ABSTRACT
Thirty-nine years ago, scrub typhus (ST), a disease, was not among the China’s notifiable diseases. However, ST has reemerged to become a growing public health issue in the southwest part of China. The major factors contributing to an increased incidence and prevalence of this disease include rapid globalization, urbanization, expansion of humans into previously uninhabited areas, and climate change. The clinical manifestation of ST also consists of high fever, headache, weakness, myalgia, rash, and an eschar. In severe cases, complications (e.g. multi-organ failure, jaundice, acute renal failure, pneumonitis, myocarditis, and even death) can occur. The diagnosis of ST is mainly based on serological identification by indirect immunofluorescence assay and other molecular methods. Furthermore, several groups of antibiotics (e.g. tetracycline, chloramphenicol, macrolides, and rifampicin) are currently effective in treating this disease. This fact suggests the need for robust early diagnostic techniques, increased surveillance, and prompt treatment, and develop future vaccine.
Introduction
Scrub typhus (ST) is a febrile illness caused by the Gram-negative coccobacillus bacteria Orientia tsutsugamushi that are transmitted to humans through the bites of infected larval mites known as “chiggers” (belonging to the family Trombiculide).Citation1,Citation2 Furthermore, this zoonotic disease predominates in the Asia-Pacific region, including China.Citation3–11
Most patients with ST that receive appropriate treatment recover from this disease. However, delayed diagnosis and improper management of ST can result in severe complications and even death. Individuals with ST have clinical characteristics that resemble other diseases, such as dengue fever, malaria, leptospirosis, typhoid and other febrile illnesses. Given the overlap of symptoms among these diseases, a definitive diagnosis of ST will depend on patients’ medical history, physical examination, detailed account of clinical features and reliable diagnostic tests. A key diagnostic indicator of ST is the presence of eschar.Citation12–14
However, in the absence of the pathognomonic eschar, ST is mainly diagnosed on the basis of laboratory testing that involves serological assays, such as indirect immunofluorescence assay (IFA) for detecting serum IgM antibodies or a four-fold increase serum antibody titers.Citation15 In addition, several molecular methods that target a specific O. tsutsugamushi gene, such as PCR, qPCR, LAMP among other tests, have been used to diagnosis ST.Citation16–22
However, it is important to note that these assays are not point-of-care methods, and they require well-equipped laboratory and technical skills.Citation23 The serological diagnosis’s main drawbacks include the possibility of serological diagnosis for ST among closely-related organisms and low sensitivity in the acute phase of illness, making it less useful for diagnosis and subsequent treatment. Nevertheless, when used in combination with molecular methods, can lead to a definitive diagnosis.Citation24 A point-of-care assay, such as an immunochromatographic test (ICT) or dipstick test that detects O. tsutsugamushi-specific antigens in the patient’s blood/plasma/serum, is ideal as a rapid and early diagnostic tool, particularly in rural settings or during emergency cases.Citation25,Citation26 Notably, ST also can be treated successfully using antibiotics; in particular, doxycycline, tetracycline, azithromycin, and rifampicin have been applied as the first line of medications for ST treatment, but that have led to few treatment failures.Citation27,Citation28 Where Chloramphenicol and tetracycline are remains a treatment option for ST patient and are resistance to conventional antibiotics,Citation27,Citation29 and Macrolide antibiotic such as azithromycin were found to be highly effective against ST,Citation30 warranting the development of an effective vaccine for the disease prevention and control in the future.Citation31,Citation32
The first case in China was identified in Guangzhou city, Guangdong Province, in the nineteenth century,Citation2 and a scientific report on ST was first published in 1885 during the Ming Dynasty.Citation33 Before 1985, ST infection was found to be endemic south of the Yangtze River;Citation4 in 1986, ST was observed in the north of Yangtze River Jiangsu Province and Shandong Province.Citation34,Citation35 In 2006, ST was detected in Henan province,Citation36 while an outbreak of ST occurred in Shandong and others part of China between 2006 to 2016.Citation37,Citation38
The prevalence of ST has substantially increased in many parts of China.Citation4,Citation39 Two species of chiggers, namely Leptotrombidium deliense and Leptotrombidium scutellare,transmit O. tsutsugamushi to humans in south and north China, respectively.Citation40 Despite efforts to prevent the spread of these pathogens, ST cases have been widely reported in many parts of China.Citation4,Citation5,Citation7,Citation31,Citation41,Citation42 To date, ST is one of the most pervasive zoonotic diseases in the country as reported by China’s Information System for Disease Control and Prevention (CISDCP).Citation5 Thus, given its current prevalence and its various effect on individual health status, there is a need to understand the epidemiological distribution and discover ways to combat this disease.
Furthermore, a more detailed analysis of the circulating O. tsutsugamushi strains in China needs to be performed since this knowledge will likely be essential to developing an of an effective vaccine and sensitive specific point-of-care diagnostic methods. The present review focuses on the epidemiological importance of O. tsutsugamushi in China. In addition, disease transmission, diagnosis, management aspects, and the current national trends of this neglected disease will also be briefly discussed.
Scrub typhus etiology
O. tsutsugamushi is the etiologic agent of ST. Moreover, the bacteria have several biological attributes that are unique. For example, O. tsutsugamushi has a single chromosome with highly repetitive sequences and an abundance of mobile genetic elements.Citation43 This bacterial strain relies on the host cell’s microtubule trafficking apparatus for intracellular motility.Citation44 O. tsutsugamushi also exits the host cell by virus-like budding that involves lipid rafts,Citation45 and these microbes have cell walls with a minimal amount of peptidoglycan content.Citation46
O. tsutsugamushi was initially isolated and identified as a Gram-negative α-proteobacterium from the family Rickettsiaceaein Japan in 1930.Citation47 However, thecausative agent of ST, is a obligate intracellular bacteria (0.5 × 1.2–3.0 µm) with a different cell wall structure and genetic makeup to other rickettsiaee. Subsequently, the classification of O. tsutsugamushi was revised, and this organism was assigned to the genus, Orientia.Citation48 Numerous serotypes of O. tsutsugamushi also exist in China, including Gilliam, Kato, Karp, Hirano/Kuroki, Shimokoshi, TA763, and Irie/Kawasaki, respectively, were reported in advance as the serotype.Citation5
Diseases transmission
Scrub typhus is primarily transmitted by the larvae (or chiggers) of the trombiculid mites.Citation41,Citation42 which belong to the genus Leptotrombidium spp.Citation1,Citation2,Citation5,Citation40,Citation49 These arthropods are the vector for O. tsutsugamushiin the Asia-Pacific region. The trombiculid mites have four main developmental stages in their lifecycle: namely egg, larva, nymph, and adult with three quiescent stages. The larvae (0.2–0.4 mm in length) can only be observed through a magnifying glass or a microscope.Citation50–54 Furthermore, these immature mites typically feed on wild rodents (R. flavipectus, R. losea, R. confucianus, and R. norvegicus), Apodemusagrarius, and Suncus murinus.Citation3,Citation55 Although humans are an incidental host of the larvae,Citation56 their transmission rate to humans can be influenced by climate change and rodent density.Citation55,Citation57,Citation58 O. tsutsugamushi is transmitted to mammalian hosts by the third-stage larvae mites, which feed on the extracellular fluid of vertebrates. Mites act as the primary reservoir for O. tsutsugamushi.Citation59 In later stage adults, mites pass on the ST-causing bacteria to their eggs in a process called transovarial transmission. Through this process, O. tsutsugamushiis maintained in the vector from one life stage to another.Citation60,Citation61 The presence of chigger mites may be strongly influenced by the environment where host species live. More explicitly, prior studies have indicated that temperature is a key factor affecting the mite development and reproduction of Leptotrombidium mites (particularly, L. delicense).Citation50,Citation52,Citation62
In addition, the onset of ST has been correlated with environmental factors. For example, the temperature is argued to drive the prevalence of ST. In particular, warmer weather conditions lead to increasing the outdoor activity levels that could create opportunities for people to be more suspected to ST infection.Citation63 Furthermore, invasive plant species, such as Leucaenaleucocephala, whichare the preferred ecological habitats of Leptotrombidium mites, could result in an overabundance of O. tsutsugamushi. Lastly, the socioeconomic changes that lead to alterations in land cover along with climate variation can also culminate in the unexpected emergence of ST in humans.Citation62–66
Clinical manifestation and diagnosis of ST
Scrub typhus is associated with a number of clinical symptoms, the most common being an initial fever, eschar, rash, and lymphadenopathy.Citation67 After a 4 to 21 day incubation period, the clinical presentation of ST begins with a fever that typically ranges from 38.5°C to 42°C. The duration of the fever persists between 1 and 12 days (the average length of the period of illness of time is 4.5 days).Citation7,Citation9,Citation68 In severe cases, serious complications such as broncho-pneumonia, myocarditis, and heart failure were observed.Citation58,Citation59,Citation63–73
In China, clinical cases of ST are characterized by the common symptoms described above along with vomiting, splenomegaly, hepatomegaly, pneumonitis, meningitis, multiple organ dysfunction, disseminated intravascular coagulation, conjunctival congestion, fatigue and anorexia.Citation69,Citation70
Although ST and dengue fever are acute febrile illnesses in Asia, they can be distinguished from one another based on the duration of fever, serum alanine aminotransferase/serum aspartate aminotransferase (ALT/AST) values, platelet count, absence of bone pain, C-reactive protein (CRP), and presence/absence of bleeding syndrome.Citation63 Furthermore, it also has been observed that lymphadenopathy and multi-organ dysfunction are common manifestations in patients with eschars. Hence, the presence of eschar can be considered an early prognostic indicator of multi-organ dysfunction in infected individuals.Citation14
While the absence of a skin rash has been reported in some cases of ST, increased levels of serum adenosine deaminase (ADA) and ferritin has been associated with complicated forms of ST, which can lead to prolonged hospitalization.Citation8,Citation74 In China, the diagnosis of ST is based on the patient’s history, clinical features and findings, and the serologic testing results.
The Chinese Ministry of Health classified ST diagnosis into three distinct categories: suspected, probable and confirmed cases. Suspected cases consist of individual who participate in outdoor activities (e.g. farming, fishing, camping, and straw collection) during the ST epidemic in China three weeks before onset of illness and presented with fever, skin rash, and lymphadenectasis. Individuals diagnosed with dengue fever, typhus fever, hemorrhagic fever, or had an uncertain history of mite bites but developed fever were excluded from this classification. Probable cases are clinically diagnosed by local physicians based on epidemiologic measures, such as travel to affected areas and contact with chiggers or rodents <3 weeks before the onset of illness. Confirmed cases are laboratory corroborated infection following the diagnosis criteria and case classification guidelines developed by the Chinese national health authorities.Citation57
The attending physicians are required by law to report all suspected and probable cases of ST to the Chinese Center for Disease Control and Prevention (China CDC). A diagnosis of ST is determined by local health care professionals in a hospital setting. In cases where eschars (a common indicator of ST) are absent, a laboratory test is mandatory to confirm diagnosis.Citation26 ST status can be assessed by direct (isolation and DNA detection) and indirect (serology) methods. Notably, Indirect immunofluorescence antibody assay(IFA) method, as known as serology gold standard can be beneficial for screening a large number of serum samples, however have limited its use in resource-restricted areas due to the test is more sensitive and requires propagation and purification of BSL3 agents as antigen for assay and fluorescence microscope. While serological diagnostic tests such as the Weil-Felix agglutination test were developed to identify ST cases, it has poor sensitivity and specificity for acute disease. It is requires paired samples.Citation26 Although the rapid diagnostic assays (RDTs) can be used for early diagnosis of ST, this latter test is particularly suitable as a diagnostic tool in rural areas of China.Citation75 In addition, several other molecular assays, such as PCR, qPCR, LAMP, and recombinase polymerase amplification can be employed to direct detectO. tsutsugamushi in clinical specimens,Citation9,Citation52,Citation71,Citation72,Citation76 which can serve as a reference culture for enhanced diagnosis.
In China, the diagnosis relies mainly on laboratory tests and clinical findings. Also, the antibody-based serological tests are basis of ST diagnosis. ST diagnostic test conducted base on diagnostic performance of IFA as gold standard reference method recommended by the WHO and Weil-Felix agglutination test; this is widely used in each level of Chinese medical institutions. ELISAs as well as provide sensitive and specific test results, and PCR assays detect antibodies against O. tsutsugamushi, where more details on the evaluation of ST diagnosis in China were recently reported by Lei Xin H., et al (2019).Citation77
Geographic distribution and current epidemiology
Scrub typhus occurs within a 13,000,000 km2 area of Asia mainly in a region known as “Tsutsugamushi Triangle” which extends from northern Japan to eastern Russia in the North and connects to northern Australia in the South and to Pakistan and Afghanistan in the West, as well as the Western Pacific islands and East Asia, including China.Citation3 It is estimated that at least one billion people in the Asia-Pacific region are at risk of contracting this disease annually.Citation5,Citation10,Citation40,Citation78 It is also important to note that the geographic range of O. tsutsugamushi and other closely-related species has extended beyond the Tsutsugamushi Triangle.Citation79-84 ST cases have been primarily found in southwest China and the southeast coastal and eastern regions of China.Citation80 The endemic of ST also report in Guangzhou province,Citation5 and in other regions in China.Citation4,Citation80 and recently reported in Jingjiang City, in south-central Jiangsu, China.Citation9 Although, the current epidemiological investigations showed the ST cases decreased significantly in China.Citation81
Epidemiology of ST in China
It is reported that ST has existed in southern China for thousands of years.Citation4,Citation5 Prior to the 1980s, cases of ST were observed mainly in the south of Yangtze River regions, (e.g. Zhejiang and Yunnan provinces).Citation83 However, from 1952 to 1989, ST was reported in six provinces: Guangdong, Fujian, Yunnan, Zhenjiang, Jiangsu, and Shandong Province,Citation35 Furthermore, over the past decade alone, ST prevalencehas increased in several areas within provinces of China.Citation79 For example, 4,865 confirmed cases were reported in 566 locations in Jiangsu Province in 2018.Citation4 The affected geographic area has rapidly expanded to encompass Taiwan, Yunnan, and Sichuan in the west, Hainan at the south, and Zhejiang and Hunan in the north, among other regions of China.Citation84 More recently, this disease has been widely reported in a number of provinces in China.Citation4,Citation5,Citation9,Citation35–39,Citation42,Citation64,Citation79,Citation85
Since the first outbreak in Shandong Province in 1986,Citation83,Citation86 ST incidence had increased in the China territories. For example, ST outbreak was reported in Jingling city, where this disease had never previously existed. Despite the current measures of prevention and control system undertaken by the China Center for Disease Control and Prevention (Chinese CDC), evidence still indicates a rapid increase in ST cases.Citation57 Notably, the number of ST reported cases in Guangdong and Yunnan was higher than in other provinces in China.Citation87 In a recent study, Yue et al. (2019) divided ST prevalence into three distinct regions in China, namely North (Anhui, Jiangsu, Shandong), Southwest (Yunnan, Sichuan), and South (Fujian, Guangdong, Guangxi, Hainan, Hunan, Jiangxi, Zhejiang) China.Citation35,Citation88
Explicitly, ST is an important public health issue in the Asia-Pacific area.Citation10,Citation89 However, the full extent of the global distribution of this disease outbreak and its burden is poorly understood.Citation90 The disease surveillance system in China was initially established in 1952.Citation80 When only two vectors of O. tsutsugamushi (Leptotrombidium deliense and L. scutellare mites) were known.Citation91,Citation92 After 1989, ST was no longer considered a notifiable infectious disease in China; however, due to recent serious outbreaks, ST has been listed as a notifiable diseases since 2009, and the physicians in China are required by law to report ST cases that are ascertained through the primary health care center, hospital or Chinese CDC.Citation57
While ST is geographically occurring in waves.Citation40,Citation93–95 For example, ST occurs at higher levels in individuals living in rural areas when compared to urban settings (), independently of their sex ().Citation38
Figure 1. The distribution of ST disease by sex (a), and the distribution of ST cases by region (b) in China between 2006 and 2016 (Li et al., 2020).Citation38
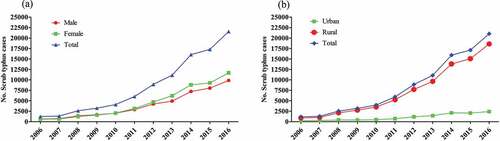
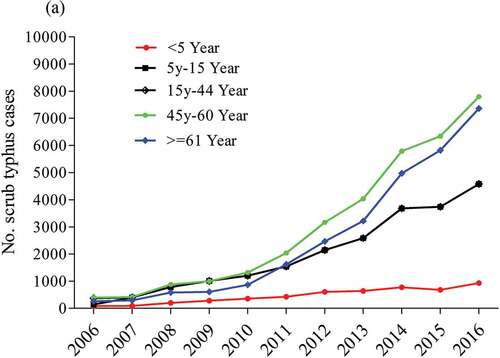
Scrub typhus occurs at higher levels in individuals at age more than 61 years, and lower ST cases occur among children under five years ().Citation38
Figure 2. Age-wise distribution of ST cases stratified by age group in China from 2006 to 2016 (Li et al, 2020).Citation38
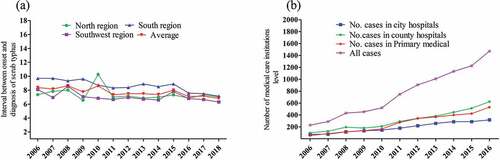
Scrub typhus occurs at higher levels in individuals living in China’s south region compared with cases in the northern part and in the southwest region ().Citation96 ST occurs at higher levels among the number of patients in county hospitals than the number of primary medical care cases ().Citation81
Figure 3. Interval and average between onset and diagnosis of ST cases in high incidence regions in China (a) and the number of medical care institutions reporting ST cases in China between 2006 and 2016 (b) based on data reported by (Yujuan et al., 2020; Xin et al. 2019).Citation77,Citation96
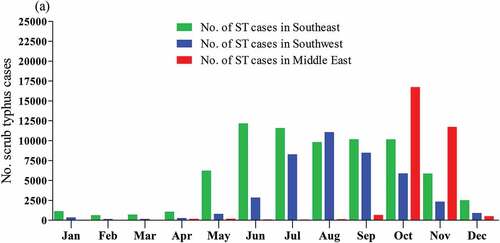
Also, ST occurs at higher seasonal variation levels in China’s Southeast and Southwest regions ()). Notably, the extended peak of ST occurs from May to October every year in southern China.Citation38,Citation77
Figure 4. Analysis of seasonal characteristics of ST the three endemic region (Southeast, Southwest and central East China between 2006 and 2018 based on data reported by (Xin et al. 2019).Citation77
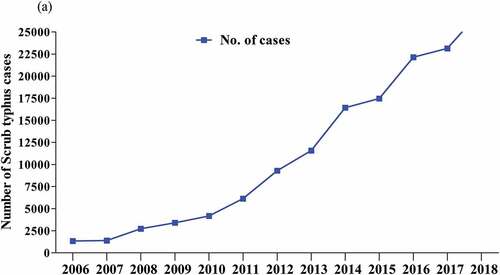
While ST is known to occur in several areas of China,Citation8,Citation97 data have indicated a dramatic increase in the number of cases across the country (), indicating this disease is a growing health problem.Citation9,Citation38,Citation65,Citation81,Citation98 Moreover, the incidence of ST is associated with an adequate temperature, and rainfall,Citation4,Citation99 breeding of rodents and mites, and a combination of climate change and geographic factors, and the expansion of humans.Citation39,Citation64,Citation100 may play roles in reemergence and the occurrence of ST. Indeed, there is a need to better understand the drivers of ST outbreaks in China. This strategy will enhance the capacity for effective bio-surveillance of ST outbreaks and mitigating safeguard public health concerns. Furthermore, a clear understanding of ST status factors will be informative for future prevention and control, including developing a reliable vaccine.
Figure 5. Scrub typhus prevalence in China between 2006 and 2018 based on data reported by (Xin et al. 2019).Citation77
Outbreaks of ST in China
Scrub typhus outbreak was reported in many provinces and cities in China, including Liaoning, Shandong, Fujian, Zhejiang, Jiangsu, and Tianjin. Moreover, ST has been designated an emerging infectious disease in northern China.Citation86 The outbreak has expanded to encompass Guangdong province and the southern parts of Shandong province,Citation71 and northern China.Citation9,Citation34,Citation37 The disease’s seasonality has led to local outbreaks of ST within Guangdong province.Citation11 Notably, ST has also become the most common vector-borne disease in southern China.Citation101,Citation102 Although ST dates back from time immemorial, this disease was not classified as an important disease until the twentieth century by the Chinese CDC Report System.Citation57Furthermore, clinically suspected ST patients are now reported directly to the municipal center or provisional Centers for Disease Control (CDC) for registration and confirmation. In 2011, ST become highly prevalent in southwest China,Citation96 and the cases were reported in many others area in China.Citation4,Citation5,Citation9,Citation70
The multiple complications associated with ST.Citation97 have placed a great burden on public health and the Chinese economy due to its widespread occurrence in recent years.Citation103 Studies conducted on the distribution of ST in China have shown that the majority of cases have occurred between June and November and are clustered in Yunnan, Guangdong, Guangxi, Fujian, and Anhui during this period. Furthermore, ST outbreaks in Guangdong and Yunnan provinces are considered the most serious.Citation87 Additional data have also indicated that the annual incidence rate in Southwest China increased by more than 21-fold from 2006 to 2017.Citation104
On the other hand, ST returning travelers becomes a life-threatening public health issue not only in China but also outside China,Citation105 with required public health interventions.Citation5 It has been reported that people who live in urban areas of China are also traveling to the rural areas to meet with their families member or for leisure and entertainment (e.g., camping, fishing, hiking, picnicking, and picking fruit) on the weekends and holidays. Therefore, these groups susceptible to ST infection spread the infections to non-endemic areas with an increased risk of ST diseases during the frequent outdoor activities in endemic areas. Reported new cases might become a challenge for physician’s inexperience with the ST clinical.Citation57 Therefore, increased ST cases among the urban population. This leads to change ST epidemiological trends in China and call for an enhanced ST disease control and prevention program strategies,Citation38,Citation57 for individuals who are traveling to rural areas of China during the disease endemicity and so that early treatment can be offered to those who return with the infection of ST.
Genetic diversity
Genetic studies of O. tsutsugamushi and a detailed panel of clinical and laboratory profiles of ST cases in China.Citation5,Citation6,Citation8,Citation106,Citation107 have been informative for reconstructing the genetic relationships among bacteria from ST patients in recent years.Citation4,Citation38,Citation108–112 For example, analyses of bacterial genomes has led to more information about bacterial strains in regions where ST outbreaks have occurred.Citation38,Citation111–113
By using molecular biologic approaches, scientists will obtain additional information regarding key bacterial genotypes in patients, rodents, and chiggers in a specific geographic region wherever an outbreak arises. In China,Citation114 many ST cases were caused by the Kawasaki bacterial strain;Citation7 however, Karp, Gilliam, and Kato bacterial types have started to appear in recent years. To date, ST outbreaks caused by the Kawasaki strain have been reported in Jiangsu and Shandong provinces,Citation110,Citation114,Citation115 while the Gilliam, Kato, and Karp strains have been observed mainly in Henan Province.Citation108 Interestingly, the Kawasaki bacterial types’ genetic profile in a patient from Jiangsu province is similar to the genetic profiles of the strain found in domestic rodents and other host species in Shandong province.Citation5,Citation116 The Gilliam strain was reported in Anhui, located in eastern China;Citation11 whereas, the Karp, Kato, Gilliam, and TA763 strains were detected in the Guangdong province located in the southern part of China.Citation6,Citation8
Although ST is considered a life-threatening disease,Citation117 it is still highly neglected and is becoming an important public health issue in ChinaCitation5,Citation8 partly due to its broad geographical distribution, large number of confirmed cases,Citation118,Citation119 and the level of genetic diversity of the causative pathogen.Citation11,Citation38,Citation110,Citation115 More detailed molecular studies are needed to development of prevention and control strategies.Citation5,Citation8 In particular, the analysis of O. tsutsugamushi genotypes and a better understanding of the pathogen’s geographic diversity could provide new information that could contribute to the fight against ST.Citation3,Citation116
Management of ST
Deviation from the disease’s normal clinical manifestations makes it more challenging to accurately diagnose ST, which can delay treatment and lead to more serious complications.Citation89 Thus, timely diagnosis can improve the success rate of treatment. To our knowledge, there is currently no vaccine available that will prevent and/or control infection by O. tsutsugamushi. This reality may be due to the enormous antigenic variation on the cell surface of O. tsutsugamushi strains.Citation120
Major antibiotics, known to be effective against O. tsutsugamushi, include tetracycline, doxycycline, azithromycin, and rifampicin. Among these antibiotics, rifampicin should be considered as the second line treatment option, since its usage can induce resistance to Mycobacterium tuberculosis.Citation121 Furthermore, antimicrobial therapy with doxycycline or azithromycin has been used to treat severe complications, such as gastrointestinal bleeding and cholecystitis, in ST patients, but this treatment course might not be appropriate in all cases.Citation122
Additionally, the failure of clinicians in a primary health care setting to recognize ST as an underlying cause of severe symptoms may lead to the delay in appropriate treatment, increasing the rate of morbidity and mortality among ST positive patients.Citation123–125
While the ST is a bacterial disease, an antibiotics (chloramphenicol, tetracycline, and doxycycline) have been used to treat the disease.Citation121,Citation125,Citation126 However, resistance to these antibiotics has been reportCitation120,Citation126-128 followed by multiple failures and deaths associated with their use.Citation129 Moreover, antibiotics have become a major global health care problem; in particular, antibiotic resistance can develop.Citation130 Indeed, the continuous use of different antibiotics to treat ST can lead to the simultaneous development of multidrug-resistant (MDR) bacterial strains.Citation27,Citation29,Citation131–133 Moreover, doxycycline resistance is argued to have contributed in increased cases’s of ST.Citation127,Citation134,Citation135 Therefore, antibiotic resistance of ST has led to considerations for the improvement of treatment protocols and the need for enhanced public health awareness.Citation75 Lastly, several proteins of O. tsutsugamushi that fall under the category of transporters, enzymes, DNA‐binding, secretory, and outer membrane proteins have been evaluated for their potential use as therapeutic agents that can target their metabolic pathways. To date, about 46 likely proteins essential for the survival of O. tsutsugamushi have been identified as therapeutic targets.Citation132
Conclusion and future vaccine developemts
Scrub typhus is one of the oldest and pernicious diseases in humans. However, the disease is still widely misdiagnosed by local clinicians in settings (e.g. rural regions) where this disease’s public health burden prevails. Therefore, health education programs should be carried out in outbreak zones to raise people’s awareness about the cause, routes of transmission and symptoms of ST. Furthermore, improved prevention and control methods, such as rapid and accurate surveillance followed by a molecular diagnostic test for individuals in affected regions in China, should be implemented. Early diagnosis is also critical for preventing the risk of severe complications and death arising from infection. Indeed, the use of molecular and serological techniques will further strengthen clinicians’ ability to diagnose ST during the initial phase of the illness. Antimicrobial therapy using doxycycline or azithromycin antibiotics in febrile patients before a diagnosis can reduce the morbidity and mortality associated with the disease. However, given the potential for antibiotic resistance, further research should focus on developing new therapeutic agents.
However, over the last 70 years, the development of an effective prophylactic vaccine against ST has been pursued. For example, a novel recombinant antigen derived from a major outer membrane protein was evaluated for its usefulness as a protective vaccine component; results indicate that this recombinant antigen can potentially play a beneficial role in combatting a wide range of O. tsutsugamushi strains.Citation136 In addition, Salmonella-derived extracellular vesicles containing full-length DNA sequences coding for several O. tsutsugamushi proteins were evaluated for their ability to induce T-cell immunity. It is believed that this new vaccine platform can be utilized to maximize T-cell response against O. tsutsugamushi.Citation137 However, despite continuous efforts to develop effective vaccines, this objective remains unfulfilled.Citation32,Citation138
The changing epidemiology of ST in China warrants an enhanced disease control and prevention program. Therefore,Citation38 with absent of vaccine ST, has been become one of the neglected diseases with an emerging and reemerging disease in many provinces in China.Citation104 There is an emphasis on the need to develop a successful ST vaccine for people in endemic areas and the world where more than one million ST cases occur each year. The fact that the different strains of O. tsutsugamushi were a broad wide challenge for future ST vaccine development where the scientific research community should focus on identifying proper immunodominant antigens or combinations, which would bring about long term broad-based protection against ST infection.Citation32
To date, there is still no effective and reliable human vaccine against ST and no point-of-care diagnostics available.Citation90 However, there is no licensed human vaccine to prevent ST infection. Therefore, attention should be paid to ST in mainland China, and effective prevention and control should be taken toward future directions of ST vaccine development. Besides the continuous surveillance of the changing process of epidemiological features and investigation on the pathogenic characteristics of ST are essential for vaccine development and prevention of the disease in China.Citation139
For example, vaccines based on antigens derived from a major outer membrane protein need to be further evaluated to determine its efficacy so that in the coming years they will be commercially available. Such vaccines, if commercialized, will become the backbone of ST prevention all over the world.
Author’s contributions
THM, TA, WL, MNW,KS: Study conception and designed, and data acquisition, and interpreted the data and wrote the first darft of the paper. HHM,RT D, WC,MCC, PW reviewed and editing. All the authors approved the final version for publication.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- Lee HI, Shim SK, Song BG, Choi EN, Hwang KJ, Park MY, Park C, Shin EH. Detection of Orientia tsutsugamushi, the causative agent of scrub typhus, in a novel mite species, Eushoengastia koreaensis, in Korea. Vector-Borne Zoonotic Dis. 2011;11(3):209–14. doi:10.1089/vbz.2009.0180.
- Lv Y, Guo XG, Jin DC. Research progress on Leptotrombidium deliense. Korean J Parasitol. 2018;56(4):313–24. doi:10.3347/kjp.2018.56.4.313.
- Kelly DJ, Fuerst PA, Ching W-M, Richards AL. Scrub typhus: the geographic distribution of phenotypic and genotypic variants of Orientia tsutsugamushi. Clin Infect Dis. 2009;48(Suppl 3):S203–30. doi:10.1086/596576.
- Zhang S, Song H, Liu Y, Li Q, Wang Y, Wu J, Wan J, Li G, Yu C, Li X, et al. Scrub typhus in previously unrecognized areas of endemicity in China. J Clin Microbiol. 2010;48(4):1241–44. doi:10.1128/JCM.01784-09.
- Zhang WY, Wang LY, Ding F, Hu WB, Soares Magalhaes RJ, Sun HL, Liu YX, Liu QY, Huang LY, Clements ACA, et al. Scrub typhus in mainland China, 2006–2012: the need for targeted public health interventions. PLoS Negl Trop Dis. 2013;7(7):e2493. doi:10.1371/journal.pntd.0002493.
- Wei Y, Huang Y, Luo L, Xiao X, Liu L, Yang Z. Rapid increase of scrub typhus: an epidemiology and spatial-temporal cluster analysis in Guangzhou City, Southern China, 2006–2012. PLoS One. 2014;9(7):e101976. doi:10.1371/journal.pone.0101976.
- Zhang L, Zhao Z, Bi Z, Kou Z, Zhang M, Yang L, Zheng L. Risk factors associated with severe scrub typhus in Shandong, northern China. Int J Infect Dis. 2014;29:203–07. doi:10.1016/j.ijid.2014.09.019.
- De W, Jing K, Huan Z, Qiong ZH, Monagin C, Min ZJ, Wen KC, Yan LJ. Scrub typhus, a disease with increasing threat in Guangdong, China. PLoS One. 2015;10(2). doi:10.1371/journal.pone.0113968.
- Hu J, Tan Z, Ren D, Zhang X, He Y, Bao C, Liu D, Yi Q, Qian W, Yin J, et al. Clinical characteristics and risk factors of an outbreak with scrub typhus in previously unrecognized areas, Jiangsu Province, China 2013. PLoS One. 2015;10:e0125999. doi:10.1371/journal.pone.0125999.
- Xu G, Walker DH, Jupiter D, Melby PC, Arcari CM. A review of the global epidemiology of scrub typhus. PLoS Negl Trop Dis. 2017;11(11):e0006062. doi:10.1371/journal.pntd.0006062.
- Cao M, Che L, Zhang J, Hu J, Srinivas S, Xu R, Guo H, Zhang Y, Wang C, Feng Y. Determination of scrub typhus suggests a new epidemic focus in the Anhui Province of China. Sci Rep. 2016;6:20737. doi:10.1038/srep20737.
- Kim HL, Park HR, Kim CM, Cha YJ, Yun NR, Kim DM. Indicators of severe prognosis of scrub typhus: prognostic factors of scrub typhus severity. BMC Infect Dis. 2019;19(1):283. doi:10.1186/s12879-019-3903-9.
- Biswal M, Zaman K, Suri V, Rao H, Kumar A, Kapur G, Sharma N, Bhalla A, Jayashree M. Use of eschar for the molecular diagnosis and genotypic characterisation of Orientia tsutsugamushi causing scrub typhus. Indian J Med Microbiol. 2018;36(3):422–25. doi:10.4103/ijmm.IJMM_18_8.
- Jamil M, Bhattacharya P, Mishra J, Akhtar H, Roy A. Eschar in scrub typhus: a study from north east India. J Assoc Physicians India. 2019;67:38–40. PMID: 31309794.
- Prakash JAJ. Scrub typhus: risks, diagnostic issues, and management challenges. Res Rep Trop Med. 2017;8:73–83. doi:10.2147/rrtm.s105602.
- Watthanaworawit W, Turner P, Turner C, Tanganuchitcharnchai A, Richards AL, Bourzac KM, Blacksell SD, Nosten F. Short report: a prospective evaluation of real-time PCR assays for the detection of Orientia tsutsugamushi and Rickettsia spp. for early diagnosis of rickettsial infections during the acute phase of undifferentiated febrile illness. Am J Trop Med Hyg. 2013;89(2):308–10. doi:10.4269/ajtmh.12-0600.
- Jiang J, Chan TC, Temenak JJ, Dasch GA, Ching WM, Richards AL. Development of a quantitative real-time polymerase chain reaction assay specific for Orientia tsutsugamushi. Am J Trop Med Hyg. 2004;70:351–56. doi:10.4269/ajtmh.2004.70.351.
- Paris DH, Blacksell SD, Newton PN, Day NPJ. Simple, rapid and sensitive detection of Orientia tsutsugamushi by loop-isothermal DNA amplification. Trans R Soc Trop Med Hyg. 2008;102:1239–46. doi:10.1016/j.trstmh.2008.04.040.
- Huber E, Ji D, Howell L, Zhang Z, Chen HW, Ching WM, Chao CC. Loop-mediated isothermal amplification assay targeting the 47-kda gene of orientia tsutsugamushi: a rapid and sensitive alternative to real-time PCR. J Med Microbiol Diagn. 2012;1(112). doi:10.4172/2161-0703.1000112.
- Paris DH, Blacksell SD, Nawtaisong P, Jenjaroen K, Teeraratkul A, Chierakul W, Wuthiekanun V, Kantipong P, Day NP. Diagnostic accuracy of a loop-mediated isothermal PCR assay for detection of Orientia tsutsugamushi during acute scrub typhus infection. PLoS Negl Trop Dis. 2011;5(9):e1307. doi:10.1371/journal.pntd.0001307.
- Qi Y, Yin Q, Shao Y, Cao M, Li S, Chen H, Shen W, Rao J, Li J, Li X, et al. Development of a rapid and visual nucleotide detection method for a Chinese epidemic strain of Orientia tsutsugamushi based on recombinase polymerase amplification assay and lateral flow test. Int J Infect Dis. 2018;70:42–50. doi:10.1016/j.ijid.2018.03.003.
- Chao CC, Belinskaya T, Zhang Z, Ching WM. Development of recombinase polymerase amplification assays for detection of orientia tsutsugamushi or rickettsia typhi. PLoS Negl Trop Dis. 2015;9(7):e0003884. doi:10.1371/journal.pntd.0003884.
- Chao CC, Belinskaya T, Zhang Z, Jiang L, Ching WM. Assessment of a sensitive qPCR assay targeting a multiple-copy gene to detect orientia tsutsugamushi DNA. Trop Med Infect Dis. 2019;4(113). doi:10.3390/tropicalmed4030113.
- Abdad MY, Abdallah RA, Fournier PE, Stenos J, Vasoo S. A concise review of the epidemiology and diagnostics of rickettsioses: rickettsia and orientia spp. J Clin Microbiol. 2018;56(8):e01728–17. doi:10.1128/JCM.01728-17.
- Saraswati K, Day NPJ, Mukaka M, Blacksell SD. Scrub typhus point-of-care testing: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2018;12(3):e0006330. doi:10.1371/journal.pntd.0006330.
- Koh GCKW, Maude RJ, Paris DH, Newton PN, Blacksell SD. Review: diagnosis of scrub typhus. Am J Trop Med Hyg. 2010;82(3):368–70. doi:10.4269/ajtmh.2010.09-0233.
- El Sayed I, Liu Q, Wee I, Hine P. Antibiotics for treating scrub typhus. Cochrane Database Syst Rev. 2018;9(2):CD002150. doi:10.1002/14651858.
- Mehlhorn H, Wu Z, Ye B. Treatment of human parasitosis in traditional Chinese medicine. Springer-Verlag Berlin Heidelberg. 2014;6:eBook. ISBN978-3-642-39824-7. doi:10.1007/978-3-642-39824-7.
- Watt G, Chouriyagune C, Ruangweerayud R, Watcharapichat P, Phulsuksombati D, Jongsakul K, Teja-Isavadharm P, Bhodhidatta D, Corcoran KD, Dasch GA, et al. Scrub typhus infections poorly responsive to antibiotics in northern Thailand. Lancet. 1996;348:9020. doi:10.1016/S0140-6736(96)02501-9.
- Lee K, Lee H, Hong J, Hur J. Roxithromycin treatment of scrub typhus. Roxithromycin treatment of scrub typhus (tsutsugamushi disease) in children. Pediatr Infect Dis J. 2003;22(2):130–33. doi:10.1097/01.inf.0000047864.80791.20.
- Hengbin G, Min C, Kaihua T, Jiaqi T. The foci of scrub typhus and strategies of prevention in the Spring in Pingtan Island, Fujian Province. Ann N Y Acad Sci. 2006;1078:188–96. doi:10.1196/annals.1374.130.
- Chattopadhyay S, Richards AL. Scrub typhus vaccines: past history and recent developments. Hum Vaccin. 2007;3:73–80. doi:10.4161/hv.3.3.4009.
- Shih-Chen I, Pen Ts’ao Kang M. A Chinese material medica of the ming dynasty. 1885. p. 1591–92.
- Liu YX, Feng D, Suo JJ, Xing YB, Liu G, Liu LH, Xiao HJ, Jia N, Gao Y, Yan H, et al. Clinical characteristics of the autumn-winter type scrub typhus cases in south of Shandong province, northern China. BMC Infect Dis. 2009;9:82. doi:10.1186/1471-2334-9-82.
- Zhang M, Wang XJ, Zhao ZT. Current epidemic status and issues on prevention and control of scrub typhus. Zhonghua Liu Xing Bing Xue Za Zhi. 2011;32:419–23. PMID:21569679. https://pubmed.ncbi.nlm.nih.gov/21569679/.
- Bian-Li X, Hao-min C, Jin Z, Sheng-Li X, Meng-lei L, Xiao-guang L, Lei C, Xi-Wu H, Zhen-Cai Z. The epidemiological investigation on the first outbreak of Tsutsugamushi Disease in Henan Province. n.d.. p. 1–3. doi:10.13515/J.cnjpm.2006.03.01. https://en.cnki.com.cn/Article_en/CJFDTOTAL-HNYF200603000.
- Ding L, Li Z, Wang XJ, Ding SJ, Zhang M, Zhao ZT. Analysis of epidemic features of scrub typhus between year 2006 and 2010 in Shandong province, China. Chin J Prev Med. 2012;46(4):S338–342. [Article in Chinese]. https://pubmed.ncbi.nlm.nih.gov/22800633/.
- Li Z, Xin H, Sun J, Lai S, Zeng L, Zheng C, Ray SE, Weaver ND, Wang L, Yu J, et al. Epidemiologic changes of scrub typhus in China, 1952–2016. Emerg Infect Dis. 2020;26(6):1091–101. doi:10.3201/eid2606.191168.
- Yu H, Sun C, Liu W, Li Z, Tan Z, Wang X, Hu J, Shi S, Bao C. Scrub typhus in Jiangsu Province, China: epidemiologic features and spatial risk analysis. BMC Infect Dis. 2018;18:372. doi:10.1186/s12879-018-3271-x.
- Watt G, Parola P. Scrub typhus and tropical rickettsioses. Curr Opin Infect Dis. 2003;16(5):429–36. doi:10.1097/00001432-200310000-00009.
- Suliang C, Cuiyin L, Chunming L, Lingyi K, Yi-Xiu G, Pei-Jun Z. First report of Tsutsugamushi disease in Hebi province: apreliminary study. Chinese J Vector Biol Contr. 2000 [accessed 2021 Jan 20];11(3):213–15. http://www.bmsw.net.cn/EN/abstract/abstract10790.shtml.
- Zhuo F, Yu D, Yu-Chen Z, Wei H, Xiao-guang Y. Clinical analysis of elderly patients with tsutsugamushi disease in Guangzhou area. 2008. p. 819–35. https://en.cnki.com.cn/Article_en/CJFDTOTAL-RDYZ200808024.htm.
- Nakayama K, Yamashita A, Kurokawa K, Morimoto T, Ogawa M, Fukuhara M, Urakami H, Ohnishi M, Uchiyama I, Ogura Y, et al. The whole-genome sequencing of the obligate intracellular bacterium orientia tsutsugamushi revealed massive gene amplification during reductive genome evolution. DNA Res. 2008;15(4):185–99. doi:10.1093/dnares/dsn011.
- Kim SW, Ihn KS, Han SH, Seong SY, Kim IS, Choi MS. Microtubule- and dynein-mediated movement of Orientia tsutsugamushi to the microtubule organizing center. Infect Immun. 2001;69(1):494–500. doi:10.1128/IAI.69.1.494-500.2001.
- Kim MJ, Kim MK, Kang JS. Involvement of lipid rafts in the budding-like exit of Orientia tsutsugamushi. Microb Pathog. 2013;63:37–43. doi:10.1016/j.micpath.2013.06.002.
- Salje J. Orientia tsutsugamushi: a neglected but fascinating obligate intracellular bacterial pathogen. PLoS Pathog. 2017;13(12):e1006657. doi:10.1371/journal.ppat.1006657.
- Katayama T, Hara M, Furuya Y, Nikkawa T, Ogasawara H. Scrub typhus (Tsutsugamushi disease) in Kanagawa Prefecture in 2001–2005. Jpn J Infect Dis. 2006;59(3):207–08. PMID: 16785710.
- Tamura A, Ohashi N, Urakami H, Miyamura S. Classification of Rickettsia tsutsugamushi in a new genus, Orientia gen. nov., as Orientia tsutsugamushi comb. nov. Int J Syst Bacteriol. 1995;45:589–91. doi:10.1099/00207713-45-3-589.
- Walch E. Over trombicula deliensis, n. sp. vermoedelijke over-brengster der Pseudotyphus, en andere Trombiculae van Deli. 1922;62(5):530–88. https://www.cabdirect.org/cabdirect/abstract/19232900994.
- Sharma P, Kakkar R, Kaore SN, Yadav VK, Sharma R. Geographical distribution, effect of season & life cycle of scrub typhus. JK Sci. 2010;12(2):63. http://www.jkscience.org/archive/volume122/GeographicalDistribution,EffectofSeason&LifeCycleScrubTyphus4.pdf.
- Traub R, Wisseman CL. Ecological considerations in scrub typhus. 2. Vector species. Bull World Health Organ. 1968;39(2):219–30. PMID: 5303405; PMCID: PMC2554568. https://www.cabdirect.org/cabdirect/abstract/19692902583.
- Peng PY, Guo XG, Ren TG, Dong WG, Song WY. An updated distribution and hosts: trombiculid mites (Acari: trombidiformes) associated with small mammals in Yunnan Province, southwest China. Parasitol Res. 2016;115(5):1923–38. doi:10.1007/s00436-016-4934-4.
- Shirai A, Saunders JP, Dohany AL, Huxsoll DL, Groves MG. Transmission of scrub typhus to human volunteers by laboratory-reared chiggers. Japanese J Med Sci Biol. 1982;35(1):9–16. doi:10.7883/yoken1952.35.9.
- Lerdthusnee K, Khuntirat B, Leepitakrat W, Tanskul P, Monkanna T, Khlaimanee N, Inlao I, Kengluecha A, Mungviriya S, Chandranoi K, et al. Scrub typhus: vector competence of Leptotrombidium chiangraiensis chiggers and transmission efficacy and isolation of Orientia tsutsugamushi. Ann N Y Acad Sci. 2003;990:25–35. doi:10.1111/j.1749-6632.2003.tb07333.x.
- Gupta V, Gautam V. Scrub typhus - a short review. J Commun Dis. 2004;36:284–89. PMID: 16506552. https://pubmed.ncbi.nlm.nih.gov/16506552/.
- Santibáñez P, Palomar AM, Portillo A, Santibáñez S, Oteo JA. The role of chiggers as human pathogens. An Overv Trop Dis. 2015. doi:10.5772/61978.
- Chinese Center for Disease Control and Prevention.. National guideline of scrub typhus control and prevention. 2009 Jan 05. [accessed 2021 May 31] http://www.chinacdc.cn/tzgg/200901/t20090105_40316.html External Link [in Chinese].
- Wei Y, Huang Y, Li X, Ma Y, Tao X, Wu X, Yang Z. Climate variability, animal reservoir and transmission of scrub typhus in Southern China. PLoS Negl Trop Dis. 2017;11(3):e0005447. doi:10.1371/journal.pntd.0005447.
- James J, Thulaseedharan NK. Scrub typhus. QJM. 2016;109(8):569. doi:10.1093/qjmed/hcw075.
- Jiacan L. Trombiculid mites of China-studies on vector and pathogen of tsutsugamushi disease. 1997. p. 1–57. [in Chinese]. doi:10.1163/_q3_SIM_00374.
- Phasomkusolsil S, Tanskul P, Ratanatham S, Watcharapichat P, Phulsuksombati D, Frances SP, Lerdthusnee K, Linthicum KJ. Transstadial and transovarial transmission of orientia tsutsugamushi in leptotrombidium imphalum and leptotrombidium chiangraiensis (acari: trombiculidae). J Med Entomol. 2009;46(6):1442–45. doi:10.1603/033.046.0628.
- Wei CY, Wang JK, Shih HC, Wang HC, Kuo CC. Invasive plants facilitated by socioeconomic change harbor vectors of scrub typhus and spotted fever. PLoS Negl Trop Dis. 2020;14(1):e0007519. doi:10.1371/journal.pntd.0007519.
- Bingkun X, Xintao C. The relationship between the temperature and the development of Trombicula akamushi var. deliensis. 1960. p. 1–7. doi:10.13343/j.cnki.wsxb.1960.01.009.
- Li T, Yang Z, Dong Z, Wang M. Meteorological factors and risk of scrub typhus in Guangzhou, southern China, 2006–2012. BMC Infect Dis. 2014;14:139. doi:10.1186/1471-2334-14-139.
- Yang LP, Liu J, Wang XJ, Ma W, Jia CX, Jiang BF. Effects of meteorological factors on scrub typhus in a temperate region of China. Epidemiol Infect. 2014;142(10):139:2217–2226. doi:10.1017/S0950268813003208.
- Kim J-H, Cheong H-K. Impacts of climate on the incidence of scrub typhus. Epidemiology. 2009;20(6):S202–203. doi:10.1097/01.ede.0000362680.19801.86.
- Brummaier T, Kittitrakul C, Choovichian V, Lawpoolsri S, Namaik-Larp C, Wattanagoon Y. Clinical manifestations and treatment outcomes of scrub typhus in a rural health care facility on the Thailand-Myanmar border. J Infect Dev Ctries. 2017;11(5):407–13. doi:10.3855/jidc.8912.
- Kim DM, Kim SW, Choi SH, Yun NR. Clinical and laboratory findings associated with severe scrub typhus. BMC Infect Dis. 2010;10(108):1–7. doi:10.1186/1471-2334-10-108.
- Silpapojakul K. Scrub typhus in the western pacific region. Ann Acad Med Singap. 1997;26:794–800.
- Ogawa M, Hagiwara T, Kishimoto T, Shiga S, Yoshida Y, Furuya Y, Aiho I, Ito T, Nemoto H, Yamamoto N, et al. Scrub typhus in Japan: epidemiology and clinical features of cases reported in 1998. Am J Trop Med Hyg. 2002;67(2). doi:10.4269/ajtmh.2002.67.162.
- Zhang M, Zhao ZT, Wang XJ, Li Z, Ding L, Ding SJ. Scrub typhus: surveillance, clinical profile and diagnostic issues in Shandong, China. Am J Trop Med Hyg. 2012;87(6):1099–104. doi:10.4269/ajtmh.2012.12-0306.
- Xiong X, Wang X, Wen B, Graves S, Stenos J. Potential serodiagnostic markers for Q fever identified in Coxiella burnetii by immunoproteomic and protein microarray approaches. BMC Microbiol. 2012;12(1):35. doi:10.1186/1471-2180-12-35.
- Kelly DJ, Richards AL, Temenak J, Strickman D, Dasch GA. The past and present threat of rickettsial diseases to military medicine and international public health. Clin Infect Dis. 2002;34:S145–S169. doi:10.1086/339908.
- Varghese GM, Abraham OC, Mathai D, Thomas K, Aaron R, Kavitha ML, Mathai E. Scrub typhus among hospitalised patients with febrile illness in South India: magnitude and clinical predictors. J Infect. 2006;52(1):56–60. doi:10.1016/j.jinf.2005.02.001.
- Luce-Fedrow A, Lehman ML, Kelly DJ, Mullins K, Maina AN, Stewart RL, Ge H, John HS, Jiang J, RichardsAL A. Review of Scrub Typhus (Orientia tsutsugamushi and Related Organisms): then, now, and tomorrow. Trop Med Infect Dis. 2018;3(1):8. doi:10.3390/tropicalmed3010008.
- Karthikeyan PA, Hoti SL, Kanungo R. Evaluation of loop-mediated isothermal amplification assay for detection of scrub typhus in patients with acute febrile illness presenting to a Tertiary Care Center in Puducherry, India. J Lab Physicians. 2019;11(1):82–86. doi:10.4103/jlp.Jlp_148_18.
- Xin HL, Yu JX, Hu MG, Jiang FC, Li XJ, Wang LP, Huang JL, Wang IF, Sun JL, Li ZJ. Evaluation of scrub typhus diagnosis in China: analysis of nationwide surveillance data from 2006 to 2016. Infect Dis Poverty. 2019;8:59. doi:10.1186/s40249-019-0566-0.
- Kim MH, Kim SH, Choi JH, Wie SH. Clinical and laboratory predictors associated with complicated scrub typhus. Infect Chemother. 2019;51(2):161–70. doi:10.3947/ic.2019.51.2.161.
- Osuga K, Kimura M, Goto H, Shimada K, Suto T. A case of tsutsugamushi disease probably contracted in Africa. Eur J Clin Microbiol Infect Dis. 1991;10:95–96. doi:10.1007/BF01964418.
- Fan MY, Walker DH, Yu SR, Liu QH. Epidemiology and ecology of rickettsial diseases in the People’s Republic of China. Rev Infect Dis. 1987;9:823–40. doi:10.1093/clinids/9.4.823.
- Wei Y, Luo L, Jing Q, Li X, Huang Y, Xiao X, Liu L, Wu X, Yang Z. A city park as a potential epidemic site of scrub typhus: a case-control study of an outbreak in Guangzhou, China. Parasit Vectors. 2014;18(7):513. doi:10.1186/s13071-014-0513-7.
- Sun WS, Ki TK, Young MK, Seog HP, Young SK, Lee DG, Yoon SA, Kim YO. Clinical role of interstitial pneumonia in patients with scrub typhus: a possible marker of disease severity. J Korean Med Sci. 2004;19(5):668–73. doi:10.3346/jkms.2004.19.5.668.
- Xin H, Sun J, Yu J, Huang J, Chen Q, Wang L, Lai S, Clements AC, Hu W, Li Z. Spatiotemporal and demographic characteristics of scrub typhus in Southwest China, 2006–2017: an analysis of population-based surveillance data. Transbound Emerg Dis. 2020;67(4). doi:10.1111/tbed.13492.
- Cordier C, Tattevin P, Leyer C, Cailleaux M, Raoult D, Angelakis E. Rickettsia sibirica mongolitimonae infection, Sri Lanka. J Infect Dev Ctries. 2017;11(8):668–71. doi:10.3855/jidc.8743.
- Wu YC, Qian Q, Magalhaes RJS, Han ZH, Haque U, Weppelmann TA, Hu WB, Liu YX, Sun YS, Zhang WY, et al. Rapid increase in scrub typhus incidence in Mainland China, 2006–2014. Am J Trop Med Hyg. 2016;94:532–36. doi:10.4269/ajtmh.15-0663.
- Kim DE, Lee SH, Park KI, Chang KH, Roh JK. Scrub typhus encephalomyelitis with prominent focal neurologic signs. Arch Neurol. 2000;5757(12):1770–72. doi:10.1001/archneur.57.12.1770.
- Ye S, Liqun F, Wuchun C. Study on the epidemiological characteristics and influencing factors of scrub typhus in the autumn-winter natural foci, from 2006 to 2013. Zhonghua Liu Xing Bing Xue Za Zhi. 2016;37(8):1112–16. doi:10.3760/cma.j..0254-6450.2016.08.012.
- Yue Y, Ren D, Liu X, Wang Y, Liu Q, Li G. Spatio-temporal patterns of scrub typhus in mainland China, 2006–2017. PLoS Negl Trop Dis. 2019;13:e0007916. doi:10.1371/journal.pntd.0007916.
- Guichang L, Dongmei L, Yan L, Yujuan Y, Xiaobo L, Yuhong G, Liang L, Qiyong L. Epidemiology of scrub typhus in China, 2006–2016. Dis Surveillance. 2018;33(2):139–43. doi:10.3784/j..1003-9961.2018.02.007.
- Bonell A, Lubell Y, Newton PN, Crump JA, Paris DH. Estimating the burden of scrub typhus: a systematic review. PLoS Negl Trop Dis. 2017;11(9). doi:10.1371/journal.pntd.0005838.
- Dun‐Qing W, Zi‐Zhong Y. Chigger mites of the genus Leptotrombidium: key to species and their distribution in China. Med Vet Entomol. 1992;6(4):389–95. doi:10.1111/j.1365-2915.1992.tb00639.x.
- Luo Y, Yin J. Progress in research of Orientia tsutsugamushi and its host and vector. Dis Surveillance. 2019;34(10):920–33. doi:10.3784/j.1003–9961.2019.10.013.
- Chen XR. Scrub typhus and O. tsutsugamushi. Beijing, China: Military Medical Science Press, 2001. p. 176–179.
- Liu Y, Yang Z, Wu Q, Sun H, Peng Z, Miao Z, Meng X. Epidemiological study of autumn-winter type scrub typhus in a new endemic focus of Fei County, Shandong Province, China. Syst Appl Acarol. 2000;5(1):25–31. doi:10.11158/saa.5.1.3.
- Jie Z, De-chang L, Zhi-jian L. Clinical features of tsutsugamushi disease among children and adults in north of Guangdong province. Acta Med Sin. 2006;19:508–09. https://en.cnki.com.cn/Article_en/CJFDTOTAL-GLYX200604009.htm.
- Yujuan Y, Yujiao W, Guichang L, Xingzhou L, Jun W, Qiyong L. Epidemiological characteristics of scrub typhus in high-incidence areas in the mainland of China, 2006–2018. Dis Surveillance. 2020;35(4):301–06. doi:10.3784/j..1003.
- Van Peene Lien PFDJC, Santana FJ, See R. Correlation of chigger abundance with temperature at a hyperendemic focus of scrub typhus. J Parasitol. 1976;62(4):653–54. doi:10.2307/3279442.
- Premaratna R, Chandrasena TG, Dassayake AS, Loftis AD, Dasch GA, De Silva HJ. Acute hearing loss due to scrub typhus: a forgotten complication of a reemerging disease. Clin Infect Dis. 2006;42(1):e6–8. doi:10.1086/498747.
- Wu YC, Qian Q, Soares Magalhaes RJ, Han ZH, Hu WB, Haque U, Weppelmann TA, Wang Y, Liu Y, Li X, et al. Spatiotemporal dynamics of scrub typhus transmission in Mainland China, 2006–2014. PLoS Negl Trop Dis. 2016;10(8):e0004875. doi:10.1371/journal.pntd.0004875.
- Liu J, Li S, Ouyang Z, Tam C, Chen X. Ecological and socioeconomic effects of China’s policies for ecosystem services. Proc Natl Acad Sci U S A. 2008;105(28):9477–82. doi:10.1073/pnas.0706436105.
- Cao M, Guo H, Tang T, Wang C, Li X, Pan X, Tang J. Spring scrub typhus, People’s Republic of China [5]. Emerg Infect Dis. 2006;12(9):1463–65. doi:10.3201/eid1209.060257.
- Deshpande GA, Mittal R, Jesudasan MR, Perakath B. Surgical manifestations of scrub typhus: a diagnostic dilemma. Natl Med J India. 2015;28(1):12–13. PMID: 26219315.
- Li W, Dou X, Zhang L, Lyu Y, Du Z, Tian L, Zhang X, Sun Y, Guan Z, Chen L, et al. Laboratory diagnosis and genotype identification of scrub typhus from pinggu district, beijing, 2008 and 2010. Am J Trop Med Hyg. 2013;89(1):123–29. doi:10.4269/ajtmh.12-0728.
- Ren J, Sun J, Wang Z, Ling F, Shi X, Zhang R, Liu Y, Chen Z, Chen E. Re-emergence of scrub typhus in Zhejiang Province, southern China: a 45-year population-based surveillance study. Travel Med Infect Dis. 2019;32:101427. doi:10.1016/j.tmaid.2019.05.013.
- Watt G, Strickman D. Life-threatening scrub typhus in a traveler returning from thailand. Clin Infect Dis. 1994;18(4):624–26. doi:10.1093/clinids/18.4.624.
- Liu YX, Jia N, Suo JJ, Xing YB, Liu G, Xiao HJ, Jia Z, Min SJ, Feng TP, Ma BS, et al. Characteristics of pediatric scrub typhus in a new endemic region of Northern China. Pediatr Infect Dis J. 2009;28(12). doi:10.1097/INF.0b013e3181af8287.107.
- Evans SM, Adcox HE, VieBrock L, Green RS, Luce-Fedrow A, Chattopadhyay S, Jiang J. Outer membrane protein A conservation among orientia tsutsugamushi isolates suggests its potential as a protective antigen and diagnostic target. Trop Med Infect Dis. 2018;3(2):63. doi:10.3390/tropicalmed3020063.108.
- Jianping L, Weiping C, Jian W. Clinical characteristics of 30 case of tsutsugamushi disease. Infect Dis Info. 2009;14:8–11.
- Zha Z, Jia_bing W, Hong L, Dong-qing Y. Clinical and epidemiological investigation of 175 tsutsugamushi disease cases. Chinese J Dis Contr Prev. 2010;14:720–22. [in Chinese]. http://en.cnki.com.cn/Article_en/CJFDTOTAL-JBKZ201008012.htm.
- Liu YX, Gao Y, Zhao ZT, Zhang JL, Yang ZQ, Bu XP, Su JJ. Amplification and typing of Sta56 gene of Orientia tsutsugamushi from Shandong province. Zhonghua Liu Xing Bing Xue Za Zhi. 2004;25(8):698–701. https://pubmed.ncbi.nlm.nih.gov/15555396/.
- Yang LP, Zhao ZT, Li Z, Wang XJ, Liu YX, Bi P. Comparative analysis of nucleotide sequences of Orientia tsutsugamushi in different epidemic areas of scrub typhus in Shandong, China. Am J Trop Med Hyg. 2008;8(6):968–72. doi:10.4269/ajtmh.
- Zhang LJ, Li XM, Zhang DR, Zhang JS, Di Y, Luan MC, Fu X. Molecular epidemic survey on co-prevalance of scrub typhus and marine typhus in Yuxi city, Yunnan province of China. Chin Med J (Engl). 2007;120(15). doi:10.1097/00029330-200708010-00004.
- Yun XL, Zhao ZT, Gao Y, Jia CQ, Zhang JL, Yang ZQ, Wang SM, Jinag BF. Characterization of Orientia Tsutsugamushi strains isolated in shandong province, China by immunofluorescence and restriction fragment length polymorphism (RFLP) analyses. Southeast Asian J Trop Med Public Health. 2004;35(2):353–57. PMID: 15691135.
- Zheng L, Yang HL, Bi ZW, Kou ZQ, Zhang LY, Zhang AH, Zhang LY, Yang L, Zhao TZ. Epidemic characteristics and spatio-temporal patterns of scrub typhus during 2006–2013 in Tai’an, Northern China. Epidemiol Infect. 2015;143(11):2451–58. doi:10.1017/S0950268814003598.
- Kim G, Ha NY, Min CK, Kim HI, Yen NTH, Lee KH, Oh I, Kang SJ, Choi SM, Kim SI, et al. Diversification of Orientia tsutsugamushi genotypes by intragenic recombination and their potential expansion in endemic areas. PLoS Negl Trop Dis. 2017;11(3):e0005408. doi:10.1371/journal.pntd.0005408.
- Ren_Jie J, Jin-Jin S, Yan_zhu Z, Hongjun Z, Hengbin G, Shouyin Z, Oh I, Kang JS, Choi MS, Kim IS, et al. Epidemiological study of scrub typhus disease in Yancheng city during 2006 to 2010. J Med Pest Control. 2011;27:1079–81.
- Paris DH, Shelite TR, Day NP, Walker DH. Review article: unresolved problems related to scrub typhus: a seriously neglected life-threatening disease. Am J Trop Med Hyg. 2013;89:301–07. doi:10.4269/ajtmh.13-0064.
- Soong L. Dysregulated Th1 immune and vascular responses in scrub typhus pathogenesis. J Immunol. 2018;200(4):1233–40. doi:10.4049/jimmunol.1701219.
- Mahajan SK. Scrub typhus. J Assoc Physicians India. 2005:954–58. https://pubmed.ncbi.nlm.nih.gov/16515236/.
- Kim DM, Yun RN, Yang YT, Lee HJ, Yang TJ, Shim KS, Choi NE, Park YM, Lee HS. Usefulness of nested PCR for the diagnosis of scrub typhus in clinical practice: a prospective study. Am J Trop Med Hyg. 2006;75(3):542–45. doi:10.4269/ajtmh.2006.75.542.121.
- Panpanich R, Garner P. Antibiotics for treating scrub typhus. Cochrane Database Syst Rev. 2002:3. doi:10.1002/14651858.cd002150.122.
- Jang MO, Jang HC, Kim UJ, Ahn JH, Kang SJ, Jung SI, Shin HY, Park KH. Outcome of intravenous azithromycin therapy in patients with complicated scrub typhus compared with that of doxycycline therapy using propensity-matched analysis. Antimicrob Agents Chemother. 2014;58(3):1488–93. doi:10.1128/AAC.01996-13.
- Mokta J, Ranjan A, Mokta K. Early clinical suspicion and early use of doxycycline reduces scrub typhus associated complications. J Assoc Physicians India. 2019;67:26–27. https://www.japi.org/article/files/early_clinical_suspicion_and_early_use.pdf.
- Crecelius EM, Burnett MW. Scrub Typhus. J Spec Oper Med. 2020;20(1):120–22. doi:10.5958/2454-2660.2019.00066.8.
- Sheehy TW, Hazlett D, Turk RE. Scrub typhus: a comparison of chloramphenicol and tetracycline in its treatment. Arch Intern Med. 1973;132(1):77–80. doi:10.1001/archinte.132.1.77.
- Brown GW, Saunders JP, Singh S, Huxsoll DL, Shirai A. Single dose doxycycline therapy for scrub typhus. Trans R Soc Trop Med Hyg. 1978;72(4):412–16. doi:10.1016/0035-9203(78)90138-4.
- Strickman D, Sheer T, Salata K, Hershey J, Dasch G, Kelly D, Kuschner R. In vitro effectiveness of azithromycin against doxycycline-resistant and - susceptible strains of Rickettsia tsutsugamushi, etiologic agent of scrub typhus. Antimicrob Agents Chemother. 1995;39(11):2406–10. doi:10.1128/AAC.39.11.2406.
- Thipmontree W, Tantibhedhyangkul W, Silpasakorn S, Wongsawat E, Waywa D, Suputtamongkol Y. Scrub typhus in northeastern Thailand: eschar distribution, abnormal electrocardiographic findings, and predictors of fatal outcome. Am J Trop Med Hyg. 2016;95(4):769–73. doi:10.4269/ajtmh.16-0088.
- Liu YX, Jia N, Xing YB, Suo JJ, Du MM, Jia N, Jia N, Gao Y, Xie LJ, Liu BW, et al. Consistency of the key genotypes of orientia tsutsugamushi in scrub typhus patients, rodents, and chiggers from a new endemic focus of Northern China. Cell Biochem Biophys. 2013;67:1461–66. doi:10.1007/s12013-013-9646-0.
- Mathai E, Rolain JM, Verghese GM, Abraham OC, Mathai D, Mathai M, Raoult D. Outbreak of scrub typhus in southern India during the cooler months. Ann N Y Acad Sci. 2003;990:359–64. doi:10.1111/j.1749-6632.2003.tb07391.x.
- Smadel JE. Influence of antibiotics on immunologic responses in scrub typhus. Am J Med. 1954;17(2):246–58. doi:10.1016/0002-9343(54)90262-4.
- Sharma D, Sharma A, Verma SK, Singh B. Targeting metabolic pathways proteins of Orientia tsutsugamushi using combined hierarchical approach to combat scrub typhus. J Mol Recognit. 2019;32(4):e2766. doi:10.1002/jmr.2766.
- Alanis AJ. Resistance to antibiotics: are we in the post-antibiotic era? Arch Med Res. 2005;36(6):697–705. doi:10.1016/j.arcmed.2005.06.009.
- Corwin A, Soderquist R, Suwanabun N, Sattabongkot J, Martin L, Kelly D, Beecham J. Scrub typhus and military operations in Indochina. Clin Infect Dis. 1999;4(15):940–41. doi:10.1086/520468.
- Harris PNA, Oltvolgyi C, Islam A, Hussain-Yusuf H, Loewenthal MR, Vincent G, Stenos J, Graves S. An outbreak of scrub typhus in military personnel despite protocols for antibiotic prophylaxis: doxycycline resistance excluded by a quantitative PCR-based susceptibility assay. Microbes Infect. 2016;18(6). doi:10.1016/j.micinf.2016.03.006.
- Kim HI, Ha NY, Kim G, Min CK, Kim Y, Yen NTH, Choi SM, Cho HN. Immunization with a recombinant antigen composed of conserved blocks from TSA56 provides broad genotype protection against scrub typhus. Emerg Microbes Infect. 2019;8(1):946–58. doi:10.1080/22221751.2019.1632676.
- Cho H, Lee WH, Kim YK, Kim KS. Extracellular vesicle-associated antigens as a new vaccine platform against scrub typhus. Biochem Biophys Res Commun. 2020;523(3):602–07. doi:10.1016/j.bbrc.2020.01.014.
- Card WI, Walker JM. Scrub-typhus vaccine field trial in South-East Asia. Lancet. 1947;249(6450):481–83. doi:10.1016/S0140-6736(47)91989-2.
- Zhang L, Bi Z, Kou Z, Yang H, Zhang A, Zhang S, Meng X, Zhng L, Zhang M, Yang H, et al. Scrub typhus caused by Orientia tsutsugamushi Kawasaki-related genotypes in Shandong Province, northern China. Infect Genet Evol J Mol Epidemiol Evol Genet Infect Dis. 2015;30:238–43. doi:10.1016/j.meegid.2014.12.036.
