ABSTRACT
Objective: The aim of the current study was to evaluate immunogenicity and safety levels of human inactivated quadrivalent influenza vaccine (QIV) which includes two A strains (A/H1N1, A/H3N2) and two B lineages (B/Victoria, B/Yamagata) in healthy adults via meta-analysis. Methods: Searches were conducted in PubMed, Cochrane Library, ClinicalTrials.gov, and EMBASE databases published in 2011–2020 according to inclusion and exclusion criteria. The purpose was to collect and perform meta-analysis of related randomized clinical trial (RCT) data concerning safety and immunogenicity levels of human QIV compared with inactivated trivalent influenza vaccine (TIV). Results: A total of 9 literatures were included. There was no significant difference in the seroconversion(SCR) and seroprotection(SPR) between QIV and TIV for influenza A strains (A/H1N1, A/H3N2) and the B lineage included in the TIV. QIV showed superior efficacy for the B lineage not included in the TIV: SCR RR of 2.20 (95%CI: 1.44–3.37, p = .0003) and SPR RR of 1.34 (95%CI: 1.10–1.63, p = .004) for B/Victoria, and SCR RR of 1.88 (95%CI: 1.53–2.31, p < .00001) and SPR RR of 1.11 (95%CI: 1.03–1.19, p = .006) for B/Yamagata, respectively. There were no significant differences between QIV and TIV for local and systemic adverse events(AE) post-vaccination. Conclusion: In adults 18–64 years old, QIV not only produced similar immunogenicity and safety levels to TIV, but also had better immunogenicity against influenza B vaccine strains not included in TIV.
KEYWORDS:
Introduction
According to the estimate of World Health Organization (WHO), up to 650,000 people die from respiratory illnesses caused by seasonal influenza every year.Citation1 Vaccination is the most important measure to prevent influenza infection. Trivalent inactivated influenza vaccine (TIV) consists of two A vaccine strains (A/H1N1 and A/H3N2) and one B vaccine strain (B).Citation2 Since 1985, the influenza B lineage (B/Yamagata) has evolved into 2 antigenically distinct lineages (B/Victoria and B/Yamagata), and there is little cross protection between them, causing the protective effect of TIV quite limited. Therefore, the inactivated quadrivalent influenza vaccine (QIV, including A/H1N1, A/H3N2, B/Victoria and B/Yamagata) was approved by WHO in 2012.Citation3 In order to systematically evaluate the immunogenicity and safety levels of QIV in adults, we systematically searched the published literatures and conducted a meta-analysis, so as to provide evidence-based basis for the formulation of QIV immunization strategies in China.
Materials and methods
Search resources and strategies
Databases, including Cochrane Library, PubMed, ClinicalTrials.gov, EMBASE, China Biology Medicine disc(CBMdisc), Chinese National Knowledge Infrastructure(CNKI), and Wanfang Data, were searched using the keywords QIV and TIV, which was limited by inclusion and exclusion criteria. The retrieval time was 2011–2020. Language was limited to English and Chinese.
Inclusion and exclusion criteria
Inclusion criteria: (1) Randomized controlled trials(RCTs); (2) Subjects that were normal people between 18 and 64 years old (ethnicity was not limited); (3) Interventions were vaccinated with QIV or TIV; (4) Control measures were blank control, self before-and-after control, or inter-group control; and (5) Outcome indicators were incidence of influenza-like symptoms, seroconversion rates (SCR), seroprotection(SPR), and so forth. Exclusion criteria: (1) Animals, pregnant and lying-in women, breast-feeding women, patients suffering from diseases affecting immune function or uncontrollable chronic diseases; (2) Subjects that had been vaccinated with QIV or TIV influenza vaccine before. This may have an impact on the immune effect; (3) Republished studies, irrelevant studies, conference abstracts, reviews, mechanism studies, and research studies irrelevant to the immune effects of influenza vaccines.
Literature screening and quality evaluation
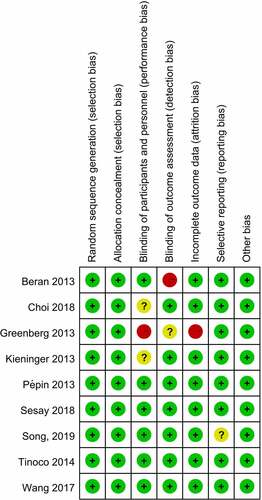
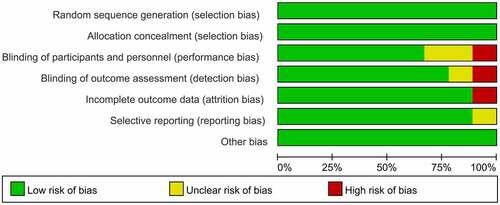
Two researchers, independently, read the abstracts and titles for screening. They then selectively read the full texts, according to inclusion and exclusion criteria. Cochrane’s Randomized Controlled Trials Bias Assessment Tool was used to evaluate study quality.Citation4 Risk of bias assessment contained 7 items, including the method of generating random sequence, process of allocation concealment, incomplete outcome data, blinding of outcome assessment, blinding of participants and personnel, selective reporting, and other bias.Citation5 The two researchers discussed divergences in literature inclusion and quality evaluation, consulting a third person if necessary.
Data extraction
The two researchers extracted all relevant data, independently entering the information into a specially designed data extraction table. Data extraction included the first author, publication time, sample size, type of vaccine strains, vaccine manufacture and study design (). The key immunogenicity variables were the number of participants of QIV and TIV at Day 0 (Pre-vaccination) and Day 21 (Post-vaccination) which met the criterion of SCR or SPR defined in next paragraph. . The key safety variables were the number of adverse events(AEs) within 7 days after vaccination of QIV or TIV. The key data were displayed in forest plots as follows.
Table 1. Characteristics of eligible trials
Analysis of immunogenicity and safety
The immunogenicity outcome indicators were SCR and SPR measured by hemagglutination inhibition test (HI) before and after vaccination (i.e., SCR is defined as the percent of the participants serum titer before vaccination ≤1:10 and serum antibody titer≥1:40, 3 weeks after vaccination, or pre-vaccination HI antibody titer <1:10 and a post-vaccination increase by a factor of four or more; SPR is defined as the percentage of participants with a post-vaccination HI antibody titer ≥1:40, 3 weeks after vaccination).Citation6,Citation7 The incidence of adverse events(AEs) within 7 days after vaccination was compared between the QIV group and TIV group. Adverse events were divided into local events and systemic events. Local events included redness, swelling and pain at the injection site. Systemic events included fatigue, headache, myalgia and fever.
Sensitivity analysis
One studyCitation8 reported the local and systemic adverse events occurring within 3 days of vaccination rather than 7 days, so the sensitivity analysis of adverse events was conducted by the method of literature exclusion. The results were acceptable when the change of pooled RR value was less than 10% after removal of one study.
Statistical analysis
The database was collected using Microsoft Excel. RevMan 5.3 software was used for meta-analysis. The current study used the Mantel-Haenszel (M-H) method (random effects model), calculating the 95% confidence interval (95%CI) and risk ratio (RR) of the SCR, SPR and adverse events(AEs) to evaluate the immunogenicity and safety levels. I2 statistics was applied to assess statistical heterogeneity. Differences between QIV and TIV were compared. Publication bias was evaluated using RevMan 5.3. Results are shown in the risk of bias summary diagram ().
Results
Basic features and quality evaluation of included studies
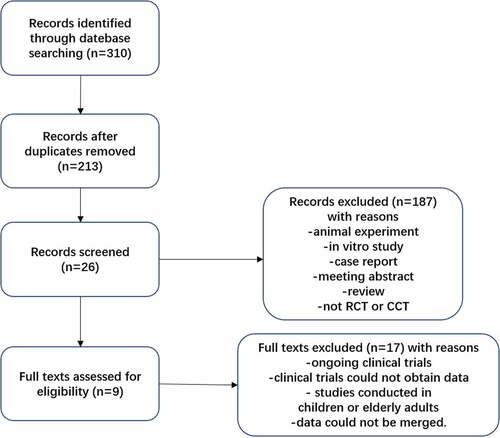
A total of 310 studies were retrieved. 9 studiesCitation8–16 were finally included, all of which were written in English. The process of literature selection is shown in . All 9 literatures were about human QIV and TIV, and serological indicators such as SCR and SPR were used to assess immune effects. Basic information of included studies is shown in . The evaluation of literature quality about randomization, blinding, performance and detection method was shown in , and there were low risks for selection bias, performance and detection bias etc.
Comparison of SCR between QIV and TIV via meta-analysis
) shows forest plots of SCR:
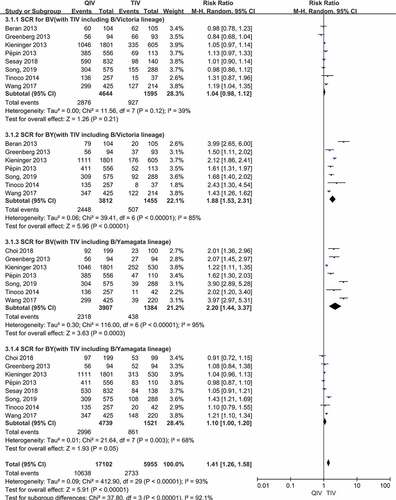
Figure 3. (a,b). Seroconversion rate (SCR) at day 21 post-vaccination, QIV versus TIV in adults between 18 and 64 years old (A strains). (SCR is defined as the percentage of participants with either a pre-vaccination HAI titer <10 and a post-vaccination HAI titer ≥40 or a pre-vaccination HI titer ≥10 and a ≥4-fold increase in HI titer after vaccination)
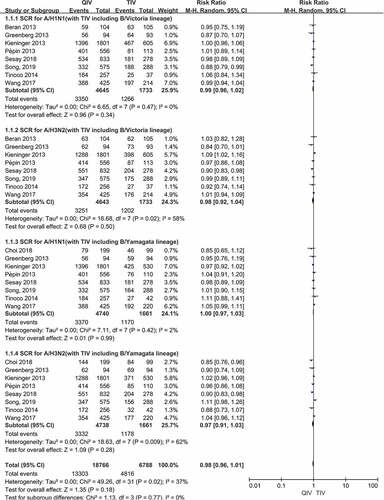
There were a total of 8 papers reported SCR data of two A vaccine strains (A/H1N1, A/H3N2) after vaccination. When comparing QIV with TIV-B/Victoria, the SCR risk ratios (RRs) were 0.99 (95%CI: 0.96–1.02, P = .34) for A/H1N1 and 0.98 (95%CI: 0.92–1.04, P = .50) for A/H3N2. Compared QIV with TIV-B/Yamagata, the SCR RRs were 1.00 (95%CI: 0.97–1.03, P = .99) for A/H1N1 and 0.97 (95%CI: 0.91–1.03, P = .28) for A/H3N2, respectively. There was no significant difference in the pooled RR values of the four A vaccine strains above, and the total SCR RRs were 0.98 (95%CI: 0.96–1.01, P = .77). The SCR forest plots of the four A vaccine strains above were shown in ).
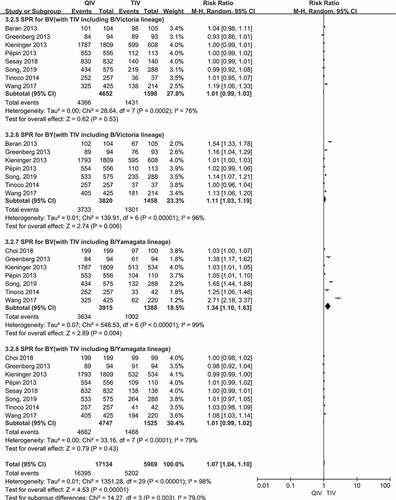
There were a total of 8 papers reported SCR data of two B lineages (B/Victoria, B/Yamagata) after vaccination. Compared QIV with TIV-B/Victoria, the SCR RRs were 1.04 (95%CI: 0.98–1.12, P = .21) for B/Victoria and 1.88 (95%CI: 1.53–2.31, P < .00001) for B/Yamagata. When comparing QIV with TIV-B/Yamagata, the RRs were 2.20 (95%CI: 1.44–3.37, P = .0003) for B/Victoria and 1.10 (95%CI: 1.00–1.20, P = .05) for B/Yamagata, respectively. Therefore, QIV showed no significant differences of SCR for the B lineage included in the TIV, whereas QIV showed superior SCR for the B lineage not included in the TIV. The SCR forest plots of the four B vaccine strains above were shown in ).
) shows forest plots of SPR:
Figure 4. (a,b). Seroprotection rate (SPR) at day 21 post-vaccination, QIV versus TIV in adults under 65 years old. (SPR is defined as the percentage of participants with a HAI titres≥40)
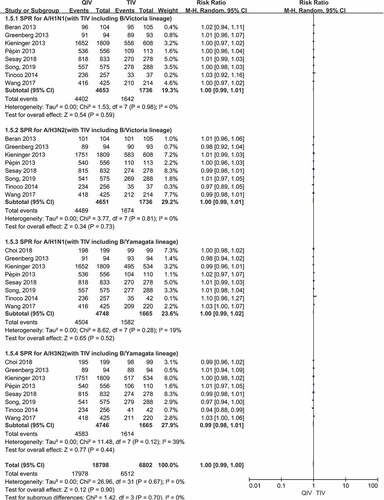
There were a total of 8 papers reported SPR data of two A vaccine strains (A/H1N1, A/H3N2) after vaccination. When comparing QIV with TIV-B/Victoria, the SPR risk ratios (RRs) were 1.00 (95%CI: 0.99–1.01, P = .59) for A/H1N1 and 1.00 (95%CI: 0.99–1.01, P = .73) for A/H3N2. Compared QIV with TIV-B/Yamagata, RRs were 1.00 (95%CI: 0.99–1.02, P = .52) for A/H1N1 and 0.99 (95%CI: 0.98–1.01, P = .44) for A/H3N2, respectively. There was no significant difference in the pooled SPR RR values of the four A vaccine strains above, () and the total SPR RRs were 1.00 (95%CI: 0.99–1.00, P = .70). The SPR forest plots of the four A vaccine strains above were shown in ).
There were a total of 8 papers reported SPR data of two B lineages (B/Victoria, B/Yamagata) after vaccination. Compared QIV with TIV-B/Victoria, the SPR RRs were 1.01 (95%CI: 0.99–1.03, P = .53) for B/Victoria and 1.11 (95%CI: 1.03–1.19, P = .006) for B/Yamagata. When comparing QIV with TIV-B/Yamagata, the SPR RRs were 1.34 (95%CI: 1.10–1.63, P = .004) for B/Victoria and 1.01 (95%CI: 0.99–1.02, P = .43) for B/Yamagata, respectively. Therefore, QIV showed no significant differences of SPR for the B lineage included in the TIV, whereas QIV showed superior SPR for the B lineage not included in the TIV. The SPR forest plots of the four B vaccine strains above were shown in ).
Meta-analysis of the safety of QIV versus TIV
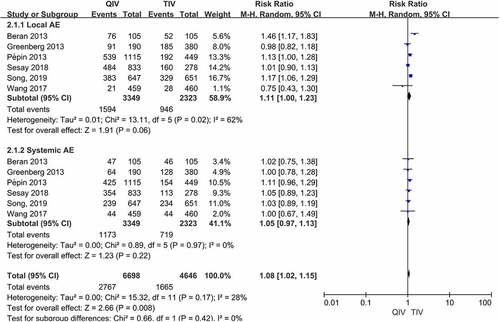
There were 6 studies reported the total number of subjects with systemic or local adverse events (AEs). When comparing QIV with TIV, pooled RRs of local AEs were 1.11 (95%CI: 1.00–1.23, P = .06) and RRs of systemic AEs were 1.05 (95%CI: 0.97–1.13, P = .22), respectively (Data were shown in ). There were no significant differences in the occurrence of any local or systemic AE compared QIV with TIV.
Sensitivity analysis
When comparing QIV with TIV, pooled RRs of local and systemic AEs were 1.13 (95%CI: 1.01–1.28) and 1.06 (95%CI: 0.97–1.15) after removal of the particular study,Citation8 respectively. The pooled RRs for local or systemic AEs were similar after the removal of the study (the change of RR value was less than 10%). We also performed sensitivity analysis on other studies, revealing no significant differences in the results after either study was excluded.
Discussion
Influenza continues to be a major public health problem worldwide. Currently licensed inactivated influenza vaccines are trivalent or quadrivalent as they contain antigens of two influenza A(H1N1, H3N2) and one or two influenza B strains (B/Victoria, B/Yamagata) that circulate in the human population.
This meta-analysis included high-quality randomized controlled trials, which strengthened the accuracy and stability of the meta-analysis results. According to the results of sensitivity analysis, there were no significant differences in the pooled RRs of local and systemic AEs after Greenberg’s studyCitation8 was excluded. We also performed sensitivity analysis on other studies, revealing no significant differences in the results after either study was excluded.
In this meta-analysis, there was no significant differences in SCR and SPR against the same vaccine strain between QIV and TIV after inoculation in adults under 65 years old, while the SCR and SPR of the unique influenza B lineage of QIV in the QIV group were significantly higher than those in the TIV group. This indicates that the current use of the TIV about influenza B vaccine strain does not have cross-protection in immunity, which means it cannot produce protective antibody against B lineage not included in the TIV. Our study also shows that QIV and TIV had equivalent efficacy against the shared three strains between the two vaccines after inoculation in 18–64 years old adults. Meanwhile, the addition of another influenza B lineage can produce good immunogenicity effects. Therefore, the promotion of QIV vaccination in adults will be more conducive to the prevention and control of influenza B.
This meta-analysis of the safety levels of QIV confirms that there’s no significant difference between QIV and TIV in the occurrence of local and systemic adverse events. So we can conclude that the safety level of QIV is as good as TIV.
Our findings above are consistent with many other researches.Citation17,Citation18 There are several limitations to this study. First, the heterogeneity of the data included in the literatures may be due to the age of the subjects, the study area, different epidemic seasons, different exposure experiences (vaccination or infection) before vaccination, different vaccine manufacturers and other factors. Second, this meta-analysis only compared non-adjuvanted and inactivated QIV with inactivated TIV, the results may not be applicable to comparisons among live attenuated influenza vaccines, subunit influenza vaccines and adjuvanted influenza vaccines.In addition, the number of studies included and overall sample sizes were small. Thus, efficacy levels of the tests may be insufficient. More relevant studies should be accumulated to further evaluate safety and immunogenicity levels of inactivated QIV in the future.
Conclusion
This meta-analysis basically shows that vaccination of inactivated QIV can not only have similar immunogenicity and safety effects to TIV, but also have better immunogenicity against the B lineage (B/Victoria or B/Yamagata) which is not included in the TIV in 18–64 years old adults.Therefore, the promotion of QIV vaccination can effectively reduce the influenza-related (especially influenza B-induced) morbidity and mortality in adults. Also, few studies examined older adults specifically and thus more research is needed in this age group. In addition, future studies of cost-effectiveness of QIV might be useful.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplemental Material
Download MS Word (2.3 MB)Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2021.1932218.
Additional information
Funding
References
- Sambala EZ, Ngcobo N, Machingaidze S, Wiyeh AB. A global review of seasonal influenza vaccine introduction: analysis of the WHO/UNICEF joint reporting form. Expert Rev Vaccines. 2019;18:859–65. doi:10.1080/14760584.2019.1640119.
- Koike ME, Gattás VL, Lucchesi MBB, Moura de Oliveira MM, Vanni T, Thomé BDC, Menang O, Socquet M, Precioso AR. Pharmacovigilance capacity strengthening for WHO prequalification: the case of the trivalent influenza vaccine manufactured by Instituto Butantan. Vaccine. 2019;37(52):7560–65. doi:10.1016/j.vaccine.2019.09.082.
- Gresset-Bourgeois V, Leventhal PS, Pepin S, Hollingsworth R, Kazek-Duret M-P, De Bruijn I, Samson SI. Quadrivalent inactivated influenza vaccine (VaxigripTetra™). Expert Rev Vaccines. 2018;17(1):1–11. doi:10.1080/14760584.2018.1407650.
- Newman P, Logie C, Lacombe-Duncan A, Baiden P, Tepjan S, Rubincam C, Doukas N, Asey F. Parents’ uptake of human papillomavirus vaccines for their children: a systematic review and meta-analysis of observational studies. BMJ Open. 2018;8(4):e019206. doi:10.1136/bmjopen-2017-019206.
- Chan JSK, Harky A. The importance of risk of bias assessment in meta-analyses: does controlling heterogeneity suffice? Eur J Cardio-thoracic Surg. 2020;58(5):1102. doi:10.1093/ejcts/ezaa174.
- Mondini G, Braga PE, Lopes MH, Sartori AMC, Miyaji KT, Infante V, Randi BA, Timenetsky MDCST, Ferreira JCDOA, Sakita NK, et al. Prospective cohort studies to evaluate the safety and immunogenicity of the 2013, 2014, and 2015 seasonal influenza vaccines produced by Instituto Butantan. Rev Inst Med Trop Sao Paulo. 2018;60:e37. doi:10.1590/s1678-9946201860037.
- Chan YJ, Lee CL, Hwang SJ, Fung CP, Wang FD, Yen DH, Tsai C-H, Chen YMA, Lee S-D. Seroprevalence of antibodies to pandemic (H1N1) 2009 influenza virus among hospital staff in a medical center in Taiwan. JCMA. 2010;73(2):62–66. doi:10.1016/S1726-4901(10)70003-4.
- Beran J, Peeters M, Dewé W, Raupachová J, Hobzová L, Devaster JM. Immunogenicity and safety of quadrivalent versus trivalent inactivated influenza vaccine: a randomized, controlled trial in adults. BMC Infect Dis. 2013;13:224. doi:10.1186/1471-2334-13-224.
- Choi WS, Noh JY, Lee J, Choi JY, Lee J-S, Kim MS, Kim HS, Bang J, Lavis N, Kim WJ, et al. Immunogenicity and safety of a split-virion quadrivalent influenza vaccine in adults 18–60 years of age in the Republic of Korea. Hum Vaccin Immunother. 2018;14(3):587–92. doi:10.1080/21645515.2017.1381808.
- Greenberg DP, Robertson CA, Noss MJ, Blatter MM, Biedenbender R, Decker MD. Safety and immunogenicity of a quadrivalent inactivated influenza vaccine compared to licensed trivalent inactivated influenza vaccines in adults. Vaccine. 2013;31(5):770–76. doi:10.1016/j.vaccine.2012.11.074.
- Kieninger D, Sheldon E, Lin WY, Yu CJ, Bayas JM, Gabor JJ, Esen M, Fernandez Roure JL, Narejos Perez S, Alvarez Sanchez C, et al. Immunogenicity, reactogenicity and safety of an inactivated quadrivalent influenza vaccine candidate versus inactivated trivalent influenza vaccine: a phase III, randomized trial in adults aged ≥18 years. BMC infectious diseases. 2013;13:343.
- Pépin S, Donazzolo Y, Jambrecina A, Salamand C, Saville M. Safety and immunogenicity of a quadrivalent inactivated influenza vaccine in adults. Vaccine. 2013;31(47):5572–78. doi:10.1016/j.vaccine.2013.08.069.
- Sesay S, Brzostek J, Meyer I, Donazzolo Y, Leroux-Roels G, Rouzier R, Astruc B, Szymanski H, Toursarkissian N, Vandermeulen C, et al. Safety, immunogenicity, and lot-to-lot consistency of a split-virion quadrivalent influenza vaccine in younger and older adults: a phase III randomized, double-blind clinical trial. Hum Vaccin Immunother. 2018;14(3):596–608. doi:10.1080/21645515.2017.1384106.
- Song JY, Lee J, Woo HJ, Wie S-H, Lee JS, Kim SW, Kim TH, Jung S-I, Noh JY, Choi WS, et al. Immunogenicity and safety of an egg-based inactivated quadrivalent influenza vaccine (GC3110A) versus two inactivated trivalent influenza vaccines with alternate B strains: a phase randomized clinical trial in adults. Hum Vaccin Immunother. 2019;15(3):710–16. doi:10.1080/21645515.2018.1536589.
- Tinoco JC, Pavia-Ruz N, Cruz-Valdez A, Aranza Doniz C, Chandrasekaran V, Dewé W, Liu A, Innis BL, Jain VK. Immunogenicity, reactogenicity, and safety of inactivated quadrivalent influenza vaccine candidate versus inactivated trivalent influenza vaccine in healthy adults aged ≥18 years: a phase III, randomized trial. Vaccine. 2014;32(13):1480–87. doi:10.1016/j.vaccine.2014.01.022.
- Wang SY, Liu SZ, Chu K, Zhao Y, Zhu FC, Hu YM, Meng FY, Li JX, Luo L, Yang JY, et al. Immunogenicity and safety of an inactivated quadrivalent influenza vaccine candidate versus inactivated trivalent influenza vaccines in participants >/=3 years of age: a double-blind, randomized, parallel-controlled phase III clinical trial in China. Expert review of vaccines. 2017;16:1155–69.
- Moa AM, Chughtai AA, Muscatello DJ, Turner RM, MacIntyre CR. Immunogenicity and safety of inactivated quadrivalent influenza vaccine in adults: a systematic review and meta-analysis of randomised controlled trials. Vaccine. 2016;34(35):4092–102. doi:10.1016/j.vaccine.2016.06.064.
- Meng Z, Zhang J, Zhang Z, Luo D, Yang X. Immunogenicity of inactivated quadrivalent influenza vaccine in adults aged 18-64 years: a systematic review and meta-analysis. Chin J Epidemiol. 2018;39:1636–41.