ABSTRACT
SARS-CoV2 infection induces various degrees of infections ranging from asymptomatic to severe cases and death. Virus/host interplay contributes substantially to these outcomes. This highlights the potential roles of the host immune system in fighting virus infections. SARS-CoV-2. We highlighted the potential roles of host immune response in the modulation of the outcomes of SARS-CoV infections. The newly emerged SARS-CoV-2 mutants complicated the control and mitigation strategies measures. We are highlighting the current progress of some already deployed vaccines worldwide as well as those still in the pipelines. Recent studies from the large ongoing global vaccination campaign are showing promising results in reducing the hospitality rates as well as the number of severe SARS-CoV-2 infected patients. Careful monitoring of the genetic changes of the virus should be practiced. This is to prepare some highly sensitive diagnostic assays as well as to prepare some homologous vaccines matching the circulating strains in the future.
Introduction
The coronavirus infectious disease-19 (COVID-19) is a newly emerged coronavirus discovered in late 2019Citation1. The etiology of this syndrome is severe acute respiratory syndrome-2 (SARS-CoV-2).Citation2 The WHO declared that SARS-CoV-2 is the causative agent of the recent modern pandemic across the globe on March 11th, 2020.Citation3 SARS-CoV-2 belongs to the subgenus Sarbecovirus, subfamily-Orthocoronavirinae within the family Coronaviridae, and the order Nidovirales. This is the seventh identified human coronavirus. The genome structure and organization of SARS-CoV is very much similar to other coronaviruses, belongs to the Betacoroanviruses along with some other important zoonotic coronaviruses (MERS-CoV and the SARS-CoV). However, the genome sequencing analysis revealed some variation among those viruses across their genomes, particularly the spike glycoprotein (S). The virus genome is a linear single-strand RNA molecule (~ 30-kilobases) in length. The 5ʹtwo-third of the viral genome encodes the non-structural proteins, which are responsible for replication and pathogenesis, immune evasion properties of viruses. While, the 3ʹ third of the viral genome encodes four essential proteins (spike glycoprotein (S), the envelope, the membrane (M), and the nucleocapsid proteins (N)) interspersed by some minor non-structural proteins. Some of these proteins play essential roles in the virus replication cycle, immune response, and immune evasion strategies possessed by the virus to hijack the host immune response. The course of SARS-CoV infection may be developed into three subsequent stages. This classification is mainly based on the clinical signs and the viral loads in the collected samples from tested patients.Citation4 During the first stage, the infected individual does not show any visible clinical signs and may have little or no viral load in their secretions. In the second stage, the patient starts to show apparent clinical manifestations with moderate viral loads detected in their secretions. In the final third stage, the affected individuals suffered from severe conditions, including the involvement of the lungs and many other organs (kidneys and intestine).Citation4 The outcomes of the infection are mainly fine-tuned by the immune status of the infected patients. The host immune response for the SARS-CoV-2 is developed in two phases.Citation5 The first phase is triggered during the acute stage of the infection to enhancing the immune response in overcoming the viral infection. While the second phase started with the progression of the inflammatory reactions and damage of the target organs which may lead to the death of the infected patient.Citation5
The biology of the SARS-CoV-2 is contributing to the control and mitigation of the virus infection and pathogenesis
SARS-CoV-2-S protein is one of the leading viral proteins that play essential roles in the virus cycle, identification of the viral tropism, especially during the virus attachment, and the procedure of viral entry into the cell. It is one of the main targets for the vaccine, therapy, and diagnostic assays for most coronaviruses, including SARS-CoV-2. The viral cycle starts with the attachment of the spike glycoprotein with the viral receptors, the angiotensinogen converting enzyme-2 (ACE-2). Some other cellular factors favor virus entry, such as basigin (BSG). This is a newly discovered mechanism for the SARS-CoV-2 entry (CD147-SP).Citation6 The S protein is inert outside the host, which undergoes activation through the furin enzyme cleavage into the S1 and S2 subunits.Citation7 This step is crucial for the success of SARS-CoV-2 infection on the cellular level. Blocking the cleavage of the spike glycoprotein will inhibit the virus infection, thus representing an attractive potential target for drug design. The furin enzymes are highly expressed in the respiratory tract; thus, once the virus exits from one cell, it will be ready to enter another cell and potentiate the virus replication.Citation8 Preventing the SARS-CoV-2 virus attachment to the host cells can be done by several strategies. Using some fusion protein inhibitors is a promising trend in halting the SARS-CoV and MERS-CoV infection both in vitro and in vivo in mouse models.Citation9 Another important host factor is the transmembrane protease, serine 2 (TMPRSS2), which potentiates the viral infection by exposing the active sites of the spike glycoprotein to interact with the host cell and mediates the virus entry.Citation10 Using the TMPRSS2 inhibitors recently proved to prevent the SARS-CoV-2 entry into the target cells. This is a new promising trend in the treatment of SARS-CoV-2.Citation10 Another essential protein is the main protease -chymotrypsin-like cysteine protease (3CLpro). It plays an essential role during coronavirus replication. The sequence of this protein is most likely highly conserved among most coronaviruses. Using some 3 CL pro inhibitors such as ledipasvir showed promising results in the inhibition of the SARS-CoV-2 replication.Citation11 This is one of the promising therapeutic approaches for COVID-19 with minimal side effects.Citation12 The N protein binds to the viral nucleic acids to form the nucleocapsid from which it acquires its name. This protein plays some essential roles during virus replication.Citation13 This protein is highly phosphorylated, thus potentiates the binding with the viral RNA compared to other RNA molecules.Citation14 The immune response plays an essential role in fine-tuning the outcomes of any infection with different pathogens. There are two arms of the immune response; the innate and the adaptive responses. In the following sections, we will elaborate on some recent advances on the immune response against SARS-CoV-2, including the humoral as well as the cell-mediated immunity.
The adaptive immune response in the context of SARS-CoV2 infection
Typically, the adaptive immune response plays an important role in controlling and clearing several viral infections.Citation15 There are two types of reactions involved in adaptive immunity, including the cellular immune response, which is mediated by the T cells, and the humoral immune response, which is an antibody-mediated response.
The T cells mediated immune response
During the coronavirus infection, the antigen-presenting cells (APC), such as dendritic cells (DCS), can pick up the viral peptides and presented them in the context of the major histocompatibility (MHC) class-2. Subsequently, the CD4 + T cells (T helper) can recognize these peptides and then subsequently being activated. Therefore, they produce several types of cytokines and chemokines. The cytokines produced by the CD4-T cells including several subsets (Th1, Th2, Th17, and T regulatory cells (T-regs)). The Th1 cells produce IFN-γ, whereas the Th2 cells produce some cytokines such as IL-4, IL-5, and IL-13 (). The T-reg cells usually produce some suppressive cytokines such as IL-10 and TGF-β. They have an essential role in the regulation and maintaining the immune response and therefore preventing autoimmunity.Citation16–18 Th17 producing some cytokines, including IL-17, IL-21, and IL-22.Citation19 Furthermore, the CD4 + T cells are activating the CD8 + T cells by providing a co-stimulatory signal.Citation20 This stimulation is achieved through the interaction between the CD40L and the CD40 on the DCS. This interaction induces an up-regulation of some ligands, including CD80 and CD86. Both are expressed by the DCs, which came in contact with the CD28 expressed by the naive CD8 T cells.Citation21 CD4 + T cells stimulate the B cells to produce some specific antibodies.Citation22 Furthermore, the CD8 T cells can destroy the virus-infected cells.Citation22 It has been shown that in the case of SARS-CoV patients, the CD8 T cells play an important role in the destruction and clearance of the infected cells within the pulmonary interstitium tissues.Citation23 Moreover, the MERS-CoV infection of mice deficient in both T and B cells showed that the virus was not cleared from the lungs in the absence of T cells compared to control animals. This suggested the importance of T cells in the clearance of coronavirus infections.Citation24 SARS-CoV infection in some CD8 deficient mice did not affect the viral load. Whereas depletion of the CD4 T cells resulted in the delay in the viral clearance and was associated with a marked reduction in the cytokines production. It also reduced the production of the viral-specific neutralizing antibodies as wells as a marked reduction in the recruitment of lymphocytes into the lung tissues. This suggests the pivotal role of the CD4 rather than the CD8 during the early stages of the SARS-CoV infection.Citation25 One study conducted on 128-SARS-CoV survived individuals revealed the convalescent samples showed that the CD8 + T cell was higher and more prominent than the CD4 + T cell. The severely affected individuals developed higher levels of central memory T cells and were associated with a higher functional activity of both CD4 and CD8-T cells. This pattern was in comparison to the mildly and moderately affected group of patients who showed a strong T cells response in about 70% of cases. This is in addition to, high level of specific antibodies against most of the structural proteins of the virus, including (S, E, M, and N). However, the Th2 cytokines, including IL-4, IL-5, and IL-10, were highly expressed in more severe and fatal cases.Citation26 Surprisingly, the role of Th17 during the SARS-CoV infection was not fully defined yet. However, the IL-17 levels were elevated in the serum of some COVID-19 patients.Citation27 The SARS-CoV-2 infection triggered an exaggerated immune response in the infected patients called cytokines storm () or macrophage activation syndrome (MAS).Citation28 It is may also be known as secondary haemophagocytic lymphohistiocytosis (sHLH).Citation29 This condition may lead to acute respiratory distress syndrome (ARDS), which contributed substantially to the outcomes of the virus infection in diseased individuals.Citation28
Cytokine storm and the severity of COVID-19
Moreover, there is a direct relationship between the cytokines storm and the severity of COVID-19 infections and fatal outcomes.Citation30 During severe COVID-19 cases, both IL-6 and IL-1 levels are dramatically increased compared to the mild or moderate groups of patients. This pattern suggests the important roles of this cytokine as a marker for the severity of clinical cases. It may also have used in assessment and the prognosis of the SARS-CoV-2 infection.Citation31,Citation32 Therefore, targeting IL-6 or its receptor (IL-6 R) with specific monoclonal antibodies, such as (Tocilizumab, Siltuximab) may result in reducing the severity of the airway inflammation and consequently reduce the severity of the disease.Citation33 Further studies are required in order to enrich our understanding of the interaction between SARS-CoV2 and the host cells. This may lead us to achieve better knowledge in regard to the cytokines response mediate by the host-infected cells and how may the virus escape from the immune recognition. Moreover, one study was conducted on the peripheral blood mononuclear cells (PBMCs) isolated from SARS-CoV recovered individuals. This study showed that SARS-specific memory T cells against most viral structural proteins, including membrane (M) and Nucleocapsid (N) protein, were detectable up to 11 years after SARS-CoV infection. These findings suggestion the SARS-CoV recovered patients may be protected against the subsequent re-infection with homologs viral strain.Citation34 This may support the theory of vaccination against SARS-CoV as an important aid in the prophylaxis of such viruses and their related viruses.
Both SARS-Co-V and SARS-CoV-2 can directly infect both the macrophages and the T cells, which contributed substantially to the pathogenicity of these viruses.Citation33,Citation35 Recent studies on some SARS-CoV-2 patients showed that the number of both CD4 + T and CD8 + T in the peripheral blood sharply decreased, although their status remained active, and the CD8 + T cells are becoming more cytotoxic.Citation36 Thus, there is an urgent need to fine-tune and control the CD8 T cell responses to minimize and avoid as much as possible any lung tissue damage and injury. Additionally, it has been revealed that the number of the T cells was decreased in the case of the severely affected SARS-CoV-2 patients, including the TH and the T reg cells. Whereas, the percentage of naïve Th cells was increased, and the memory Th was decreased in severe cases of SARS-CoV-2 infection.Citation37 T cells were reported to express the SARS-CoV-2 receptors, such as ACE-2 and CD147.Citation33,Citation37 This might be one of the main reasons behind the dramatic decrease in the total numbers of T-cells count in the case of the severely affected SARS-CoV-2 patients.
Mapping and identification of neutralizing epitopes in SARS-CoV-2 and its applications in the vaccines and diagnostic assays development
During MERS-CoV infection, the CD8-T-cell production was largely increased during the early stage of the infection and was usually associated with severe cases of infection; however, during the recovery phase, the Th1 cell was notably observed.Citation38 Interestingly, both SARS-CoV and MESR-CoV can produce specific CD4 memory cells against some conserved epitopes shared by both viruses. Therefore, mice were protected against the challenge of the wild types of these viruses. These shared epitopes induce neutralizing antibodies that can cross-react with MESR-CoV and SARS-CoV, suggesting the potential role of these conserved peptides as vaccines against most of the coronaviruses.Citation39 Since most of these epitopes recognized the antigenic counterparts within most structural proteins of both SARS-CoV and MERS-CoV, a similar approach may be adopted in the case of the SARS-CoV-2. Mapping these neutralizing epitopes within the three viruses (SARS-CoV, MERS-CoV, and SARS-CoV-2) may pave the way for several potential vaccines against SARS-CoV-2. In a similar trend, it may highlight the promising trends of using serum and plasma of the recovered patients as potential therapeutic interventions against SARS-CoV-2 infection. A new study showed a promising trend of using some single antibody domains isolated from Illama immunized with the coronaviruses spike protein in vitro.Citation40 These potent antibodies protected the cells from infection with the SARS-CoV-2 infection by preventing the virus entry into the cells.Citation40
In conclusion, although the three coronavirus candidates (SARS-CoV, MERS-CoV, and SARS-CoV-2) are belonging to the same family, there is a differential display of their downstream immunological profiles, which contribute to the severity and outcomes of the viral infection for each virus.
The B cells mediated immune response
The B cell immune response plays key role in the protection against the subsequent SARS-CoV-2 infection through the production of viral-specific antibodies.Citation41 These antibodies were able to neutralize the viral infectivity, therefore, prevent the virus attack to the host cells.
Although few serological studies were conducted on the SAR-CoV-2 at this time, some studies were conducted to monitor the antibody levels in some patients. These studies showed that the specific IgM antibody reached its peak 9-day post-infection. While the IgG level increased in about two weeks and lasted up to 20 days after infection. The serum containing antibodies collected from confirmed COVID-19 patients appeared to cross-reactive with SARS-CoV and not with other coronaviruses members. Moreover, in vitro studies on sera from those patients were also able to neutralize the SARS-CoV infectivity in cell culture. This suggests cross-reactivity between both SARS-CoV and SARS-CoV-2.Citation33 Another study was conducted on one patient infected with SARS-CoV-2 to monitor the curve of neutralizing antibodies of IgM and IgG classes against (S) and (N) proteins of SARS-CoV. This study revealed that the level of these antibodies in sera of this patient was low on day four after infection, which increased at day 9, then dropped to non-detectable levels on day 20 in the case of IgG.Citation42 In the same context, another independent study also reported that at day 0, the titters of the IgM and the IgG were undetectable or even very low then increased by the fifth day of infection, whereas the IgG levels were increased in all the patients.Citation43 However, in the case of SARS-CoV infection, the starting of the antibody production against the virus is varied among different classes of antibodies. In the case of IgM, it ranged from 3 to 42 days post-infection. Whereas in the case of the IgG, it ranges from 5 to 47 days.Citation44 In conclusion, there is a differential display in the kinetic of various classes of antibodies between SARS-CoV and SARS-CoV-2. These data may explain at least in part the differential clinical outcomes of infection of those two viruses.
Immunity to the reinfection in the context of SARS-CoV-2
It is not clear whether the primary infection with SARS-CoV-2 may provide long-term protection against the re-infection or not yet. The re-infection of monkeys on day 28 after the initial SARS-CoV-2 infection revealed the same result in the protection of these animals against the second round of infection with the virus ().Citation45 This was suggesting the potential roles of the immune response in the protection against re-infection with the SARS-CoV; however, these findings require further clarification in case of human infection. Thus, longitudinal cohort studies are urgently required to clarify whether the primary SARS-CoV-2 infection may provide enough long-lasting protection upon any subsequent infection.
Applications of convalescent sera and monoclonal antibodies in the treatment of COVID-19
The human convalescent serum is considered a potential opportunity for the treatment of COVID-19 patients. This may be achieved through the collection of sera from the recovered COVID-19 patients where the neutralizing antibodies are present against SARS-CoV-2 and administered to some new patients to help them overcome the active infection, especially in the more severe cases.Citation46 Additionally, it has been reported that the administration of SARS-CoV-2 convalescent sera from recovered patients helped in the alleviation and subsidy of severing active COVID-19 infections.Citation31,Citation47 Although human convalescent serum is a promising approach in the treatment of SARS-CoV-2 infection, there are some limitations related to this method. These concerns include the possibility of transferring other pathogens to the recipient patients, the potential activation of some antibody-dependent enhancement (ADE). This process may suppress the anti-innate immune responses as it was reported in some in vitro studies done earlier on SARS-CoV.Citation48 Other factors related to the adjustment and fine-tuning of the administered volumes, titers, and time of transfusion of plasma from the donor to the recipient patients.Citation49 The convalescent serum approach, using passive immunization by specific monoclonal antibodies is more specific, safe, and has a low risk of transfer other potential pathogens. Furthermore, using cocktail monoclonal antibodies (Mab) of the SARS-CoV-2 that are able to recognize several epitopes may increase the efficiency of these neutralizing (Mab) antibodies.
Kinetics of SARS-CoV-2 memory cell responses
Regarding B cell response, a recent study reported that all serum patients relative to uninfected controls had a significant proportion of specific B memory cells against RBD and NCP. These B cell populations have both un-switched class (CD27+ IgM IgD) and switched class cells (CD27+ IgD) which contained IgG expressing B memory cells.Citation50 Effective and predominant B memory cells response are induced by SARS-CoV-2 infections, which contained IgM and IgG-specific B memory cells against RBD and NCP. Furthermore, the B memory cells against RBD are nearly CD27+ and correlated positively with T follicular helper cells (Tfh).Citation50 Serum antibodies have decreased gradually following SARS-CoV-2 clearance. However, specific memory B cells are detected over time in a stable proportion.Citation50
It has shown that memory response against SARS-CoV-2 lasts for 8 months and spike-specific IgG antibodies were stable and circulated for up to 6 months post-SARS-CoV-2 infection. Also, B memory cell counts were abundant at 6 months rather than the first month especially post-viral symptoms appearance. The proportion of RBD-specific IgG was 88% between 6 and 8 months.Citation51 On the other hand, IgA levels were assessed in most COVID-19 patients, started to increase on day 27, and disappeared by 3 months. B cells appeared on day 16 early after the onset of viral symptoms and increased and become stable in the following 4–5 months.Citation51 10–30% of memory B cells in recovery patients were specifically for RBD antigens. Antibody titers (IgG) decreased after 6–8 months post symptoms onset among individuals but is highly heterogeneous among them.Citation51 Another study has demonstrated that CD19 + B cell numbers were higher in severe COVID-19 than mild cases. Memory B cells were low in severe and critical cases, but the plasma cells were high, due to the disease severity. Overall the total number of B cells in COVID-19 patients (different categories (mild, severe, and critical) was lower than healthy donors, suggesting that preexisting memory B cells specific for other coronavirus were activated and differentiated to atypical memory (CD27−) and/or plasma cells.Citation52 Taking together, memory B cells might be represented the long-lived humoral response than serum antibodies and could be used as robust and substitute markers of humoral immunity in immunization studies.Citation50 In contrary to other studies that reported a decrease in B cell numbers during COVID-19,Citation53,Citation54 there was no reduction of B cells in all patients and serum that collected within 14 days of symptoms appearance.Citation50
Based on the presented research about the SARS-CoV-2 memory cells, it is concluded that both previous infections and vaccination against the virus may provide a considerable level of immunity against the virus however, large-scale studies are required to map the kinetics of the memory cells among the infected and vaccinated individuals.
Some SARS-CoV-2 immune evasion strategies
Both SARS-CoV and MERS-CoV evade the immune response in many ways.Citation23,Citation55 Here, we are presenting some immune evasion strategies posed by those closely related viruses to the SARS-CoV-2. These evasion strategies help the SARS-CoV-2 to hijack the host immune response and contribute to the progress and promotion of its replication cycle. These strategies may also contribute to the lethality and virulence of SARS-CoV-2 infected patients. The SARS-CoV-2 induces severe pathology in many vital organs of the affected patients, especially lungs, kidneys, and, heart, brain, intestines.Citation36 There might be some virulent virus factors produced by the virus to induce this multi-organ failure.
Meanwhile, the SARS-CoV-2 utilizes some unique immune evasion strategies that may contribute to the virulence and lethality of this virus. Recent studies revealed SARS-CoV-2 infection-induced differential display of some crucial blood and immune markers. The infected patients had an increase in the neutrophils, IL-6, and the serum reactive protein (CRP). However, those patients showed a 35% reduction in the total numbers of circulating lymphocytes.Citation56 These parameters act as biomarkers for the SARS-CoV-2 infection. They may be used for monitoring the progress of the treatment of infected patients. Both SARS-CoV and MERS-CoV possess some immune evasion strategies through the production of the double-membrane vesicles, which contain the virus replication complex. Those viruses escape the host immune system detection through the replication inside these vesicles.Citation57 Another interesting phenomenon is the inhibition of the IFN-I pathway by the MERS-CoV- ORF-4a.Citation58
Furthermore, both MERS-CoV and influenza virus (H5N1) decrease the expression of the antigen presentation.Citation59 The SARS-CoV-2 infection triggers similar pathways to ensure the success of its replication cycle; however, further studies are required to confirm these findings. In the same context, the MERS-CoV- 4b interferes with the NF-κB pathway resulting in the counteraction of the host’s innate immune response against the virus infection.Citation60 A new study showed the coexistence of the viral RNA in saliva and specific antibodies in sera of patients recovered from SARS-CoV-2 for up to 40 days after the recovery of some infected patients. These findings suggest some unique immune evasion strategies adapted by the virus to hijack the host immune response, thus remain active even after the recovery of the patient.Citation61 More studies are required to confirm these findings and to reveal the mechanism behind these phenomena.
Roles of various SARS-CoV-2 proteins in the immune evasion and hijacking the host immune response
Recent studies showing that various SARS-CoV-2 proteins including some structural and the nonstructural proteins may contribute to the virus immune evasion strategies () which enhance the virus replication and promotes its pathogenesis and spread.Citation62,Citation63,Citation65,Citation67,Citation69,Citation70 SARS-CoV-2-ORF8 plays important functions during virus replication and in viral immune evasion.Citation67 This may affect the downstream pathogenesis of the virus. It mediates the degradation of the MHC-class-I and contributes to the marked inhibition of the IFNs production. These effects fine-tune the outcomes of the virus infection.Citation67 SARS-CoV-ORF-3 plays an important role during the virus infection and pathogenesis through its actions on the JAK-STAT, chemokine, and cytokine- pathways.Citation66 Mutations within the SARS-CoV-ORF-3 are associated with high case fatality rates among the affected individuals.Citation66 Both SARS-CoV-2-NSP-2 and NSP-6 act synergistically to inhibit the INFs production in a much more efficient way than the SARS-CoV and MERS-CoV.Citation63 There is an active interaction between the NSP16 and the NSP-10 in the context of SARS-CoV-2 infection. This interaction creates some unique binding pocket sites for the virus activation and contributes to the viral immune evasions.Citation65 SARS-CoV-NSP-1 binds to various host cell ribosomes subunits resulting in the shutdown of some host cell genes both in-vitro and in vivo.Citation62 SARS-CoV-2-ORF-9 C is a transmembrane protein that recently showed to play important immune evasion roles during the virus infection.Citation71 It is the first coronavirus-ORF-9 C to induces its downstream effects through tackling the interferon signaling pathways.Citation68,Citation69 The SARS-CoVNSp-15
Table 1. Summary of some immune evasion effects of various SARS-CoV-2 proteins
Play important roles in the RNA processing and host immune evasion. Repurposing of some drugs using the in silico analysis showed that targeting the NSP-15 of the virus may have promising trends in the control of SAR-CoV-2 infection.Citation72 SARS-CoV2-PLpro (papin like protease) plays important role in the process of post-translation modification of viral and host cell proteins. It also contributes to the regulation of the IFN and NFKB pathways which enhancing the progression of virus replication and immune evasion.Citation64 Thus, targeting this protein may represent one of the promising trends in controlling virus infection within the host.Citation64 The SARS-CoV-2-S protein undergoes a process of posttranslational glycosylation which plays important role in the virus infection, pathogenesis, and host immune system evasion.Citation68 Recent studies showing the SARS-CoV-2-M protein possesses sugar transporter-like which have a substantial impact on virus replication and host immune evasion.Citation69
SARS-CoV-2 vaccine candidates
Since the emergence of SARS-CoV-2, scientists all over the world and vaccine development companies are in a battle working day and night to achieve this milestone. The rationale behind the vaccination strategy is to immunize the at-risk people, especially the immunocompromised, elderly, and health care workers in the front line of combating this virus. Although there are six other human coronaviruses identified from long time, there are no available licensed vaccines against any of them despite some new trials for vaccines against MERS-CoV recently developed and are in the clinical trials.Citation73,Citation74 Typically, vaccine development is a lengthy procedure and requires many steps, including laboratory testing preclinical and clinical trials. At this time, to speed up the procedures of making successful SARS-CoV-2 vaccines. Perhaps some of the preclinical studies were performed in parallel with clinical trials; however, many obstacles were hampering these procedures, such as the regulatory authorities must assess the vaccine manufacturing process and preclinical data to ensure the safety of volunteer persons.Citation75
History of the development, testing, evaluation, and approval of some SARS-CoV-2 vaccines
There are many strategies for vaccine preparation and development for many viruses. Some of these strategies may be applicable for coronaviruses, including (i) the live-inactivated, (ii) the subunit vaccines, and (iii) the nucleic acid-based vaccines. On Jan 23rd, 2020, the Coalition for Epidemic Preparedness Innovations (CEPI) has declared the funding to develop some vaccines against SARS-CoV-2 using three different approaches, including DNA, mRNA, and molecular clamp.Citation76 As of April 2020, more than 20 potential SARS-CoV-2 vaccine candidates have been published worldwide (). As of April 2020, several COVID-19 vaccine candidates were approved and currently in the clinical trials at different phases. Currently, there are at least 10 SARS-CoV-2 approved vaccines and deployed in many parts across the world. Finally, we now have several SARS-CoV-2 vaccines approved and deployed in one of the largest global vaccination campaigns ever. As of Dec 23rd, 2020, there are 2013 vaccine candidates for SARS-CoV-2 under development. Almost 44 out of them are in varying stages of the clinical trials.Citation86 A strong network of international coordination and cooperation between academia, vaccine developers, public health institutes, and government bodies urgently needed to make sure that an effective vaccine may be developed as soon as possible to stop the spread of SARS-CoV-2 across the globe thus the current SARS-CoV-2 pandemic can be contained.
Table 2. Top ranked SARS-CoV-2 vaccines
Some potential nucleic acid-based SARS-CoV-2 vaccines
The nucleic acid-based vaccines are the most advanced platforms for the response to emerging viral pathogens. For example, during the Zika virus outbreak, nucleic acid-based vaccines were the first vaccine candidates that entered clinical trials in less than 1 year after the emergence of the epidemic.Citation87 Some SARS-CoV-2 nucleic acid-based vaccine platforms are developed. The Moderna Therapeutics, CureVac, and Shanghai East Hospital (Tongji University) Stermirna Therapeutics are exploring the mRNA vaccine platforms.Citation56
Some SARS-CoV-2 mRNA deployed vaccines
The mRNA-1273 vaccine was developed by Moderna Therapeutics and the US National Institute of Allergy and Infectious Diseases (NIH).Citation88 This COVID-19 vaccine RNA-1273 is an mRNA vaccine expressing COVID-19 spike protein. This vaccine phase I clinical trial was initiated at Kaiser Permanente Washington Health Research Institute (KPWHRI) in Seattle. Simply, the vaccine was constructed to encode the SARS-CoV-2 gene and encapsulated with lipid nanoparticles for delivery into the host cells.Citation89 This vaccine showed a high degree of prevention of the severe COVID-19 cases up to 94.1% (). This was a great leap for lowering the number of hospitalizations among infected patient especially severe cases require admission to the intensive care units.Citation89 Another promising vaccine is the BNT162b2 mRNA. This vaccine developed through the nucleoside-modified RNA encoding the full-length SARS-CoV-S gene coupled with some lipid nanoparticle.Citation77 This vaccine is showing 95% protection against the development of severe COVID-19 cases.Citation77
Both vaccines are currently deployed in many parts int eh world. They administered on a prime-boost regimen 21 days apart.Citation90
Some recombinant SARS-CoV-2 vaccines
Another approved vaccine established by the Cansino Biologics Inc, the Academy of Military Medical Sciences of China, named Ad5-nCoV. This vaccine is mainly based on a vector containing a defective adenovirus-5 that expresses the spike protein of the SARS-CoV-2. Finally, the University of Oxford in combination with AstraZeneca company, developed and deployed the ChAdOx1-nCoV-19 vaccine.Citation91 This approach has been used recently to develop a vaccine against MERS-CoV.Citation73 This ChAdOx1-nCoV-19 vaccine showed promising results in protecting some rhesus macaques from experimental infections with the SARS-CoV-2.Citation88 The clinical human trials for these vaccines showed a high rate of protection (88%) against the severe COVID-19 cases when administered in a prime-boost regimen ().Citation91 These studies suggested that the maximum performance of this vaccine can be achieved in a prime-boost regimen 28 days apart.Citation92
Some potential live virus and recombinant SARS-CoV-2 vaccines
One potential common vaccine candidate for coronaviruses may be developed based on the similarities between the T-cell epitopes of SARS-CoV and MERS-CoV. It may also depend on the possibility of cross-reactivity between these viruses.Citation43 A recent study identified some candidate vaccines design based on the high degree of genetic similarity between the SARS-CoV and the SARS-CoV-2.Citation93 By screening SARS-CoV-derived B and T cell epitopes, they identified some promising candidates derived from the spike (S) and nucleocapsid (N) proteins that showed a high degree of similarity to SARS-CoV-2 proteins.Citation93 It is well documented that the spike (S) glycoprotein or S protein is the primary inducer of neutralizing antibodies and T-cell responses in several SARS-CoV vaccines. This includes the use of full-length S protein or S1 receptor-binding domain (RBD) and expression in virus-like particles (VLP), DNA, or viral vectors.Citation94 The Johnson & Johnson Company has recently released its SARS-CoV-2 vaccine using the Janssen’s-AdVac® adenoviral vector used previously to develop their Ebola virus vaccine. This vaccine has been approved by many countries. The University of Hong Kong has also launched a modified nasal spray influenza vaccine expressing a surface antigen from the SARS-CoV-2 protein. Besides, Codagenix has established a “codon de-optimization” vector method to abolish the virus virulence and to explore this SARS-CoV-2 vaccine strategy.Citation95 This type of vaccine has proven to be highly effective and provides long-lasting protection. One of the significant advantages of the whole virus vaccine is the activation of the T-cells responses, including CD4+ and CD8+ T, offering specific neutralizing antibodies, and memory B cell responses. Furthermore, it can trigger toll-like receptors (TLRs), such as TLR 3, TLR 7, TLR-8, and TLR 9. However, the attenuated pathogen may cause disease, as was reported with the oral polio vaccine.Citation96 A recent study used the recombinant parainfluenza virus-5 (PIV-5) backbone for the delivery of the MERS-CoV-S protein. This study showed excellent protection of the mice challenged with lethal MERS-CoV after a single intranasal administration of this recombinant vaccine.Citation97 This approach could be one of the new promising vaccination approaches for the SARS-CoV-2.
SARS-CoV-2 inactivated vaccines
The inactivated vaccine is one of the classical vaccination strategies developed a long time ago and was applicable for many pathogens, including respiratory pathogens such as the influenza virus.Citation98 One of the main advantages of this approach is the preservation of the spike protein conformational epitopes in their original forms. This will result in the production of specific neutralizing antibodies against the original virus, as in the case of MERS-CoV.Citation98 Several inactivated SARS-CoV-2 vaccine candidates were tested for their efficacy and side effects.Citation99 Those two vaccines were developed by both the Beijing Institute of Biological Products Co., Ltd and the Wuhan Institute of Biological Products Co., Ltd. The clinical trials of those two vaccines revealed no safety concerns reported in the people who received those two vaccines.Citation99 The Sinovac Life Sciences, Beijing, China, developed an inactivated SARS-CoV-2 vaccine called CoronaVac.Citation100 Both phase I and II clinical trials for the CoronaVac revealed potential efficacy and safety in the vaccinated individuals.Citation100 Another recent study reported the encouraging trend of a new inactivated SARS-CoV-2 vaccine candidate prepared by the (PiCoVacc) company. They used the purified ten SARS-CoV-2 strains then inactivate them by beta-Propriolactone. This study showed this vaccine protects the non-human primates against the circulating strains of SARS-CoV-2.Citation97
Some SARS-CoV-2 subunit vaccines
The subunit vaccines usually consist of purified antigens in the form of saccharides or conjugated proteins. They are generally prepared from viral synthetic peptides or recombinant proteins. The spike protein is the crucial player in viral pathogenesis, as well as the immune response. It is an ideal target for vaccine preparation using various strategies as well as diagnostic assays.Citation55,Citation101 The DNA vaccine is much safer than other types of vaccines due to the absence of any microbial reversion and side effects. The key antigen in the subunit vaccine should be capable of inducing protective immune responses against a specific pathogen.Citation97 This vaccine candidate was used against SARS-CoV to stimulate a potent immune response against the spike proteins; as a result, neutralizing the binding site of viral protein and preventing its interaction with its specific receptor ACE2.Citation102 Some SARS-CoV-2 subunit vaccines are in the clinical stage, such as the vaccine developed by Queen’s land University using Rapid Response Technology, the ‘Molecular clamp’ vaccine platform.Citation103 This technology can present the target vaccine straight forward to the immune system to induce a specific antibody response against the SARS-CoV-2.Citation103 Meanwhile, the
Clover Biopharmaceuticals is also working on developing a highly purified recombinant SARS-CoV-2-S protein subunit-trimer vaccine called (S-Trimer) through using their Trimer-Tag© technology.Citation104 Another example of the SARS-CoV-2 potential subunit vaccine is the immunogenic virus-like nanoparticles developed by Novavax Company. This type of vaccine is based on the expression of the recombinant SARS-CoV-2-S-protein. Moreover, the Texas Children’s Hospital Center for Vaccine Development has developed another subunit vaccine against SARS-CoV-2 infection conjugated with alum, which incorporates the receptor-binding domain (RBD) of the viral spike protein.Citation105 Although most of these SARS-CoV-2 subunit vaccines are safe and elicit a specific immune response, they trigger low immunogenicity levels. Thus, to overcome this problem and to enhance the effectiveness of most subunit vaccines, immune response-enhancing adjuvant should be considered as in the case of using alum adjuvant above. Meanwhile, specific delivery systems must be convenient to have an optimal response.Citation106
Some potential SARS-CoV-2 DNA vaccines
The DNA vaccine technology was well documented and had significant progress in the field of vaccinology of many pathogens, including some other coronaviruses.Citation107 Instead of having antigen substances in DNA – based vaccines, it is better to have a genetic component responsible for antigen production by the host. The DNA vaccines are mimicking natural viral infections. Both of them can trigger the antigen-presenting cells (APCs) to increase the expression of MHC-I as well as the intracellular mediated response via CD8 + T cells.Citation107 Furthermore, the antibody-mediated response was confirmed during the use of DNA vaccines.Citation107 To increase the efficacy of the DNA vaccines and to ensure a high delivery of them to the target cells, some components should be mixed with vaccines such as adjuvants, lipid complexes, and micro-particles.Citation108 Additionally, the INO-4800 (DNA vaccine) from in ovo has developed a vaccine using DNA plasmid encoding SARS-CoV-2-S protein delivered by electroporation. Shenzhen Geno-Immune Medical Institute has also been approved for phase I clinical trials for two vaccine candidates, LV-SMENP-DC and COVID-19 artificial antigen-presenting cells (APC). LV-SMENP-DC is composed of dendritic cells (DCs) modified with a lentiviral vector expressing synthetic mini-genes based on selected viral proteins to activate antigen-specific cytotoxic T cells.Citation88
The emergence of new SARS-CoV-2 escape mutants and the fine-tuning of the vaccines and vaccination strategies
Several SARS-CoV-2 mutants were reported recently in many countries, especially the UK, South Africa, Brazil, and India.Citation109,Citation110 These mutants have higher transmissibility and replication potential than the viruses reported in the early phase of the pandemic.109,Citation110 Most of these mutations occur within the RBD of the S protein of the virus. These mutations enable the virus to bind firmly to its receptors; the ACE.Citation111 These mutants are posing significant risk to human health in many aspects. First, the high replication potentials make these mutants spread much faster in a certain population. Second, these mutants resulted in an increase in the number of hospitalization as well as the number of severe cases. This contributed to the high case fatality rates among the affected populations. Third, some of these mutants escaped the action of neutralizing antibodies generated because of both natural infections with older versions of the virus or antibodies from some vaccinated individuals with some of the recently deployed vaccines.Citation112 On the other hand, the new mutants may affect the sensitivity of the already developed diagnostic assays against SARS-CoV-2.Citation113 Several studies are recently showing that some of the mRNA-1273 vaccine-based SARS-CoV-2 vaccines may tolerate these escape mutations and are able to induce a potent reduction in the virus infectivity.Citation114 We believe the gold standard is the vaccination of the individuals against the homologous strains and variants of the virus is for achieving the required immune response; thus, protection against the natural infection may be granted. Careful and continuous monitoring of the virus is necessary at the current sage to prepare effective vaccines against the most recent circulating strains of the virus.
Conclusions
The host immune system is one of the key players in the battle against COVID-19. SARS-CoV2 adapts many unique evasion strategies to escape the effects of the host immune responses. The continuous emergence of new viral mutants making the virus several steps ahead of us in its control and mitigation. Through understanding various aspects of SARS-CoV/host immune system interaction, we may unrevealed some novel approaches for its control and prevention. Developments of various types of vaccines and immunotherapeutic are great steps toward the control of SARS-CoV-2. However, careful, and vigilant monitoring of the virus and its evasion strategies are necessary to contain SARS-CoV-2 and put an end to the current pandemic in the near future.
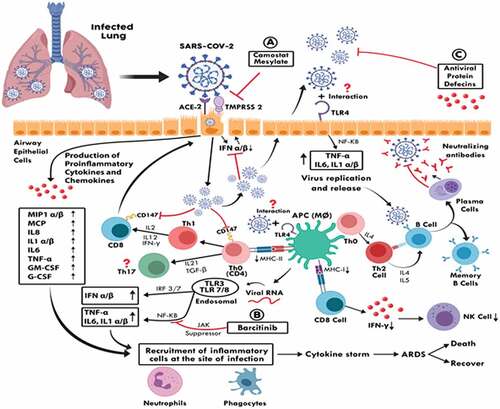
The virus enters the host cells after an interaction between the spike protein (S) and the ACE-2 host cell receptor in the presence of the TMPRSS2 ligands. The SASR-CoV-2 inhibits the early production of type-I Interferon, including IFN alpha, beta. The antigen-presenting cells (APC), such as macrophages that present antigenic peptides of the virus to the Th0 cells further lead to T cell activation and differentiation to different CD4 T cell subsets (i.e., Th1, Th2, and Th17). The Th1 produces various cytokines such as IFN-gamma, IL-2, and IL-12, which all can activate the CD8 T cells response. The Th2 cells exert their functions via the production of cytokines, including IL-4, IL-5, which activate the B-cell response and result in activation and differentiation into effector plasma cells and memory B cells. This can produce specific neutralizing antibodies against the common SARS-CoV-2 structural proteins, including (S, N, and M) proteins. The SARS-CoV-2 presented by macrophages in combination with MHC-1 will be recognized by CD8 T cells that can produce IFN-gamma, which plays an essential role in the process of viral clearance.
SARS-CoV-2 can infect the T cells (CD4 and CD8) and lead to a reduction in their total numbers. The pattern recognition receptors, including the Toll-like receptors, can recognize both the extracellular and the intracellular invaded pathogens. The TLR-4 expresses at the surface of airway epithelial cells, macrophages may also recognize the (s) protein, and once the virus releases its genomic RAN material into the cytoplasm during replication. The endoplasmic RNA sensors such as TLR-3, TLR-7, and TLR-8 can further recognize it; this may result in the induction of both the cytokines and the chemokines. Moreover, the infected host cells produce a wide range of pro-inflammatory cytokines and chemokines that also able to recruit several inflammatory cells at the site of infection. The level of their production is associated with the severity of the COVI-19 infection. Thus, the acute respiratory distress syndrome (ARDS) may lead in most cases to the death of the infected individuals; however, some patients may also recover. Red arrows showing inhibitory pathways, while the black arrows indicate an active pathway.
The virus enters the host cells after an interaction between the spike protein (S) and the ACE-2 host cell receptor in the presence of the TMPRSS2 ligands.Citation10 The SASR-570 inhibits the early production of type-I Interferon, including IFN alpha, beta.Citation115 The antigen-presenting cells (APC), such as macrophages that present antigenic peptides of the virus to the Th0 cells that are further lead to T cell activation and differentiation to different CD4 T cell subsets (i.e., Th1, Th2, and Th17).Citation116 The Th1 produces various cytokines such as IFN-gamma, IL-2, and IL-12, which all can activate the CD8 T cells response.Citation117 The Th2 cells exert their functions via the production of cytokines, including IL-4, IL-5, which activate the B-cell response and result in activation and differentiation into effector plasma cells and memory B cells.Citation112 This can produce specific neutralizing antibodies against the common SARS-CoV-2 structural proteins, including (S, N, and M) proteins. The SARS-CoV-2 presented by macrophages in combination with MHC-1 will be recognized 580 by CD8 T cells that can produce IFN-gamma, which plays an essential role in the process of viral clearance. SARS-CoV-2 can infect the T cells (CD4 and CD8) and lead to a reduction in their total numbers.Citation118 The pattern recognition receptors, including the Toll-like receptors, can recognize both the extracellular and the intracellular invaded pathogens. The TLR-4 expresses at the surface of airway epithelial cells, macrophages may recognize the (s) protein, and once the virus releases its genomic RAN material into the cytoplasm during replication.Citation119 The endoplasmic RNA sensors such as TLR-3, TLR-7, and TLR-8 can further recognize it;Citation120 this may result in the induction of both the cytokines and the chemokines. Moreover, the infected host cells produce a wide range of pro-inflammatory cytokines and chemokines that also able to recruit several inflammatory cells at the site of infection. The level of their production is associated with the severity of the COVI-19 infection. Thus, the acute respiratory distress syndrome (ARDS) may lead in most cases to the death of the infected individuals; however, some patients may also recover.Citation121 Red arrows showing inhibitory pathways, while the black arrows indicate an active pathway.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–33. doi:10.1056/NEJMoa2001017.
- Coronaviridae Study Group of the International Committee on Taxonomy of V. The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–44. doi:10.1038/s41564-020-0695-z.
- WHO. WHO director-general’s opening remarks at the media briefing on COVID-19-11 March 2020. 2020; [accessed 2020 March 11]. https://webarchiveorg/web/20200311212521/https://wwwwhoint/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020.
- Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–69. doi:10.1001/jama.2020.1585.
- Shi Y, Wang Y, Shao C, Huang J, Gan J, Huang X, Bucci E, Piacentini M, Ippolito G, Melino G, et al. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27:1451–54. doi:10.1038/s41418-020-0530-3.
- Ulrich H, Pillat MM. CD147 as a target for COVID-19 treatment: suggested effects of azithromycin and stem cell engagement. Stem Cell Rev Rep. 2020. doi:10.1007/s12015-020-09976-7.
- Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–92 e6. doi:10.1016/j.cell.2020.02.058.
- Wang Q, Zhang Y, Wu L, Niu S, Song C, Zhang Z, Lu G, Qiao C, Hu Y, Yuen KY, et al. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell. 2020;181(4):894–904.e9. doi:10.1016/j.cell.2020.03.045. Epub 2020 Apr 9.
- Xia S, Liu M, Wang C, Xu W, Lan Q, Feng S, Qi F, Bao L, Du L, Liu S, et al. Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res. 2020;30:343–55. doi:10.1038/s41422-020-0305-x.
- Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu N-H, Nitsche A, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–80 e8. doi:10.1016/j.cell.2020.02.052.
- Bello M, Martinez-Munoz A, Balbuena-Rebolledo I. Identification of saquinavir as a potent inhibitor of dimeric SARS-CoV2 main protease through MM/GBSA. J Mol Model. 2020;26:340. doi:10.1007/s00894-020-04600-4.
- Chen YW, Yiu CB, Wong KY. Prediction of the SARS-CoV-2 (2019-nCoV) 3C-like protease (3CL (pro)) structure: virtual screening reveals velpatasvir, ledipasvir, and other drug repurposing candidates. F1000Res. 2020;9:129. doi:10.12688/f1000research.22457.2.
- Schoeman D, Fielding BC. Coronavirus envelope protein: current knowledge. Virol J. 2019;16:69.
- Fehr AR, Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol. 2015;1282:1–23.
- Rosendahl Huber S, van Beek J, de Jonge J, Luytjes W, van Baarle D. T cell responses to viral infections - opportunities for Peptide vaccination. Front Immunol. 2014;5:171. doi:10.3389/fimmu.2014.00171.
- Murphy K, Weaver C. Janeway’s immunobiology. Garland Sci. 2016. ISBN: 978-3-662-56003-7.
- Zajac A, Harrington L. Immune response to viruses: cell-mediated immunity. In: Encyclopedia of virology. Ed Elsevier Ltd; 2008. doi:10.1016/B978-012374410-4.00799-8. Corpus Corpus ID: 81055973.
- Cecere TE, Todd SM, Leroith T. Regulatory T cells in arterivirus and coronavirus infections: do they protect against disease or enhance it? Viruses. 2012;4:833–46. doi:10.3390/v4050833.
- Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annu Rev Immunol. 2009;27:485–517. doi:10.1146/annurev.immunol.021908.132710.
- Peng Y, Mentzer AJ, Liu G, Yao X, Yin Z, Dong D. Broad and strong memory CD4 (+) and CD8 (+) T cells induced by SARS-CoV-2 in UK convalescent COVID-19 patients. bioRxiv. 2020;21(11):1336–1345. doi:10.1038/s41590-020-0782-6. Epub 2020 Sep 4.
- Zhang S, Zhang H, Zhao J. The role of CD4 T cell help for CD8 CTL activation. Biochem Biophys Res Commun. 2009;384:405–08. doi:10.1016/j.bbrc.2009.04.134.
- Gupta S, Su H, Narsai T, Agrawal S. SARS-CoV-2-associated T-cell responses in the presence of humoral immunodeficiency. Int Arch Allergy Immunol. 2021;182:195–209. doi:10.1159/000514193.
- Maloir Q, Ghysen K, Louis R, Guiot J. Acute respiratory distress revealing antisynthetase syndrome. Rev Med Liege. 2018;73:370–75.
- Zhao J, Li K, Wohlford-Lenane C, Agnihothram SS, Fett C, Zhao J, Gale MJ, Baric RS, Enjuanes L, Gallagher T, et al. Rapid generation of a mouse model for Middle East respiratory syndrome. Proc Natl Acad Sci U S A. 2014;111:4970–75. doi:10.1073/pnas.1323279111.
- Chen J, Lau YF, Lamirande EW, Paddock CD, Bartlett JH, Zaki SR, Subbarao K. Cellular immune responses to severe acute respiratory syndrome coronavirus (SARS-CoV) infection in senescent BALB/c mice: CD4+ T cells are important in control of SARS-CoV infection. J Virol. 2010;84(3):1289–301. doi:10.1128/JVI.01281-09.
- Li CK-F, Wu H, Yan H, Ma S, Wang L, Zhang M, Tang X, Temperton NJ, Weiss RA, Brenchley JM, et al. T cell responses to whole SARS coronavirus in humans. J Immunol. 2008;181:5490–500. doi:10.4049/jimmunol.181.8.5490.
- Wu D, Yang XO. TH17 responses in cytokine storm of COVID-19: an emerging target of JAK2 inhibitor Fedratinib. J Microbiol Immunol Infect. 2020. doi:10.1016/j.jmii.2020.03.005.
- Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–34. doi:10.1016/S0140-6736(20)30628-0.
- McGonagle D, Sharif K, O’Regan A, Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020:102537. doi:10.1016/j.autrev.2020.102537.
- Kim JS, Lee JY, Yang JW, Lee KH, Effenberger M, Szpirt W, Kronbichler A, Shin JI. Immunopathogenesis and treatment of cytokine storm in COVID-19. Theranostics. 2021;11:316–29. doi:10.7150/thno.49713.
- Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, Wang F, Li D, Yang M, Xing L, et al. Treatment of 5 critically Ill patients with COVID-19 with convalescent plasma. JAMA. 2020. doi:10.1001/jama.2020.4783.
- Wan S, Yi Q, Fan S, Lv J, Zhang X, Guo L, Lang C, Xiao Q, Xiao K, Yi Z, et al. Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP). medRxiv. 2020 Feb 10:20021832.
- Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si H-R, Zhu Y, Li B, Huang C-L, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–73. doi:10.1038/s41586-020-2012-7.
- Ng O-W, Chia A, Tan AT, Jadi RS, Leong HN, Bertoletti A, Tan Y-J. Memory T cell responses targeting the SARS coronavirus persist up to 11 years post-infection. Vaccine. 2016;34:2008–14. doi:10.1016/j.vaccine.2016.02.063.
- Perlman S, Dandekar AA. Immunopathogenesis of coronavirus infections: implications for SARS. Nat Rev Immunol. 2005;5:917–27. doi:10.1038/nri1732.
- Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020. doi:10.1016/S2213-2600(20)30076-X.
- Wang K, Chen W, Zhou Y-S, Lian J-Q, Zhang Z, Du P, Gong L, Zhang Y, Cui HY, Geng JJ, et al. SARS-CoV-2 invades host cells via a novel route: CD147-spike protein. bioRxiv. 2020 Dec 4;5(1):283. doi:10.1038/s41392-020-00426-x.
- Shin HS, Kim Y, Kim G, Lee JY, Jeong I, Joh JS, Kim H, Chang E, Sim SY, Park J-S, et al. Immune responses to middle east respiratory syndrome coronavirus during the acute and convalescent phases of human infection. Clin Infect Dis. 2019;68:984–92. doi:10.1093/cid/ciy595.
- Zhao J, Zhao J, Mangalam AK, Channappanavar R, Fett C, Meyerholz DK, Agnihothram S, Baric R, David C, Perlman S, et al. Airway memory CD4(+) T cells mediate protective immunity against emerging respiratory coronaviruses. Immunity. 2016;44:1379–91. doi:10.1016/j.immuni.2016.05.006.
- Wrapp D, De Vlieger D, Corbett KS, Torres GM, Wang N, Van Breedam W, Roose K, van Schie L, Hoffmann M, Pöhlmann S, et al. Structural basis for potent neutralization of betacoronaviruses by single-domain camelid antibodies. Cell. 2020;181(5):1004–15 e15. doi:10.1016/j.cell.2020.04.031.
- Cromer D, Juno JA, Khoury D, Reynaldi A, Wheatley AK, Kent SJ, Davenport MP. Prospects for durable immune control of SARS-CoV-2 and prevention of reinfection. Nat Rev Immunol. 2021. doi:10.1038/s41577-021-00550-x.
- Haveri A, Smura T, Kuivanen S, Österlund P, Hepojoki J, Ikonen N, Pitkäpaasi M, Blomqvist S, Rönkkö E, Kantele A, et al. Serological and molecular findings during SARS-CoV-2 infection: the first case study in Finland, January to February 2020. Euro Surveill. 2020;25(11):2000266. doi:10.2807/1560-7917.ES.2020.25.11.2000266.
- Zhang W, Du R-H, Li B, Zheng X-S, Yang X-L, Hu B, Wang -Y-Y, Xiao G-F, Yan B, Shi Z-L, et al. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect. 2020;9(1):386–89. doi:10.1080/22221751.2020.1729071.
- Chen X, Zhou B, Li M, Liang X, Wang H, Yang G, Wang H, Le X. Serology of severe acute respiratory syndrome: implications for surveillance and outcome. J Infect Dis. 2004;189(7):1158–63. doi:10.1086/380397.
- Bao L, Deng W, Gao H, Xiao C, Liu J, Xue J, Lv Q, Liu J, Yu P, Xu Y, et al. Reinfection could not occur in SARS-CoV-2 infected rhesus macaques. bioRxiv. 2020 Mar 13:990226. doi:10.1101/2020.03.13.990226.
- Casadevall A. Pirofski L-a. The convalescent sera option for containing COVID-19. J Clin Invest. 2020;130:1545–48.
- Duan K, Liu B, Li C, Zhang H, Yu T, Qu J, Zhou M, Chen L, Meng S, Hu Y, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Nat Acad Sci. 2020Apr 28;117(17):9490–9496.
- Wang S-F, Tseng S-P, Yen C-H, Yang J-Y, Tsao C-H, Shen C-W, Chen K-H, Liu F-T, Liu W-T, Chen YMA, et al. Antibody-dependent SARS coronavirus infection is mediated by antibodies against spike proteins. Biochem Biophys Res Commun. 2014;451(2):208–14. doi:10.1016/j.bbrc.2014.07.090.
- Shanmugaraj B, Siriwattananon K, Wangkanont K, Phoolcharoen W. Perspectives on monoclonal antibody therapy as potential therapeutic intervention for Coronavirus disease-19 (COVID-19). Asian Pac J Allergy Immunol. 2020;38:10–18. doi:10.12932/AP-200220-0773.
- Hartley GE, Edwards ESJ, Aui PM, Varese N, Stojanovic S, McMahon J, Peleg AY, Boo I, Drummer HE, Hogarth PM, et al. Rapid generation of durable B cell memory to SARS-CoV-2 spike and nucleocapsid proteins in COVID-19 and convalescence. Sci Immunol. 2020;5(54):eabf8891. doi:10.1126/sciimmunol.abf8891.
- Dan JM, Mateus J, Kato Y, Hastie KM, Yu ED, Faliti CE, Grifoni A, Ramirez SI, Haupt S, Frazier A, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371:eabf4063. doi:10.1126/science.abf4063.
- Sosa-Hernández VA, Torres-Ruíz J, Cervantes-Díaz R, Romero-Ramírez S, Páez-Franco JC, Meza-Sánchez DE, Juárez-Vega G, Pérez-Fragoso A, Ortiz-Navarrete V, Ponce-de-León A, et al. B cell subsets as severity-associated signatures in COVID-19 patients. Front Immunol. 2020:11. doi:10.3389/fimmu.2020.611004.
- Mathew D, Giles JR, Baxter AE, Oldridge DA, Greenplate AR, Wu JE, Alanio C, Kuri-Cervantes L, Pampena MB, D’Andrea K, et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science. 2020;369:eabc8511.
- Oliviero B, Varchetta S, Mele D, Mantovani S, Cerino A, Perotti CG, Ludovisi S, Mondelli MU. Expansion of atypical memory B cells is a prominent feature of COVID-19. Cell Mol Immunol. 2020;17:1101–03. doi:10.1038/s41423-020-00542-2.
- Mubarak A, Alturaiki W, Hemida MG. Middle east respiratory syndrome coronavirus (MERS-CoV): infection, immunological response, and vaccine development. J Immunol Res. 2019;2019:6491738. doi:10.1155/2019/6491738.
- Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–13. doi:10.1016/S0140-6736(20)30211-7.
- Snijder EJ, van der Meer Y, Zevenhoven-Dobbe J, Onderwater JJ, van der Meulen J, Koerten HK, Mommaas AM. Ultrastructure and origin of membrane vesicles associated with the severe acute respiratory syndrome coronavirus replication complex. J Virol. 2006;80(12):5927–40. doi:10.1128/JVI.02501-05.
- Niemeyer D, Zillinger T, Muth D, Zielecki F, Horvath G, Suliman T, Barchet W, Weber F, Drosten C, Muller MA, et al. Middle East respiratory syndrome coronavirus accessory protein 4a is a type I interferon antagonist. J Virol. 2013;87(22):12489–95. doi:10.1128/JVI.01845-13.
- Menachery VD, Schafer A, Burnum-Johnson KE, Mitchell HD, Eisfeld AJ, Walters KB, Nicora CD, Purvine SO, Casey CP, Monroe ME, et al. MERS-CoV and H5N1 influenza virus antagonize antigen presentation by altering the epigenetic landscape. Proc Natl Acad Sci U S A. 2018;115(5):E1012–E21. doi:10.1073/pnas.1706928115.
- Canton J, Fehr AR, Fernandez-Delgado R, Gutierrez-Alvarez FJ, Sanchez-Aparicio MT, Garcia-Sastre A, Perlman S, Enjuanes L, Sola I. MERS-CoV 4b protein interferes with the NF-kappaB-dependent innate immune response during infection. PLoS Pathog. 2018;14:e1006838. doi:10.1371/journal.ppat.1006838.
- Yang JR, Deng DT, Wu N, Yang B, Li HJ, Pan XB. Persistent viral RNA positivity during recovery period of a patient with SARS-CoV-2 infection. J Med Virol. 2020. doi:10.1002/jmv.25940.
- Thoms M, Buschauer R, Ameismeier M, Koepke L, Denk T, Hirschenberger M, Kratzat H, Hayn M, Mackens-Kiani T, Cheng J, et al. Structural basis for translational shutdown and immune evasion by the Nsp1 protein of SARS-CoV-2. Science. 2020;369:1249–55. doi:10.1126/science.abc8665.
- Xia H, Cao Z, Xie X, Zhang X, Chen JY, Wang H, Menachery VD, Rajsbaum R, Shi P-Y. Evasion of type i interferon by SARS-CoV-2. Cell Rep. 2020;33(1):108234. doi:10.1016/j.celrep.2020.108234.
- Shin D, Mukherjee R, Grewe D, Bojkova D, Baek K, Bhattacharya A, Schulz L, Widera M, Mehdipour AR, Tascher G, et al. Papain-like protease regulates SARS-CoV-2 viral spread and innate immunity. Nature. 2020;587(7835):657–62. doi:10.1038/s41586-020-2601-5.
- Vithani N, Ward MD, Zimmerman MI, Novak B, Borowsky JH, Singh S, Bowman GR. SARS-CoV-2 Nsp16 activation mechanism and a cryptic pocket with pan-coronavirus antiviral potential. Biophys J. 2021. doi:10.1016/j.bpj.2021.03.024.
- Majumdar P, Niyogi S. ORF3a mutation associated with higher mortality rate in SARS-CoV-2 infection. Epidemiol Infect. 2020;148:e262. doi:10.1017/S0950268820002599.
- Zinzula L. Lost in deletion: the enigmatic ORF8 protein of SARS-CoV-2. Biochem Biophys Res Commun. 2021;538:116–24. doi:10.1016/j.bbrc.2020.10.045.
- Ramirez Hernandez E, Hernandez-Zimbron LF, Martinez Zuniga N, Leal-Garcia JJ, Ignacio Hernandez V, Ucharima-Corona LE, Pérez Campos E, Zenteno E. The role of the SARS-CoV-2 S-protein glycosylation in the Interaction of SARS-CoV-2/ACE2 and immunological responses. Viral Immunol. 2021;34:165–73. doi:10.1089/vim.2020.0174.
- Thomas S. The structure of the membrane protein of SARS-CoV-2 resembles the sugar transporter SemiSWEET. Pathog Immun. 2020;5:342–63. doi:10.20411/pai.v5i1.377.
- Zhou T, Tsybovsky Y, Gorman J, Rapp M, Cerutti G, Chuang G-Y, Katsamba PS, Sampson JM, Schön A, Bimela J, et al. Cryo-EM structures of SARS-CoV-2 spike without and with ACE2 reveal a pH-dependent switch to mediate endosomal positioning of receptor-binding domains. Cell Host Microbe. 2020;28(6):867–79 e5. doi:10.1016/j.chom.2020.11.004.
- Lu F. SARS-CoV-2 ORF9c: a mysterious membrane-anchored protein that regulates immune evasion? Nat Rev Immunol. 2020;20:648. doi:10.1038/s41577-020-00449-z.
- Khan RJ, Jha RK, Singh E, Jain M, Amera GM, Singh RP, Muthukumaran J, Singh AK. Identification of promising antiviral drug candidates against non-structural protein 15 (NSP15) from SARS-CoV-2: an in silico assisted drug-repurposing study. J Biomol Struct Dyn. 2020:1–11. doi:10.1080/07391102.2020.1814870.
- DrugBank. ChAdOx1 nCoV-19; 2020.
- Folegatti PM, Bittaye M, Flaxman A, Lopez FR, Bellamy D, Kupke A, Mair C, Makinson R, Sheridan J, Rohde C, et al. Safety and immunogenicity of a candidate Middle East respiratory syndrome coronavirus viral-vectored vaccine: a dose-escalation, open-label, non-randomised, uncontrolled, phase 1 trial. Lancet Infect Dis. 2020;20(7):816–26. doi:10.1016/S1473-3099(20)30160-2.
- Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020;38:1–9. doi:10.12932/AP-200220-0772.
- Lu S. Timely development of vaccines against SARS-CoV-2. Emerg Microbes Infect. 2020;9:542–44. doi:10.1080/22221751.2020.1737580.
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–15. doi:10.1056/NEJMoa2034577.
- Soiza RL, Scicluna C, Thomson EC. Efficacy and safety of COVID-19 vaccines in older people. Age Ageing. 2021;50(2):279–83. doi:10.1093/ageing/afaa274.
- Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111.
- Logunov DY, Dolzhikova IV, Shcheblyakov DV, Tukhvatulin AI, Zubkova OV, Dzharullaeva AS, Kovyrshina AV, Lubenets NL, Grousova DM, Erokhova AS, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397(10275):671–81. doi:10.1016/S0140-6736(21)00234-8.
- Sadoff J, Le Gars M, Shukarev G, Heerwegh D, Truyers C, de Groot AM, Stoop J, Tete S, Van Damme W, Leroux-Roels I, et al. Interim results of a phase 1-2a trial of Ad26.COV2.S Covid-19 vaccine. N Engl J Med. 2021. doi:10.1056/NEJMoa2034201.
- Jones I, Roy P. Sputnik V COVID-19 vaccine candidate appears safe and effective. Lancet. 2021;397(10275):642–43. doi:10.1016/S0140-6736(21)00191-4.
- Palacios R, Patino EG, de Oliveira Piorelli R, Conde M, Batista AP, Zeng G, Xin Q, Kallas EG, Flores J, Ockenhouse CF, et al. Double-blind, randomized, placebo-controlled phase III clinical trial to evaluate the efficacy and safety of treating healthcare professionals with the adsorbed COVID-19 (Inactivated) vaccine manufactured by Sinovac - PROFISCOV: a structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21(1):853. doi:10.1186/s13063-020-04775-4.
- Xia S, Duan K, Zhang Y, Zhao D, Zhang H, Xie Z, Li X, Peng C, Zhang Y, Zhang W, et al. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA. 2020;324(10):951–60. doi:10.1001/jama.2020.15543.
- Mahase E. Covid-19: novavax vaccine efficacy is 86% against UK variant and 60% against South African variant. BMJ. 2021;372:n296. doi:10.1136/bmj.n296.
- Zhao J, Zhao S, Ou J, Zhang J, Lan W, Guan W, Wu X, Yan Y, Zhao W, Wu J, et al. COVID-19: coronavirus vaccine development updates. Front Immunol. 2020;11:602256. doi:10.3389/fimmu.2020.602256.
- Garg H, Mehmetoglu-Gurbuz T, Joshi A. Recent advances in zika virus vaccines. Viruses. 2018:10. doi:10.3390/v10110631.
- van Doremalen N, Lambe T, Spencer A, Belij-Rammerstorfer S, Purushotham J, Port J, Avanzato VA, Bushmaker T, Flaxman A, Ulaszewska M, et al. ChAdOx1 nCoV-19 vaccination prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature. 2020 Oct;586(7830):578–582. doi:10.1038/s41586-020-2608-y. Epub 2020 Jul 30.
- Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–16. doi:10.1056/NEJMoa2035389.
- Meyer M, Wang Y, Edwards D, Smith GR, Rubenstein AB, Ramanathan P, Mire CE, Pietzsch C, Chen X, Ge Y, et al. mRNA-1273 efficacy in a severe COVID-19 model: attenuated activation of pulmonary immune cells after challenge. bioRxiv. 2021. doi:10.1101/2021.01.25.428136.
- Voysey M, Costa Clemens SA, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE, et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397:881–91.
- Ramasamy MN, Minassian AM, Ewer KJ, Flaxman AL, Folegatti PM, Owens DR, Voysey M, Aley PK, Angus B, Babbage G, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396:1979–93. doi:10.1016/S0140-6736(20)32466-1.
- Ahmed SF, Quadeer AA, McKay MR. Preliminary identification of potential vaccine targets for the COVID-19 coronavirus (SARS-CoV-2) based on SARS-CoV immunological studies. Viruses. 2020:12. doi:10.3390/v12030254.
- Dhama K, Sharun K, Tiwari R, Dadar M, Malik YS, Singh KP, Chaicumpa W. COVID-19, an emerging coronavirus infection: advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics. Hum Vaccin Immunother. 2020Jun 2;16(6):1232–1238. doi:10.1080/21645515.2020.1735227. Epub 2020 Mar 18.
- Shieber J. Codagenix raises $20 million for a new flu vaccine and other therapies. Tech Crunch; [ accessed 2020 Feb 28]. https://techcrunchcom/2020/01/13/codagenix-raises-20-million-for-a-new-flu-vaccine-and-other-therapies/. Return to ref 23 in article 2020.
- Plotkin S. Vaccines: correlates of vaccine-induced immunity. Clin Infect Dis. 2008;47:401–09. doi:10.1086/589862.
- Qiang GLB, Mao H, Wang L, Xu K, Yang M, Li Y, Zhu L, Wang N, Lv Z, Gao H, et al. Rapid development of an inactivated vaccine for SARS-CoV-2. Science. 2020 Jul 3;369(6499):77–81. doi:10.1126/science.abc1932. Epub 2020 May 6.
- Zverev VV, Katlinskii AV, Kostinov MP, Zhirova SN, Erofeeva MK, Stukova MA, Korovkin SA, Mel’nikov SI, Semchenko AV, Mironov AN, et al. Comparative clinical trial of vaccines against avian influenza. Zh Mikrobiol Epidemiol Immunobiol. 2007;(3):10–6.
- Yang ZN, Zhao YY, Li L, Gao HD, Cai Q, Sun XX, Zhang FS, Su JF, Zhang YN, Shu X, et al. Evaluation of safety of two inactivated COVID-19 vaccines in a large-scale emergency use. Zhonghua Liu Xing Bing Xue Za Zhi. 2021;42:1–6. doi:10.3760/cma.j.cn112338-20210325-00249.
- Zhang Y, Zeng G, Pan H, Li C, Hu Y, Chu K, Han W, Chen Z, Tang R, Yin W, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:181–92. doi:10.1016/S1473-3099(20)30843-4.
- Hemida MG, Ba Abduallah MM. The SARS-CoV-2 outbreak from a one health perspective. One Health. 2020:100127. doi:10.1016/j.onehlt.2020.100127.
- Zhu X, Liu Q, Du L, Lu L, Jiang S. Receptor-binding domain as a target for developing SARS vaccines. J Thorac Dis. 2013:S142–S8. doi:10.3978/j.2072-1439.2013.06.06.
- Pang J, Wang MX, Ang IYH, Tan SHX, Lewis RF, Chen JI, Gutierrez RA, Gwee SXW, Chua PEY, Yang Q, et al. Potential rapid diagnostics, vaccine and therapeutics for 2019 novel coronavirus (2019-nCoV): a systematic review. J Clin Med. 2020:9. doi:10.3390/jcm9030623.
- Biopharmaceuticals C. Clover initiates development of recombinant subunit-trimer vaccine for Wuhan coronavirus (2019-nCoV). 2020:2020. AP news Press release,01/28/2020, avaliable at https://apnews.com/press-release/pr-businesswire/fe24e251ddb843a9b091561a06f8eead
- Zhang Y, Zhao W, Mao Y, Chen Y, Wang S, Zhong Y, Su T, Gong M, Du D, Lu X, et al. Site-specific N-glycosylation characterization of recombinant SARS-CoV-2 spike proteins. Mol Cell Proteomics. 2021;20:100058. doi:10.1074/mcp.RA120.002295.
- McKee A, MacLeod M, Kappler J, Marrack P. Immune mechanisms of protection: can adjuvants rise to the challenge? BMC Biol. 2010;8:37. doi:10.1186/1741-7007-8-37.
- Donnelly JJ, Wahren B, Liu MA. DNA vaccines: progress and challenges. J Immunol. 2005;175:633–39. doi:10.4049/jimmunol.175.2.633.
- Ferraro B, Morrow MP, Hutnick NA, Shin TH, Lucke CE, Weiner DB. Clinical applications of DNA vaccines: current progress. Clin Infect Dis. 2011;53:296–302. doi:10.1093/cid/cir334.
- Yuan M, Huang D, Lee CD, Wu NC, Jackson AM, Zhu X, Liu H, Peng L, van Gils MJ, Sanders RW, et al. Structural and functional ramifications of antigenic drift in recent SARS-CoV-2 variants. bioRxiv. 2021 May 20;eabh1139. doi:10.1126/science.abh1139.
- Zhou D, Dejnirattisai W, Supasa P, Liu C, Mentzer AJ, Ginn HM, Zhao Y, Duyvesteyn HME, Tuekprakhon A, Nutalai R, et al. Evidence of escape of SARS-CoV-2 variant B.1.351 from natural and vaccine-induced sera. Cell. 2021;184(9):2348–2361.e6. doi:10.1016/j.cell.2021.02.037.
- Liu Z, VanBlargan LA, Bloyet LM, Rothlauf PW, Chen RE, Stumpf S, Zhao H, Errico JM, Theel ES, Ellebedy A, et al. Landscape analysis of escape variants identifies SARS-CoV-2 spike mutations that attenuate monoclonal and serum antibody neutralization. bioRxiv. 2020Nov 8;2020.11.06.372037. doi:10.1101/2020.11.06.372037.
- Weisblum Y, Schmidt F, Zhang F, DaSilva J, Poston D, Lorenzi JC, Muecksch F, Rutkowska M, Hoffmann -H-H, Michailidis E, et al. Escape from neutralizing antibodies by SARS-CoV-2 spike protein variants. Elife. 2020:9. doi:10.7554/eLife.61312.
- Hemida MG. The next-generation coronavirus diagnostic techniques with particular emphasis on the SARS-CoV-2. J Med Virol. 2021. doi:10.1002/jmv.26926.
- Wu K, Werner AP, Moliva JI, Koch M, Choi A, Stewart-Jones GBE, Bennett H, Boyoglu-Barnum S, Shi W, Graham BS, et al. mRNA-1273 vaccine induces neutralizing antibodies against spike mutants from global SARS-CoV-2 variants. bioRxiv. 2021 Jan 25;2021.01.25.427948. doi:10.1101/2021.01.25.427948.
- Felgenhauer U, Schoen A, Gad HH, Hartmann R, Schaubmar AR, Failing K, Drosten C, Weber F. Inhibition of SARS-CoV-2 by type I and type III interferons. J Biol Chem. 2020;295:13958–64. doi:10.1074/jbc.AC120.013788.
- Yu J, Yuan X, Chen H, Chaturvedi S, Braunstein EM, Brodsky RA. Direct activation of the alternative complement pathway by SARS-CoV-2 spike proteins is blocked by factor D inhibition. Blood. 2020;136:2080–89. doi:10.1182/blood.2020008248.
- Volpatti LR, Wallace RP, Cao S, Raczy MM, Wang R, Gray LT, Alpar AT, Briquez PS, Mitrousis N, Marchell TM, et al. Polymersomes decorated with SARS-CoV-2 spike protein receptor binding domain elicit robust humoral and cellular immunity. bioRxiv. 2021. doi:10.1101/2021.04.08.438884.
- Li S, Jiang L, Li X, Lin F, Wang Y, Li B, Jiang T, An W, Liu S, Liu H, et al. Clinical and pathological investigation of patients with severe COVID-19. JCI Insight. 2020Jun 18;5(12):e138070. doi:10.1172/jci.insight.138070.
- Shirato K, Kizaki T. SARS-CoV-2 spike protein S1 subunit induces pro-inflammatory responses via toll-like receptor 4 signaling in murine and human macrophages. Heliyon. 2021;7:e06187. doi:10.1016/j.heliyon.2021.e06187.
- Root-Bernstein R. Innate receptor activation patterns involving TLR and NLR synergisms in COVID-19, ALI/ARDS and sepsis cytokine storms: a review and model making novel predictions and therapeutic suggestions. Int J Mol Sci. 2021:22. doi:10.3390/ijms22042108.
- Zuin M, Rigatelli G, Zuliani G, Roncon L. Fatigue as long-term consequence of ARDS in COVID-19 patients. Anaesth Crit Care Pain Med. 2021;40:100787. doi:10.1016/j.accpm.2020.10.016.