combination with ipilimumab versus ipilimumab alone in patients with advanced, unresectable melanoma. J Clin Oncol Off J Am Soc Clin Oncol. 2018;36(17):1658–67. doi:10.1200/JCO.2017.73.7379.
- Puzanov I, Milhem MM, Andtbacka RHI, Minor DR, Hamid O, Li A, Chastain M, Gorski K, Anderson A, Vanderwalde AM, et al. Primary analysis of a phase 1b multicenter trial to evaluate safety and efficacy of talimogene laherparepvec (T-VEC) and ipilimumab (ipi) in previously untreated, unresected stage IIIB-IV melanoma. J Clin Oncol. 2014;32(15_suppl):9029–9029. doi:10.1200/jco.2014.32.15_suppl.9029.
- Dias JD, Hemminki O, Diaconu I, Hirvinen M, Bonetti A, Guse K, Escutenaire S, Kanerva A, Pesonen S, Löskog A, et al. Targeted cancer immunotherapy with oncolytic adenovirus coding for a fully human monoclonal antibody specific for CTLA-4. Gene Ther. 2012;19(10):988–98. doi:10.1038/gt.2011.176.
- Manson G, Mear J-B, Herbaux C, Schiano J-M, Casasnovas O, Stamatoullas A, Deau B, Schmitt A, Garnier G, Regny C, et al. Long-term efficacy of anti-PD1 therapy in Hodgkin lymphoma with and without allogenic stem cell transplantation. Eur J Cancer. 2019;115:47–56. doi:10.1016/j.ejca.2019.04.006.
- Zhang Y, Joe G, Hexner E, Zhu J, Emerson SG. Alloreactive memory T cells are responsible for the persistence of graft-versus-host disease. J Immunol Baltim Md 1950. 2005;174(5):3051–58. doi:10.4049/jimmunol.174.5.3051.
- Lilly CL, Villa NY, Lemos De Matos A, Ali HM, Dhillon J-KS, Hofland T, Rahman MM, Chan W, Bogen B, Cogle C, et al. Ex vivo oncolytic virotherapy with myxoma virus arms multiple allogeneic bone marrow transplant leukocytes to enhance graft versus tumor. Mol Ther Oncolytics. 2017;4:31–40. doi:10.1016/j.omto.2016.12.002.
- Villa NY, McFadden G. Virotherapy as potential adjunct therapy for graft-vs-host disease. Curr Pathobiol Rep. 2018;6(4):247–63. doi:10.1007/s40139-018-0186-6.
- Villa NY, Wasserfall CH, Meacham AM, Wise E, Chan W, Wingard JR, McFadden G, Cogle CR. Myxoma virus suppresses proliferation of activated T lymphocytes yet permits oncolytic virus transfer to cancer cells. Blood. 2015;125(24):3778–88. doi:10.1182/blood-2014-07-587329.
