ABSTRACT
M-M-R®II (M-M-R II) is routinely used in many countries at 12–15 months with a second dose at 4 to 6 years of age. However, the vaccine may need to be administered at other ages due to delays in the immunization schedule or in certain situations such as outbreaks or international travel. A systematic literature review was conducted to evaluate efficacy, immunogenicity and safety of M-M-R II among 6- to 11-month-olds and persons ≥7 years of age. A search for randomized controlled trials (RCTs) was conducted in 2019 including Medline, Embase and Cochrane CENTRAL. Only one study reported seroconversion rates after one dose in infants at 9 months of age: 87.4% (measles), 92.3% (mumps), and 91.2% (rubella); no safety data were reported. Seven studies reported immunogenicity and safety data for M-M-R II at ≥7 years of age. Seroconversion rates ranged from 96%-100% (measles), 65%-100% (mumps), and 91%-100% (rubella). Rates of selected adverse events ranged from 5.2%-8.7% for fever (≥38°C or ≥38.1°C), 2%-33.3% for injection site reactions, and 0.4% for measles/rubella-like rash (one study). No efficacy studies were found. This literature review identified RCTs with evidence to support that M-M-R II is immunogenic and well tolerated in individuals ≥7 years of age.
Introduction
Measles, mumps and rubella are highly contagious, vaccine preventable viral diseases that can affect susceptible children, adolescents, and adults. Each virus can be acquired through airborne (measles) or droplet transmission (measles, mumps, and rubella). Some outbreaks of measles and mumps continue to occur globally, mostly among unvaccinated or undervaccinated individuals. Although many individuals with mild symptoms recover after a few days, infection can lead to serious complications and, in rare cases, death.Citation1
High uptake and timely vaccination against measles, mumps and rubella is critical to curtail these diseases. M-M-R®II (measles, mumps, and rubella vaccine live; Merck & Co., Inc., Kenilworth, NJ, USA [henceforth referred to as M-M-R II]; also known as MMRvaxPRO in the EU) is a combination vaccine indicated for active immunization for the prevention of measles, mumps, and rubella in individuals 12 months of age and older. M-M-R II has been exclusively used in children in the U.S. for over 40 years, and has resulted in a ≥ 96% reduction in the rate of all 3 diseases.Citation2 Measles and rubella have been eliminated from the US due to high vaccine coverage rates.Citation3,Citation4 Mumps continues to circulate in the context of outbreaks primarily in close contact settings, though the disease is largely under control.Citation5
M-M-R II is the only MMR vaccine registered in the U.S. In the U.S. and many other countries, the first dose of MMR vaccine is routinely recommended at 12 to 15 months of age and the second dose is routinely recommended at 4 to 6 years of age (although the second dose may be administered at any time ≥4 weeks after the first dose). MMRV (ProQuad®, Merck & Co., Inc., Kenilworth, NJ, USA), the only quadrivalent vaccine in use that contains measles, mumps, rubella and varicella, has been available since 2005 in the U.S. The Advisory Committee on Immunization Practices (ACIP) recommends the use of MMRV vaccine for the second dose of MMR and varicella at 4 to 6 years of age, in the context of its general preference for combination vaccines.Citation6
Worldwide, the vast majority of individuals who receive MMR vaccine routinely receive two doses between 12 months and 6 years of age. In certain circumstances, MMR vaccine is recommended outside of the standard age ranges. For example, infants 6 to 11 months of age may be vaccinated in outbreak situations or before international travel, and persons ≥7 years of age may be vaccinated as a catch-up or during outbreaks.Citation7–9 In countries with high measles prevalence, the World Health Organization (WHO) considers 9 months as optimal timing for the first dose of measles vaccination for protection of susceptible infants against measles (recognizing that this dose does not count as part of the 2-dose regimen).Citation9 The safety, immunogenicity, and efficacy of MMR vaccine outside the routine age of administration are important to understand, especially in light of the continued endemicity of measles in some regions and continued outbreaks in regions where measles has been eliminated.Citation10,Citation11
For this reason, a systematic literature review (SLR) was conducted to identify clinical trial and real-world data on the use of M-M-R II by age group (6 to 11 months, ≥12 months to 6 years, and ≥7 years). For the current analysis, randomized controlled trials (RCTs) reporting immunogenicity, efficacy and/or safety data for use at 6 to 11 months and ≥7 years of age were reviewed.
Methods
A detailed research plan for the SLR was pre-specified in a protocol in line with Cochrane guidelines.Citation12 A search limited to the English language was conducted on May 15, 2019 in Medline, Embase and Cochrane CENTRAL, without time or geographic restriction. The search strategy was adapted to the requirements of each database. In addition, a comprehensive search for gray literatureFootnote1 was performed between June 24 and July 22, 2019, including a trial registry (clinicaltrials.gov), conferences of eight societies, other databases and various Internet sources (WHO, Centers for Disease Control and Prevention, European Center for Disease Prevention and Control, Eurosurveillance, National Immunization Technical Advisory Groups [NITAG], PATH Vaccine Resource Library, and The Technical Network for Strengthening Immunization Services [TechNet-21]).
Two teams of reviewers systematically screened the search results independently against pre-defined eligibility criteria. At the end of the screening, consensus meetings were held to reach agreement on inclusion or exclusion of data, with a team of third reviewers consulted in case of disagreement. Studies using M-M-R II or MMRvaxPRO with or without concomitant use of other vaccines were included (studies using monovalent and MMRV vaccines and other brands of MMR were excluded). RCTs were selected for inclusion if they reported efficacy, immunogenicity or safety outcomes. Data from studies in which the vaccine was administered by a nonstandard route of administration (aerosol, jet injector) were not included in this report.
Results
The initial systematic literature review returned a total of 122 references pertaining to 88 studies (75 RCT and 13 observational studies). Eight studies, one for 6 to 11 months of age and seven for individuals ≥7 years of age, met the inclusion criteria and provided immunogenicity or safety data. No study reported efficacy data in these age groups ().
Figure 1. PRISMA flow diagram of bibliographic and gray literature searches (SLR overall).
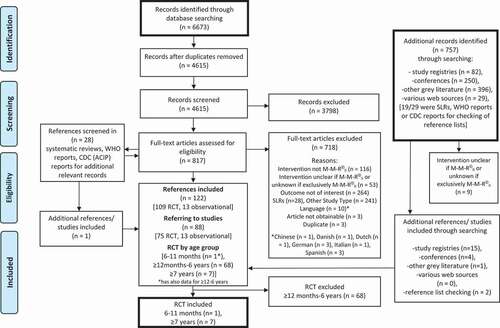
Only one double-blind RCT evaluated the immunogenicity of M-M-R II in infants <12 months of age ().Citation13 The trial was conducted between 1992 and 1994 in the U.S. and enrolled 990 children who were 7 months of age at enrollment. The infants were randomized to receive a single dose of vaccine at either 9 (n = 285), 12 (n = 358) or 15 (n = 347) months. Measles seroconversion rates were higher in children vaccinated at 15 months of age (98.3%) and 12 months (95.3%) than they were in those vaccinated at 9 months (87.4%). This was similar for rubella (96.4% at 15 months, 94.9% at 12 months, and 91.2% at 9 months), while for mumps the rates were nearly identical in each of the groups (93.0% at 15 months, 89.8% at 12 months, and 92.3% at 9 months), respectively. In an additional analysis comparing children whose mothers were born in 1964 and later to children of mothers born in 1963 or earlier (pre-vaccination era), the seroconversion rates among children vaccinated at 9 months were higher in children whose mothers were born in the post-vaccination era for measles (93.6% vs 83.5%) and rubella (95.3% vs 88.6%), but not for mumps (92.4% vs 92.2%). No safety data were reported in this study.Citation13
Table 1. Immunogenicity of M-M-R II in individuals 6–11 months and ≥7 years of age
Seven studies reported data for M-M-R II administered at ≥7 years of age (6 studies without and 1 study with concomitant administration of other vaccines). The vaccine was given as a second dose in five of the studies, as a first or second dose in one study, and in one study this was not specified. These studies were conducted in Brazil (1), Mexico (3), Sweden (1), the U.S., and U.S./Europe (1).Citation14–20 The mean age of participants ranged from 7 to 26 years ().
One observer-blinded RCT conducted in 2014–2015 in 3 countries (U.S., Estonia, and Slovakia) evaluated immunogenicity and safety of a second dose of M-M-R II in individuals ≥7 years of age (mean age 25.6 years [standard deviation (SD) 13.8]). The seroresponse rates were 99.1% for measles, 99.5% for mumps, and 99.8% for rubella 42 days post-dose 2; a subgroup analysis of the immunogenicity data by age group was not presented.Citation20
In a study conducted in Sweden in 1999, children received a second dose of M-M-R II at 11–12 years of age after receiving their first dose at 2 years of age. There was a ≥ 4-fold increase in antibody levels in 5.7% of children for measles, 24.1% for mumps, and 39.7% for rubella after the second dose compared to baseline (among individuals with detectable antibodies prior to second dose). Among the subjects with no detectable antibody at baseline, all seroconverted for measles, mumps, and rubella approximately 6 weeks after the administration of the second dose.Citation18
Three studies conducted in Mexico evaluated the administration of M-M-R II in participants ≥7 years of age.Citation15–17 In study 1, seroconversion rates after a second dose of M-M-R II in individuals 6 to 7.5 years of age were 100% for measles, mumps and rubella at one month post-vaccination and were 100%, 90.3% and 100%, respectively, at one year post-vaccination.Citation15 In study 2, seroconversion rates 2 months post-vaccination among individuals 18 to 25 years of age were 96% for measles, 65% for mumps, and 100% for rubella, respectively.Citation16 In a third study among individuals 9 to 14 years of age, seroconversion rates at 12 weeks post-vaccination were 100% for measles, 95% for mumps, and 97.5% for rubella.Citation17
In a study conducted in Brazil during 1996 in school children 6 to 12 years of age (mean 8.9 years), all children had the opportunity to be previously vaccinated at either 9 months of age or during a mass vaccination campaign in 1992 (the exact vaccine administered for the first dose was not specified). The overall seropositivity rates increased 21–30 days after receipt of M-M-R II compared to prevaccination (from 78.1% to 99.5% for measles, 73.5% to 94.5% for mumps, and 53.9% to 91.3% for rubella).Citation14
The duration of safety follow-up as well as type of events assessed and their definitions varied from study to study. Across these six studies, the rates of selected adverse events ranged from 5.2%-8.7% for fever (defined as ≥38°C or ≥38.1°C), and 2%-33.3% for any injection site reaction (pain, redness and/or swelling). Only one study reported measles/rubella-like rash; the rate was 0.4% after dose 2 ().
Table 2. Safety of M-M-R II in individuals ≥7 years of age
Only one study reported concomitant administration of M-M-R II with other vaccines in subjects ≥7 years of age. An open-label US-based RCT was conducted from 1996 to 1997 in children 11 to 12 years of age (n = 197). One group received hepatitis B (HepB), tetanus and diphtheria toxoid (Td) and M-M-R II concomitantly at visit 1, while another group received HepB at visit 1 and Td and M-M-R II at a later visit (4.5 months). Seropositivity rates for all relevant antigens were over 98% in both groups. A higher rate of headache (31.5% vs 8.6%, p = .001) was found in the group receiving all three vaccines at the same visit compared to the group receiving Td and M-M-R II at 4.5 months; a higher rate of injection site tenderness (24.7% vs. 9.1%, p = .005) was found in the group receiving all three vaccines at the same visit compared to the group receiving HB only. However, both concomitant and non-concomitant administration of HepB with Td and M-M-R II were considered to be well tolerated.Citation19
Discussion
Although M-M-R II has been used for over 40 years to prevent measles, mumps and rubella, this review identified only 8 studies that assessed immunogenicity and safety of M-M-R II outside the age range typically recommended for immunization. The evidence from seven RCTs conducted in persons ≥7 years of age suggests that M-M-R II is immunogenic and well tolerated in older age group.
In the one study conducted in infants 6 to 11 months of age, the vaccine appeared to be less immunogenic when given at 9 months of age as compared to 12 and 15 months of age. This is likely due to the persistence of maternal antibodies that interfere with vaccine response. This phenomenon was less pronounced for younger mothers, whose own immunity to measles, mumps and rubella was likely vaccine-induced rather than due to natural infection. The decreased immunogenicity of M-M-R II in infants 6 to 11 months of age will likely be less of an issue in the future, as virtually all mothers today have vaccine-induced immunity.Citation13
Administration of M-M-R II at ≥7 years of age may be indicated due to delays in completing the routine schedule, international travel, or for outbreak control. The vaccine may also need to be administered to adults lacking proof of immunity or to healthcare personnel, military service members, immigrants, and travelers at higher risk.Citation8 In 2017, the ACIP recommended a third dose of MMR to individuals who had already received two doses, but who have an increased risk for mumps during an outbreak.Citation22 This systematic review of RCTs shows that in these situations, M-M-R II can be expected to be well tolerated and immunogenic.
This review found a limited amount of safety data for M-M-R II administration outside the routinely recommended age. Safety data reported in trials varied in terms of duration of follow-up and method of data collection. No adverse events indicating a new safety signal were reported in these studies, and overall, M-M-R II was considered well tolerated in persons ≥7 years of age. No safety data were assessed or reported in the one study we identified in infants 6 to 11 months of age.
Although our search was extensive, including sources such as clinicaltrials.gov, conference abstracts, WHO, CDC websites in addition to bibliographic databases, we found few RCTs outside the recommended age ranges for administration of M-M-R II. This review focuses exclusively on M-M-R II and did not include all the measles, mumps and rubella vaccines available globally limiting the generalizability of the findings to potentially M-M-R II only. This review would be of particular interest to countries who currently use and those that plan to incorporate M-M-R II or require WHO-prequalification for inclusion of MMR vaccine into the immunization schedules. This report only focused on RCTs conducted for M-M-R II and did not include real-world evidence studies such as epidemiological studies or surveillance studies and did not report the vaccine effectiveness in the real-world setting. Many low- and middle-income countries have introduced or are considering the introduction of MR or MMR vaccines into their routine childhood immunization programs and some programs provide or consider providing the first dose of measles vaccine at earlier age (prior to 12 months).
In light of the potential risk for future outbreaks of measles, mumps, and/or rubella, it is important to understand the safety and immunogenicity of M-M-R II outside the routine age ranges recommended by the ACIP and other healthcare authorities. M-M-R II is indicated for use in children 12 months of age and older. This SLR supports the safety and immunogenicity of M-M-R II outside the ACIP recommended age windows for routine administration, particularly among persons ≥7 years of age. Future research may be warranted to better understand the efficacy, immunogenicity and safety of M-M-R II should it need to be administered to children younger than 12 months of age. The data support the use of M-M-R II across a wider age range, which may be important when implementing vaccine catchup programs or to prevent future outbreaks in susceptible populations.
Disclosure of potential conflicts of interest
MP, ER, LPS, are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA and stockholders of Merck & Co., Inc., Kenilworth, NJ, USA. ES, MN, LK are employees of Certara, Loerrach, Germany, and were consultants for Merck & Co., Inc., Kenilworth, NJ, USA and paid for their services. SL and BK are former employees of Merck & Co., Inc., GM is an investigator on Merck clinical trials and GM, JF are consultants for Merck & Co., Inc., Kenilworth, NJ, USA.
Additional information
Funding
Notes
1. Grey literature is literature that is not formally published in sources such as books or journal article.Citation23 It is defined as “Information produced on all levels of government, academics, business and industry in electronic and print formats not controlled by commercial publishing i.e. where publishing is not the primary activity of the producing body”Citation24–26.
References
- Centers for Disease Control and Prevention (CDC). Complications of measles. [Internet]; 2020 [accessed 2020 October 30]. https://www.cdc.gov/measles/symptoms/complications.html .
- Centers for Disease Control and Prevention (CDC). Epidemiology and prevention of vaccine-preventable diseases. Appendix E: data and statistics. Impact of Vaccines in the 20th and 21st Centuries; 2020 [accessed 2020 Nov 17]. https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/appendices/e/impact.pdf .
- Centers for Disease Control and Prevention (CDC). Measles cases and outbreaks. [Internet]; 2020 [accessed 2020 October 30]. https://www.cdc.gov/measles/cases-outbreaks.html .
- Centers for Disease Control and Prevention (CDC). Rubella in the U.S. [Internet]; 2020 [accessed 2020 Nov 17]. https://www.cdc.gov/rubella/about/in-the-us.html .
- Centers for Disease Control and Prevention (CDC). Mumps cases and outbreaks. [Internet]; 2020 [accessed 2020 Nov 17]. https://www.cdc.gov/mumps/outbreaks.html .
- Marin M, Broder KR, Temte JL, Snider DE, Seward JF. Use of combination measles, mumps, rubella, and varicella vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2010 May 7;59(RR–3):1–12.
- European Centre for Disease Prevention and Control (ECDC). ECDC vaccine scheduler: recommended vaccinations: measles, Mumps, Rubella [Internet]. [accessed 2020 Dec 14]. https://vaccine–schedule.ecdc.europa.eu/Scheduler/ByDisease?SelectedDiseaseId=8&SelectedCountryIdByDisease=−1;https://vaccine–schedule.ecdc.europa.eu/Scheduler/ByDisease?SelectedDiseaseId=9&SelectedCountryIdByDisease=−1;https://vaccine–schedule.ecdc.europa.eu/Scheduler/ByDisease?SelectedDiseaseId=10&SelectedCountryIdByDisease=−1 .
- Centers for Disease Control and Prevention (CDC). Measles, mumps, and rubella (MMR) vaccination: what everyone should know [Internet]. [accessed 2020 December 14]. https://www.cdc.gov/vaccines/vpd/mmr/public/index.html .
- World Health Organization (WHO). Measles vaccines: WHO position paper - April 2017. Wkly Epidemiol Rec [Internet]. 2017 Apr 28;92(17):205–27. http://apps.who.int/iris/bitstream/10665/255149/1/WER9217.pdf?ua=1 .
- Zucker JR, Rosen JB, Iwamoto M, Arciuolo RJ, Langdon-Embry M, Vora NM, Rakeman JL, Isaac BM, Jean A, Asfaw M, et al. Consequences of undervaccination — measles outbreak, New York City, 2018–2019. N Engl J Med. 2020;382(11):1009–17. doi:10.1056/NEJMoa1912514.
- Kuehn BM. Drop in vaccination causes surge in global measles cases, deaths. Jama. 2021 Jan 19;325(3):213.
- Higgins JPTTJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane handbook for systematic reviews of interventions version 6.1 (updated September 2020). [Internet]; 2020 www.training.cochrane.org/handbook .
- Redd SC, King GE, Heath JL, Forghani B, Bellini WJ, Markowitz LE. Comparison of vaccination with measles-mumps-rubella vaccine at 9, 12, and 15 months of age. J Infect Dis. 2004 May 01;189(SUPPL. 1):S116–S22. doi:10.1086/378691.
- Dos Santos BA, Stralioto SM, Siqueira MM, Ranieri TS, Bercini M, Schermann MT, Wagner MB, Silveira TR. Prevalence of antibodies against measles, mumps, and rubella before and after vaccination of school-age children with three different triple combined viral vaccines, Rio Grande do Sul, Brazil, 1996. Revista Panamericana De Salud Publica/Pan Am J Public Health. 2006Nov;20(5), 299–306.
- Diaz Ortega JL, Castaneda D, Arellano Quintanilla DM, Martinez D, Trumbo SP, Fernandez de Castro J. Antibody persistence in children aged 6-7 years one year following booster immunization with two MMR vaccines applied by aerosol or by injection. Vaccine. 2017 May 25;35(23):3116–22. doi:10.1016/j.vaccine.2017.04.027.
- Diaz-Ortega JL, Bennett JV, Castaneda D, Martinez D, Fernandez de Castro J. Antibody persistence in young adults 1 year after MMR immunization by aerosol or by subcutaneous route. Vaccine. 2010 Oct 18;28(44):7228–32. doi:10.1016/j.vaccine.2010.08.055.
- Sarno MJ, Blase E, Galindo N, Ramirez R, Schirmer CL, Trujillo-Juarez DF. Clinical immunogenicity of measles, mumps and rubella vaccine delivered by the Injex jet injector: comparison with standard syringe injection. Pediatr Infect Dis J. 2000;19(9):839–42. doi:10.1097/00006454-200009000-00006.
- Gothefors L, Bergstrom E, Backman M. Immunogenicity and reactogenicity of a new measles, mumps and rubella vaccine when administered as a second dose at 12 y of age. Scand J Infect Dis. 2001;33(7):545–49. doi:10.1080/00365540110026593.
- Cassidy WM, Jones G, Williams K, Deforest A, Forghani B, Virella G, Venters C. Safety and immunogenicity of concomitant versus nonconcomitant administration of hepatitis B, tetanus-diphtheria, and measles-mumps-rubella vaccines in healthy eleven- to twelve-year-olds. J Adolesc Health. 2005 Mar;36(3):187–92. doi:10.1016/j.jadohealth.2004.02.021.
- Abu-Elyazeed R, Jennings W, Severance R, Noss M, Caplanusi A, Povey M, Henry O. Immunogenicity and safety of a second dose of a measles-mumps-rubella vaccine administered to healthy participants 7 years of age or older: a phase III, randomized study. Hum Vaccin Immunother. 2018 Nov 02;14(11):2624–31. doi:10.1080/21645515.2018.1489186.
- Dos Santos BA, Ranieri TS, Bercini M, Schermann MT, Famer S, Mohrdieck R, Maraskin T, Wagner MB. An evaluation of the adverse reaction potential of three measles-mumps-rubella combination vaccines. Revista Panamericana De Salud Publica/Pan Am J Public Health. 2002 Oct 01;12(4):240–46.
- Marin M, Marlow M, Moore KL, Patel M. Recommendation of the advisory committee on immunization practices for use of a third dose of mumps virus-containing vaccine in persons at increased risk for mumps during an outbreak. Mmwr. Morbidity Mortality Weekly Rep. 2018 Jan 12;67(1):33–38. doi:10.15585/mmwr.mm6701a7.
- Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JAC, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. Bmj. 2011 Oct;18(343):d5928. doi:10.1136/bmj.d5928.
- Grey Literature International Steering Committee (GLISC). Guidelines for the production of scientific and technical reports: how to write and distribute grey literature, Version 1.0. 2006; [accessed 2019 Sep 12]. http://eprints.rclis.org/7469/1/nancy.pdf .
- Farace DJ, Schöpfel J. Introduction grey literature. 0.1 definitions. Grey literature in library and information studies. Berlin; New York: De Gruyter Saur; 2010. 1. https://library.oapen.org/handle/20.500.12657/45661?show=full
- Schöpfel J. Towards a Prague definition of grey literature. at: twelfth international conference on grey literature: transparency in grey literature grey tech approaches to high tech issues; 2010 Dec 6-7; Prague (Czech Republic). 2019. https://archivesic.ccsd.cnrs.fr/sic_00581570 .
