ABSTRACT
Although the administration of the Bacillus Calmette–Guérin (BCG) vaccine is generally safe, lymphadenitis, the most common complication of BCG vaccination, can occur. Here, we describe the epidemiological characteristics and incidence trends of BCG lymphadenitis in Shanghai, China, among a population with a high burden of tuberculosis. A total of 56 cases of adverse events following immunization (AEFI) after BCG vaccination were reported in Shanghai, including 51 cases of BCG lymphadenitis (91.07%), from 2010 to 2019. The general incidence of BCG lymphadenitis was 173 per 1,000,000 doses in Shanghai from 2010 to 2019. A nonsignificant increase of 58.81% per year was observed between 2010 and 2012 (t = 0.93; p = .40), followed by a significant decline of 28.00% per year from 2012 to 2019 (t = −4.27; p < .01). Seven batches of BCG vaccines triggered three or more BCG lymphadenitis cases, for 27 (52.94%) cases in total. We identified two patients with immunodeficiency of chronic granulomatous disease, one of whom died four years later after BCG vaccination and another of whom was still being treated after two transplants. The average total care cost of the 47 recovered cases was 11,336 RMB (range: 2,637–33,861 RMB). Due to the high burden of BCG lymphadenitis, especially in children with immunodeficiency, it is suggested that government departments should strengthen healthcare provider training, assign specific nurses to perform BCG vaccination, monitor vaccinated individuals actively and timely detect abnormal signals so as to reduce the incidence of BCG lymphadenitis.
Introduction
The World Health Organization (WHO) recommends that all healthy newborns in countries or regions with high incidence rates of tuberculosis receive one dose of the Bacillus Calmette–Guérin (BCG) vaccine, the only effective measure against tuberculosis.Citation1 The BCG vaccine has been used to protect against tuberculosis in China since 1954 and was included in the national immunization program in 1978. Since then, more than one billion doses have been administered.Citation2 A systematic review and meta-analysis showed that the effectiveness of BCG vaccination for tuberculosis in newborns was 59%, which could reduce the risk of severe tuberculosis by 90%.Citation3 Also, BCG vaccination reduced the incidence of tuberculosis meningitis by 73% and the incidence of miliary tuberculosis by 77%.Citation4
The BCG vaccine uses a live attenuated strain of Mycobacterium bovis which still has antigenic activity. After intradermal injection, this organism starts multiplying rapidly at the site of inoculation and is later transported through the lymphatics to the regional lymph glands; this is followed by hematogenous dissemination, resulting in the creation of very small foci in different organs.Citation5 Therefore, following BCG vaccination, pathological reactions will occur both in the inoculation site and in regional lymph nodes, the vast majority of people will form scar at the inoculation site, and subclinical lymphadenitis can also be spontaneously reduced. However, a few people develop extensive local ulcers or regional lymphadenitis at the site of inoculation. In addition to the complications mentioned above, disseminated infection may even occur in cases with primary immunodeficiency diseases after BCG inoculation.Citation6 From 2009 to 2010, the reported incidence of adverse BCG reactions in China was 38.84 per 1,000,000 doses, with case of BCG lymphadenitis accounting for 67.67%, followed by local abscess (6.67%), allergic rash (4.59%), and systemic dissemination of BCG-induced infection (0.31%).Citation7 BCG lymphadenitis and rare systemically spread infection caused by BCG vaccination greatly affect the level of public confidence in the safety of BCG vaccine due to its severity, slow recovery and long course of disease. As far as we know, BCG lymphadenitis cases in China are mainly reported by individuals,Citation8 with rare epidemiological characteristics and trends observed. Here, we conducted a study to describe the epidemiological characteristics and incidence trends of BCG lymphadenitis in Shanghai, China, and to provide a scientific data basis for BCG vaccination and treatment strategy.
Methods
Data collection
China’s adverse events following immunization (AEFI) monitoring system was launched in 2005. In this study, the cases of adverse events following BCG vaccination in the central area of Shanghai (Xuhui and Huangpu districts) were documented in China’s AEFI monitoring system from 2005 to 2019. Information regarding these cases, including demographic characteristics, BCG vaccination, diagnosis and treatment, outcome of the disease, and compensation from the government, was collected. Hospitals’ obstetric clinics are responsible for the BCG vaccination of newborns in Shanghai. For premature low-weight infants and newborns with certain diseases, BCG vaccination should be performed in a designated clinic in each district after the infant reaches a minimum weight (> 2.5 kg). The doses and numbers of BCG vaccinations performed in each clinic were collected from the Shanghai vaccination rate monitoring system. From 2005 to 2019, the BCG vaccine used in Shanghai was produced by two companies, the Shanghai Institute of Biological Products (Shanghai, China) and the Chengdu Institute of Biological Products (Chengdu, China).
Diagnostic criteria for BCG lymphadenitis
Patients with isolated axillary (or supraclavicular/cervical) lymph node enlargement without fever or other constitutional symptoms within two years of BCG vaccination were clinically diagnosed with BCG lymphadenitis.Citation5,Citation9 Puncture of enlarged lymph nodes or acid-fast stain of secretions is helpful in the diagnosis of BCG lymphadenitis.Citation9
Statistical analysis
BCG lymphadenitis cases were classified and analyzed according to the BCG vaccination date. Chi-square test was used to compare varying incidence rate of BCG lymphadenitis caused by different factors. Since no BCG lymphadenitis cases were reported in Shanghai from 2005 to 2009, the data analysis performed was limited to the period from 2010 to 2019. Annual percentage changes (APCs) of BCG lymphadenitis, from 2010 to 2019, were analyzed using Joinpoint regression models (Joinpoint; Division of Cancer Control and Population Sciences, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA).Citation10 The APC represents the slope of a given trend, where a value of less than 0 indicates a downward trend year by year and that of greater than 0 indicates an upward trend year by year. The significance test chosen for a change in trend was the t-test of interval slope, and a p-value of less than 0.05 was considered to be statistically significant. Data were analyzed with the Statistical Package for the Social Sciences version 18.0 (IBM Corporation, Armonk, NY, USA) and Joinpoint version 4.3.1.
Results
Epidemiological characteristics of BCG lymphadenitis
A total of 56 cases of AEFI after BCG vaccination were reported in Shanghai, including 51 cases of BCG lymphadenitis, accounting for 91.07%, with three cases of local abscess and two cases of disseminated BCG lymphadenitis from 2010 to 2019. No cases of BCG AEFI were reported between 2005 and 2009.
The general incidence of BCG lymphadenitis was 173 per 1,000,000 doses in Shanghai from 2010 to 2019. Most cases of BCG lymphadenitis occurred after BCG vaccination in the hospital obstetrics clinic for newborns (96.08%). Only two cases of BCG lymphadenitis occurred after vaccination in a designated BCG vaccination clinic for infants. The incidence of BCG lymphadenitis in newborns was lower than that in infants after BCG vaccination (171 per 1,000,000 doses vs. 256 per 1,000,000 doses), but there was no statistically significant difference between the two incidence rates (p = .321).The incidence of BCG lymphadenitis in Xuhui district was significantly higher than that in Huangpu district (219 per 1,000,000 doses vs. 67 per 1,000,000 doses; p < .01). The incidence of BCG lymphadenitis was significantly higher from 2010 to 2014 period than from 2015 to 2009 period (266 per 1,000,000 doses vs. 76 per 1,000,000 doses; p < .01), as shown in .
Table 1. Epidemiological characteristics of BCG lymphadenitis cases in Shanghai, 2010–2019
Impact of vaccine manufacturer and batch number
Among the 51 cases of BCG lymphadenitis reported in Shanghai from 2010 to 2019, 37 cases were caused by BCG vaccines produced by the Shanghai Institute of Biological Products (accounting for 72.55%). The incidence of lymphadenitis after using of BCG vaccines produced by the Shanghai Institute of Biological Products was higher than that when using vaccines produced by the Chengdu Institute of Biological Products (234 per 1,000,000 doses vs. 102 per 1,000,000 doses; p < .01). These two vaccine manufacturers supplied BCG simultaneously in 2010, 2011, 2014, 2015 and 2019. There were no statistical differences in the incidence of BCG lymphadenitis in these five years between these two producers (147 per 1,000,000 doses vs. 170 per 1,000,000 doses Supplementary Figure 1).
The 51 BCG lymphadenitis cases involved 24 batches of vaccines produced by the Shanghai Institute of Biological Products and the Chengdu Institute of Biological Products. Seven batches of BCG vaccines caused three or more BCG lymphadenitis cases, totaling 27 (52.94%) cases in all, as shown in .
Clinical characteristics of cases
The average age of onset of lymphadenitis among the 51 BCG lymphadenitis cases was 4.10 months. The average time between vaccination and the onset of lymphadenitis was 130.02 days, as shown in . The time between vaccination and lymphadenitis onset was one to six months in 80.39% of the cases; meanwhile, in 13.73% of the cases, the time was more than six months, while, in the remaining 5.88% of the cases, the time was less than one month. Among the 51 BCG lymphadenitis cases, 35 cases were diagnosed by lymph node puncture and acid-fast staining, including 33 cases whose smear staining was positive (64.71%). In 39 cases (76.47%), enlarged lymph nodes were found only in the ipsilateral axillary region after BCG vaccination, while in the remaining 12 cases (23.53%), enlarged lymph nodes were also found in other regions, such as the neck, clavicle and groin. Except for two cases where the lymph node number was not available, 32 cases (62.75%) presented only one enlarged lymph node, while the remaining 17 cases (33.33%) presented two or more enlarged lymph nodes. Six cases did not report the diameter of the enlarged lymph nodes, 42 cases (82.35%) had enlarged lymph nodes with a diameter greater than 10 mm, and the remaining three cases had enlarged lymph nodes with a diameter of less than 10 mm.
Table 2. Clinical characteristics of BCG lymphadenitis cases in Shanghai from 2010 to 2019
Only in seven cases (13.73%) the enlarged lymph nodes ruptured during the follow-up period. Four cases underwent drainage surgery (7.84%), 18 cases received antituberculosis therapy (35.29%), and 36 cases received thymosin to improve immunity during treatment (70.59%). There were two patients with immunodeficiency of chronic granulomatous disease, one of whom died four years later after BCG vaccination. The postmortem diagnoses of this death case were septic shock, acute respiratory distress syndrome, severe pneumonia, metabolic acidosis, acute liver failure, acute suppurative tonsillitis, acute upper gastrointestinal bleeding, chronic granulomatosis, harmful effects of the BCG vaccine and hypoglycemia. Another case is still undergoing treatment after two transplants. Of the remaining 49 cases, 48 cases were cured (94.12%) and one case remains under treatment.
Trends of BCG lymphadenitis incidence
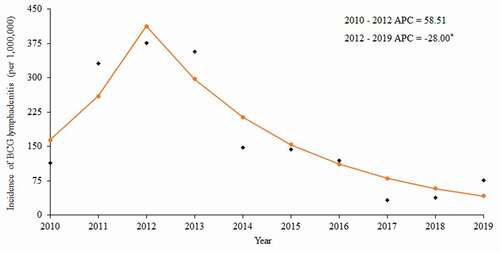
During the period of 2010 to 2019, cases of BCG lymphadenitis occurred in Shanghai in all years except 2018, with a reported rate ranging from 33 per 1,000,000 doses to 376 per 1,000,000 doses. The Joinpoint software regression model detected one joinpoint in 2012. A nonsignificant increase of 58.81% per year was observed between 2010 and 2012 (t = 0.93; p = .40), followed by a significant decline of 28.00% per year from 2012 to 2019 (t = −4.27; p < .01) ().
Figure 1. Trends of BCG lymphadenitis incidence in Shanghai from 2010 to 2019.
Burden of BCG lymphadenitis
A total of 47 cases of lymphadenitis after BCG vaccination submitted compensation applications after recovery. The average total cost of these 47 cases was 11,336 RMB (range: 2,637–33,861 RMB), with the average medical cost being 9,604 RMB (range: 1,347–25,415 RMB). Since 2015, patients with BCG lymphadenitis in Shanghai can be compensated for transportation costs arising from medical treatment in addition to medical costs. The average cost of transportation for 20 cases was 3,656 RMB (range: 302–9,670 RMB), as shown in .
Table 3. List of BCG lymphadenitis case costs in Shanghai from 2010 to 2019
Discussion
During 2010 to 2019, BCG lymphadenitis accounted for 91.06% of AEFIs after BCG vaccination in Shanghai, China, similar to the monitoring results reported by a hospital (91.58%) during 2007 to 2016,Citation11 but higher than the AEFI monitoring data of China (84.26%)Citation5 and South Korea (67.05%)Citation12 and the results of other studies (61.33%–80.43%).Citation13–15 During the study period, the incidence of BCG lymphadenitis was 173 per 1,000,000 doses, higher than the national level of BCG lymphadenitis (33 per 1,000,000).Citation7 China’s AEFI monitoring system is a passive surveillance system. The higher AEFI reporting sensitivity in eastern and central regions, including Shanghai, than that in western China may be the main reason for why the incidence of BCG lymphadenitis in Shanghai was higher than the national average level.Citation7 The incidence of BCG lymphadenitis reported in our study was significantly lower than that reported in Babor, Iran from 2011 to 2013 (0.93%),Citation16 in Latvia from 2005 to 2013 (0.11%),Citation17 in France in 2007 (0.1%),Citation18 and in Georgia in the United States in 2002 (1.12 cases per 1,000 people).Citation9 The incidence of BCG lymphadenitis in our study may be underestimated due to the use of passive surveillance data. Therefore, although the reported incidence in Shanghai was higher than the national level, the AEFI monitoring sensitivity still needs to be strengthened, especially for certain rare adverse reactions.
Among the BCG lymphadenitis cases we detected in Shanghai, two patients had a clear history of chronic granuloma, of whom one died and the other remained uncured after two transplant treatments. In addition to these two cases and one newly reported case, all of the remaining 48 BCG lymphadenitis cases have recovered. Our work reveals that, in nearly one in seven BCG lymphadenitis cases, enlarged lymph nodes become purulent and rupture. According to the Shanghai vaccination work norms, BCG lymphadenitis with lymph node enlargement or rupture should be treated with antituberculosis treatment.Citation19 This is based on the opinion that simple lymphadenitis may have a risk of turning into pyogenic lymphadenitis and isoniazid is recommended.Citation20 Therefore, more than one third of cases were treated with antituberculosis therapy in this study. However, the efficacy of antituberculous therapy in treating local complications of BCG is unclear.Citation21 Studies have shown that antituberculosis drugs are ineffective in accelerating the resolution of BCG lymphadenitis or preventing suppuration.Citation5 For nonsuppurative lymphadenitis, the conservative approach of observation and waiting is recommended.Citation14,Citation22 Puncture aspiration is the only effective treatment that can improve the healing of suppurative nodules.Citation23 In addition, antituberculosis medication may induce liver lesions in 8% of cases.Citation24 Therefore, the treatment of BCG lymphadenitis in Shanghai needs to be further optimized, such as with the use of antituberculosis dugs. More than 70% of the reported cases used immunotherapy, whose effectiveness needs to be further verified. Due to the long course and heavy burden of BCG lymphadenitis cases, it is necessary to take certain measures to reduce the incidence of BCG lymphadenitis while better standardizing the treatment.
The causes of BCG lymphadenitis include virulence of the vaccine strain, uniformity and concentration of bacterial fluid, vaccine dose, age of vaccination, immune response to vaccine, vaccination method, and vaccination technique.Citation5 Studies showed that low age at vaccination was a high risk factor for adverse events.Citation18 However, the incidence of BCG lymphadenitis in newborns was lower than that in infants (171 per 1,000,000 doses vs. 256 per 1,000,000 doses) in our study. Surveillance data from Taiwan, China, also showed that there were more cases of lymphadenitis when BCG vaccination was performed above five months of age as with at birth, but with a shorter course of disease.Citation25 More than half of the cases were related to seven concentrated vaccination batch numbers. Chinese monitoring data also showed clustering characteristics of some batch numbers related to BCG lymphadenitis.Citation7 A study by Soh et al. found that the sharp increase in BCG lymphadenitis might be related to the vaccine batch number, after excluding vaccine administration–related and host–related factors.Citation26 After the vaccine strain was switched, more BCG lymphadenitis cases were found in the United States,Citation9 Saudi Arabia,Citation27 South Korea,Citation12 and Gaza.Citation15 The Denmark II strain has been used in China since 1993.Citation2 Therefore, monitoring of BCG–related AEFIs should be strengthened when considering vaccine strain changes. The risk of BCG lymphadenitis is higher with subcutaneous BCG vaccination than with intradermal vaccination.Citation12 The BCG vaccination method used in China is intradermal vaccination which is more stringent. Nurses are prone to inject BCG vaccine into the subcutaneous or intramuscular region when they are not skilled, which leads to an increased risk of BCG lymphadenitis. Therefore, it is recommended to implement a standardized BCG vaccination protocol, such as strengthening personnel training and selecting fixed inoculation personnel, to avoid adverse events and variations. In addition, government departments should also strengthen monitoring for AEFIs and adjust the vaccine varieties in a timely fashion when signals indicating increasing AEFI cases are observed so as to reduce the incidence of BCG lymphadenitis.
Our study showed that the incidence of BCG lymphadenitis in Shanghai increased rapidly from 2010 to 2012 but has decreased since 2012. A rising trend in BCG lymphadenitis incidence was also observed in Hong Kong, China, from 0.43 per 10,000 doses in 2007 to 3.26 per 10,000 doses in 2011.Citation28 The incidence of BCG lymphadenitis in Latvia also increased from 2005 to 2013, reaching a peak in 2011.Citation17 Since the strain of BCG used in China has not been altered for some time, we believe that the following factors may contribute to the increase in incidence observed in our study. First, there was aggregation among some batches, and it cannot be ruled out that some batches of BCG vaccine produced from 2010 to 2013 might have caused a sharp increase in the incidence. Secondly, the Shanghai Public Health Clinical Center has been designated to diagnose and treat BCG-induced AEFI cases in Shanghai since 2009. Communication between cases in this hospital might have made more patients aware of the compensation policy for BCG lymphadenitis cases, which led to more BCG lymphadenitis cases being reported to the Centers for Disease Control (CDC) on their own initiative. Perhaps because affected patients did not know where to report or for some other unknown reasons, we did not receive any reports of BCG lymphadenitis cases between 2005 and 2009. In addition, in order to find BCG lymphadenitis cases earlier and avoid delays in treatment, all obstetrics departments in Xuhui district give notes to newborns’ parents about the characteristics of BCG lymphadenitis, the designated hospital for treatment, reporting procedures for CDC, and other pertinent information after BCG vaccination. This may have led to more cases being reported to the CDC, which is why the incidence in Xuhui district was higher than that in Huangpu district. A study also reported a five–fold increase in the incidence of BCG lymphadenitis with active hospital-based surveillance as compared with passive surveillance.Citation29 Since 2012, the incidence of BCG lymphadenitis in Shanghai has decreased significantly, and the incidence in the first five years (2010–2014) was 3.5 times that recorded in the more recent five years (2015–2019). In addition to factors of vaccine manufacturers, fixed BCG vaccination nurses in obstetrics departments in Xuhui district since 2014 cannot be excluded as one of the reasons leading to the trend of incidence decline.Citation30
This study has several limitations. First, our AEFI monitoring protocol is passive, which will underestimate the incidence of BCG lymphadenitis to a certain extent. Second, due to differences in BCG vaccination procedures and vaccine strains in different countries and regions, the representativeness of our results is limited. Third, this study did not include the medical costs of the two immunodeficient BCG lymphadenitis cases and indirect losses such as missed work costs other than direct medical costs and transportation costs which resulted in an underestimation of the disease burden.
In conclusion, BCG lymphadenitis accounted for the majority of AEFI cases caused by BCG vaccination in Shanghai from 2010 to 2019. Although the incidence of BCG lymphadenitis in Shanghai was higher than the national surveillance level, it was still lower than that obtained through active surveillance. Due to the long course of disease and heavy disease burden caused by BCG lymphadenitis, especially in children with immunodeficiency, it is suggested that government departments should strengthen the training of healthcare providers, assign specific nurses to perform BCG vaccination, monitor vaccinated patients actively, and timely detect abnormal signals so as to reduce the incidence of BCG lymphadenitis. Additionally, guidelines for the treatment of BCG lymphadenitis should be created and implemented to avoid overtreatment.
Disclosure of potential conflicts of interest
No potential conflicts of interest in preparing this article.
Supplemental Material
Download TIFF Image (37.3 KB)Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2021.1938922.
Additional information
Funding
References
- The World Health Organization. BCG vaccines: WHO position paper. Weekly Epidemiological Rec 2018;93:73–6.
- Xia XZ, Luo HM. Practical vaccination manual. Beijing (China): People’s Health Press; 2010.
- Mangtani P, Abubakar I, Ariti C, Beynon R, Pimpin L, Fine PE, Rodrigues LC, Smith PG, Lipman M, Whiting PF, et al. Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin Infect Dis. 2014;58:470–80. doi:10.1093/cid/cit790.
- Trunz BB, Fine P, Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet. 2006;367:1173–80. doi:10.1016/S0140-6736(06)68507-3.
- Goraya JS, Virdi VS. Bacille Calmette-Guerin lymphadenitis. Postgrad Med J. 2002;78:327–29. doi:10.1136/pmj.78.920.327.
- Norouzi S, Aghamohammadi A, Mamishi S, Rosenzweig SD, Rezaei N. Bacillus Calmette-Guerin (BCG) complications associated with primary immunodeficiency diseases. J Infect. 2012;64:543–54. doi:10.1016/j.jinf.2012.03.012.
- Li KL, Liu DW, Wu WD, Xu DS, Zhen JS, Cao LS, Cao L, Yuan P, Wang HQ. Analysis on surveillance of adverse events following immunization for Bacille Calmette-Guerin vaccine in China, 2009-2010. Chin J Vacc Immunization. 2012;18:252–60.
- Wu WD, Liu DW, Li L. Literature review of adverse events following immunization of Bacillus Calemtte-Guerin vaccine. Zhongguo Yi Miao He Mian Yi. 2009;15:491–95.
- Kuchukhidze G, Kasradze A, Dolakidze T, Baliashvili D, Merabishvili T, Blumberg HM, Kempker RR. Increase in lymphadenitis cases after shift in BCG vaccine strain. Emerg Infect Dis. 2015;21:1677–79. doi:10.3201/2109.150289.
- Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–51. doi:10.1002/(SICI)1097-0258(20000215)19:3<335::AID-SIM336>3.0.CO;2-Z.
- Rermruay R, Rungmaitree S, Chatpornvorarux S, Brukesawan C, Wittawatmongkol O, Lapphra K, Phongsamart W, Kongstan N, Khumcha B, Chokephaibulkit K. Clinical features and outcomes of Bacille Calmette-Guerin (BCG)-induced diseases following neonatal BCG Tokyo-172 strain immunization. Vaccine. 2018;36:4046–53. doi:10.1016/j.vaccine.2018.05.098.
- Roh EJ, Lee YK, Lee MH, Kim MK, Kim TE, Lee SG, Chung EH. Investigation of adverse events following bacille Calmette-Guerin immunization using immunization safety surveillance system in Korea Centers for Disease Control and Prevention. Clin Exp Vaccine Res. 2020;9:133–45. doi:10.7774/cevr.2020.9.2.133.
- Mahmoudi S, Khaheshi S, Pourakbari B, Aghamohammadi A, Keshavarz Valian S, Bahador A, Sabouni F, Ramezani A, Mamishi S. Adverse reactions to Mycobacterium bovis bacille Calmette-Guerin vaccination against tuberculosis in Iranian children. Clin Exp Vaccine Res. 2015;4:195–99. doi:10.7774/cevr.2015.4.2.195.
- Venkataraman A, Yusuff M, Liebeschuetz S, Riddell A, Prendergast AJ. Management and outcome of Bacille Calmette-Guerin vaccine adverse reactions. Vaccine. 2015;33:5470–74. doi:10.1016/j.vaccine.2015.07.103.
- Daoud W. Control of an outbreak of BCG complications in Gaza. Respirology. 2003;8:376–78. doi:10.1046/j.1440-1843.2003.00489.x.
- Barari-Savadkouhi R, Shour A, Masrour-Roudsari J. A study of the incidence of BCG vaccine complications in infants of Babol, Mazandaran (2011-2013). Caspian J Internal Med. 2016;7:48–51.
- Engelis A, Kakar M, Meiksans R, Petersons A. BCG-SSI((R)) vaccine-associated lymphadenitis: incidence and management. Medicina. 2016;52:187–91. doi:10.1016/j.medici.2016.05.001.
- Dommergues MA, de La Rocque F, Guy C, Lecuyer A, Jacquet A, Guerin N, Fagot JP, Boucherat M, d’Athis P, Cohen R. Local and regional adverse reactions to BCG-SSI vaccination: a 12-month cohort follow-up study. Vaccine. 2009;27:6967–73. doi:10.1016/j.vaccine.2009.09.073.
- Shanghai Municipal Health Commission. Shanghai guidelines on vaccination work. 2017 ed. Shanghai; 2017.
- Liu EY, Zhou L, Zhao SY, Li HM. The effects and adverse reactions of Bacillus Calmette-Guérin. Chin J Pract Pediatr. 2016;31:347–49.
- Riordan A, Cole T, Broomfield C. Fifteen-minute consultation: bacillus Calmette-Guerin abscess and lymphadenitis. Arch Dis Child Educ Pract Ed. 2014;99:87–89. doi:10.1136/archdischild-2013-304457.
- Cuello-Garcia CA, Perez-Gaxiola G, Jimenez Gutierrez C. Treating BCG-induced disease in children. The Cochrane Database of Systematic Reviews. 2013;CD008300. doi:10.1002/14651858.CD008300.pub2.
- Banani SA, Alborzi A. Needle aspiration for suppurative post-BCG adenitis. Arch Dis Child. 1994;71:446–47. doi:10.1136/adc.71.5.446.
- Wondwossen A, Waqtola C, Gemeda A. Incidence of antituberculosis-drug-induced hepatotoxicity and associated risk factors among tuberculosis patients in Dawro Zone, South Ethiopia: a cohort study. Int J Mycobacteriol. 2016;5:14–20. doi:10.1016/j.ijmyco.2015.10.002.
- Huang W, Chiu NC, Chi H, Huang FY, Huang CY. Inoculation age of Bacillus Calmette-Guerin Tokyo-172 strain and vaccine-related adverse reactions in Taiwan birth cohort of 2012-2017. Clin Infect Dis. 2020. doi:10.1093/cid/ciaa1235.
- Soh SB, Han PY, Tam KT, Yung CF, Liew WK, Tan NW, Chong CY, Thoon KC. Investigations into an outbreak of suppurative lymphadenitis with BCG vaccine SSI((R)) in Singapore. Vaccine. 2014;32:5809–15. doi:10.1016/j.vaccine.2014.08.032.
- Alrabiaah AA, Alsubaie SS, Bukhari EI, Gad A, Alzamel FA. Outbreak of Bacille Calmette-Guerin-related lymphadenitis in Saudi children at a university hospital after a change in the strain of vaccine. Ann Saudi Med. 2012;32:4–8. doi:10.5144/0256-4947.2012.4.
- Lam TS, Leung YH, Tsang HL, Choi KW, Wong TY, Wong MH, Chuang SK. Bacille-Calmette-Guerin vaccine-associated suppurative lymphadenitis in Hong Kong Special Administrative Region (China), 2004 to 2012. West Pac Surveillance Response J. 2013;4:39–40. doi:10.5365/wpsar.2013.4.1.001.
- Thoon KC, Soh SB, Liew WK, Gunachandran A, Tan NW, Chong CY, Yung CF. Active surveillance of adverse events following childhood immunization in Singapore. Vaccine. 2014;32:5000–05. doi:10.1016/j.vaccine.2014.07.020.
- Zhou Q, Wu QS, Huang Q. Application of health care failure mode and effact analysis in BCG inoculation process of the newborn. Nurs J Chin PLA. 2017;34:61–64.
