ABSTRACT
Objective
Hepatitis-B virus (HBV) infection is an important health problem worldwide. HBV vaccine application varies according to the birth weight and gestational week in the neonatal period. This study aimed to reconsider delaying the administration of the HBV vaccine because the birth weight of newborns was very low.
Methods
The newborns with very low birth weight in the study group were babies weighing less than 2000 g in the postnatal first month and at the time of administering HBV vaccine. Babies born at term from mothers who did not receive an HBV vaccine, had negative hepatitis B surface antibody levels, and were given HBV vaccine at birth were included in the study as a control group. The antibody levels against HBV vaccine were compared between these two groups.
Results
The retrospective study included 60 participants (32 men and 28 women) grouped as control first vaccine weight (first vaccine weight was >2000 g, control group, n = 30) and case vaccine weight (first vaccine weight was <2000 g, case group, n = 30). The mean birth weight was 2976 ± 84.8 g and 1054 ± 44.5 g in the control and case groups, respectively. The first vaccine weight was 2030–3780 g and 960–1900 g in the control and case groups, respectively. The mean antibody level was 297.8 ± 76.3IU/mL and 309.7 ± 56.3IU/mL in the <1500 g and >1500 g groups, respectively. No significant difference was found in hepatitis antibody levels between the groups.
Conclusion
Further studies in larger samples are needed to confirm the efficacy and efficiency of postponement of hepatitis B vaccination in babies with a birth weight of <2000 g.
Introduction
Hepatitis B virus (HBV) infection is an important health problem worldwide. It can cause acute and chronic hepatitis, cirrhosis, and liver cancer. Chronic HBV infection is present in more than 350 million individuals worldwide. A total of 600,000 people die annually due to HBV infection. The disease in adulthood improves to a great extent; however, the disease in childhood becomes chronic by 90%. More than 80% of hepatocellular cancers occur as a result of HBV infection. Therefore, vaccination is very important for the control of HBV infection.Citation1
HBV is transmitted via close contact with infected people (horizontal transmission) and from mother to baby (vertical transmission) in the perinatal period. Vertical transmission from mother to baby mostly (90%) occurs during delivery.Citation1
In the United States, about 20,000 babies are born each year from HBV-infected pregnant women, and about 5500 are chronically infected with HBV without immunoprophylaxis. The prevalence of acute HBV infection among children in the USA decreased by 98% between 1990 and 2014 as a result of universal vaccination against HBV.Citation2 According to the data of the World Health Organization, the prevalence among low birth weight (LBW; ˂2500 g) newborns varies between 5% and 19% in developed and developing countries.Citation3–7 The prevalence among LBW newborns was 10% compared with the Turkey Demographic and Health Survey 2018 data.Citation8
The HBV vaccine should be administered in three doses in the postnatal first month or when the birth weight reaches 2000 g in preterm babies born to mothers who are definitely not known to be HBV carriers.Citation2
HBV vaccination is administered in “0, 1 and 6th month” according to the routine vaccination schedule for children in Turkey. The first vaccine is administered on the day of birth of the infant. The second hepatitis B vaccine are administered at least 1 month after the first one; the third vaccine at least 4 months after the first one and at least 2 months after the second one. However, in babies whose mothers are HBsAg negative and whose birth weight is <2000 g, HBV vaccination is delayed until the baby is more than 2000 g or 1 month old.
Newborns admitted to the Neonatal Intensive Care Unit (NICU) may have additional comorbidities such as infection, respiratory distress, feeding difficulty, and jaundice. This situation may cause impaired immunological responses.Citation9
Anti-HBs shows that the vaccine is protective.Citation10 An anti-HBs level of 10 (IU/mL) in healthy individuals is considered protective against acute and chronic diseases.Citation11
Some previous studies supported low anti-HBs response in premature infants who received HBV vaccine; however, the difference in seropositivity rates between preterm and term groups was generally not statistically significant. These studies did not include quantitative analyses, and studies on HBV vaccination of LBW newborns were insufficient.Citation12–14
Studies on the serological response, especially immune memory, in babies born with very LBW (VLBW) (1500 g) who are administered HBV vaccine are limited.
In this study, the immunity of newborns born with VLBW (˂1500 g) was compared with those of term infants who were given HBV vaccine at birth. Thus, it aimed to reconsider the delay of HBV vaccine application according to birth weight and gestational week.
Materials and methods
This retrospective study was performed in the newborn units of University Hospital between July 2018 and September 2020. The study was approved by the local ethics committee (No.: 2020.226.09.13).
Inclusion criteria
The newborns were those whose weight was less than 2000 g in the postnatal first month and at the time of administering HBV vaccine. The anti-HBs levels were measured in these newborns in the study group 1 month after vaccination (before the second dose of HBV vaccine).
All these babies were born with VLBW (<1500 g) to mothers who did not receive HBV vaccine and had negative anti-HBs levels.
Babies born at term from mothers who did not receive HBV vaccine and had negative anti-HBs levels were given HBV vaccination at birth and included in the study as a control group.
The anti-HBs levels of the newborns in the control group were measured in the first month (before the second dose of the HBV vaccine).
Consecutive and living cases were selected for the study group, considering compliance with the criteria. Consecutive cases were also selected for the control group.
Exclusion criteria
Babies of mothers who were given HBV vaccine or had hepatitis B infection, syndromic babies, babies with immunodeficiency, babies with an actual weight more than 2000 g when HBV vaccine was administered were excluded from the study.
Thirty newborns with birth weight <2 kg constituted the case group, and another 30 newborns with birth weight ≥2 kg constituted the control group.
Demographic characteristics such as maternal age, gestational week, birth weight, Apgar cores, anti-HBs plasma levels 1 month after the first dose and before the second dose of HBV vaccine in both groups, weight at the time of vaccination, sex, type of delivery, diagnosis in the newborn intensive care unit, and accompanying comorbidities were obtained from the file records of the hospital.
HBV immunization protocol
Hepatitis B vaccine (rDNA) (Serum Institute of India Pvt Ltd.) (0.5 mL/10 µg) was administered intramuscularly to the anterior lateral area of the thigh to all infants.
Anti-HBs test (seroprevalence research) protocol
Semi-quantitative analysis of anti-hepatitis B (anti-HBs) antibodies for hepatitis B was performed using a commercially available Elecsys Anti-HBs II Reagent Kit (Roche, IND, USA) for electrochemiluminescent immunoassay (ECLIA) following the manufacturer’s protocols. Adequate antibody response was defined as an antibody titer of ≥10 international units (IU)/dL.
Statistical analysis
Statistical analyses were performed using GraphPad Prism (ver. 7.00). For the nonparametric data set, the two-tailed Mann–Whitney U test was used to determine the P values. Data sets having a P value <.005 were considered as significantly different from the compared data set. All the data were presented as means with the standard error of the mean (mean ± SEM).
Results
The study included 60 participants (32 men and 28 women) grouped as control first vaccine weight (first vaccine weight was >2000 g, control group, n = 30) and case vaccine weight (first vaccine weight was <2000 g, case group, n = 30). However, 40% of the patients were women (n = 12) and 60% were men (n = 18). Further, 60% in the case group showed first vaccine weight less than 1500 g (n = 18), and only 10% were less than 1000 g (n = 3). Also, 33.3% had cesarean delivery (n = 10) in the case group and only 6.6% (n = 2) in the control group. The maternal age was 18–46 years in the control group and 19–37 years in the case group. The mean maternal age was 28.77 ± 6.5 in the control group and 28.03 ± 4.7 years in the case group. The mean birth weight was 2976 ± 84.8 g and 1054 ± 44.5 g in the control and case groups, respectively. The first vaccine weight was 2030–3780 g in the control group and 960–1900 g in the case group ().
Table 1. Diagnostic data of the subjects in the study (Mean ± SEM)
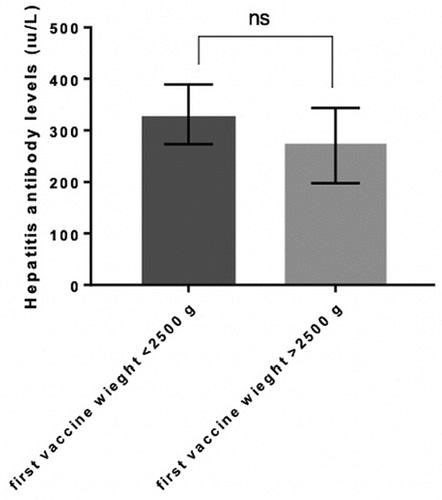
Hepatitis B antibody levels showed no significant difference between the case and control groups (, P > .005) and varied from 2 to 1000 IU/mL in both groups. The mean antibody level was 276.7 ± 66.8 and 335.5 ± 61.72 in the control and case groups, respectively (). The antibody levels of newborns with 2500 and 1500 g vaccine weight were also compared against the remaining participants. However, no significant difference was observed between <2500 g and >2500 g and <1500 g and >1500 g groups in terms of hepatitis antibody levels ( and ; P > .005). Additionally, when the data were grouped as first vaccine weight less than 1500 g and more than 1500 g, the antibody levels were closer to each other compared with the other groups (). The mean antibody level was 297.8 ± 76.3 IU/mL and 309.7 ± 56.3 IU/mL in the <1500 g and >1500 g groups. Moreover, the mean antibody level was 331.4 ± 58 IU/mL and 270.7 ± 72.87 IU/mL in the <2500 g and >2500 g groups ().
Figure 1. Hepatitis antibody level comparing between normal (>2000 g) and the case (<2000 gr). P value <.005 is accepted as significantly different and data presented as means with standard error of the mean.
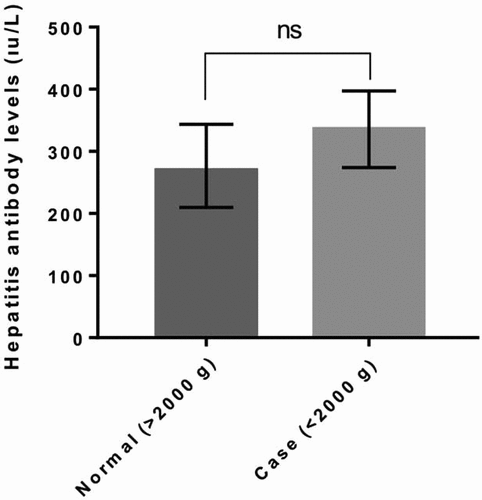
Figure 2. Hepatitis antibody level comparing between first vaccine weight >2500 g and first vaccine weight <2500 gr. P value <.005 is accepted as significantly different and data presented as means with standard error of the mean.
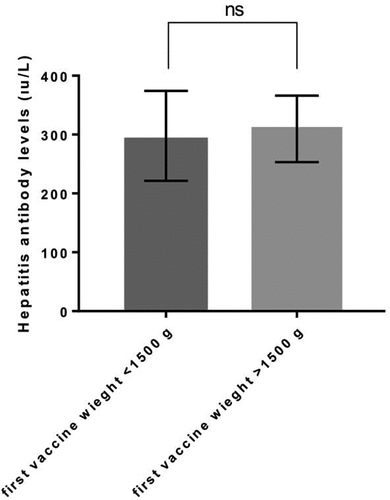
Figure 3. Hepatitis antibody level comparing between first vaccine weight >1500 g and first vaccine weight <1500 gr. P value <.005 is accepted as significantly different and data presented as means with standard error of the mean.
Antenatal steroids and postnatal surfactant were administered to all patients in the case group. Moreover, 30 babies had comorbidities during admission to the NICU: 30, respiratory distress syndrome; 30, administration of intravenous antibiotics (at least for 5 days); 27, jaundice; 14, blood transfusion; 8, pulmonary hypertension; 7, intracranial bleeding; 5, hydrocephalus; 4, necrotizing enterocolitis; 3, neonatal seizure ().
The control group comprised 30 babies, with comorbidities in 5 during admission to the NICU: 3, jaundice; 1, pneumonia; and 1, pneumothorax. Further, 25 babies in the control group had no problems.
Discussion
This study was novel in evaluating the immune response to HBV vaccine within a month following the vaccination in a VLBW infant population.
Infections acquired from HbsAg-positive mother in infancy constitute 40% of all infections and have great clinical and epidemiological importance worldwide.Citation15,Citation16 Many studies reported lower seroconversion rates and anti-HBs concentrations in VLBW and extremely LBW infants administered HBV vaccine. Considering the findings of these studies, the American Academy of Pediatrics recommended a policy of delaying the first HBV vaccine dose until the weight of 2000 g or age of 2 months in babies born to HBsAg-negative mothers and weighing less than 2000 g.Citation15,Citation16 The current recommendation states that it is appropriate to postpone HBV vaccination in babies whose mothers are HBsAg negative and whose birth weight is <2000 g until the baby weighs more than 2000 g or is 1 month old.Citation2 Hence, a standard dosage of HBV vaccine is used for all infants weighing more than 2000 g. The fact that anti-HBs titers were sufficient in VLBW newborns, in whom high antibody levels were detected during follow-up, has raised questions about the adequacy of previous practices regarding HBV vaccination, suggesting that this situation should be reviewed.
Previous studies reported that gestational age was a much more objective parameter than weight to evaluate the immune response in preterm newborns, and concluded that seroprotection rates were generally lower in preterm newborns.Citation17–19 In contrast, Sood et al. reported a relationship between the decrease in immunological response and LBW in the evaluation performed for hepatitis B vaccine according to birth weight in premature newborns.Citation20 There are also several studies reporting the immune response to HBV vaccine is not correlated with the birth weight in both term and preterm infants.Citation13,Citation21 The present study found that birth weight did not affect the immune response of the HBV vaccine, and the protective antibody level was as much as in healthy-term newborns.
In this study, two infants whose birth weight was less than 1000 g, and were born in 28 gestational weeks, were found to have adequate immune response to their first HBV vaccine at the first month of life. HBs antigen and HBs antibodies were negative in their mothers. These results attracted our attention on the antibody response occurred at the age of 28 weeks and in preterm newborns weighing less than ˂1000 g. Anti-HBs level was found to be >10 mIU/mL which is protective against HBV, in most of the infants of case group, so the protection rate 1 month after the first dose of HBV vaccine, was determined as 93,3%. Anti-HBs titer was less than 10 mIU/mL only in two patients from the case group. In the control group, the anti-HBs titer in three newborns was less than 10 mIU/mL. Further, 27 (27/30, protection 90%) newborns had anti-HBs levels >10 mIU/mL.
Although the number of preterm or LBW infants not stated in their study, Süleyman et al. reported that the anti-HBs levels of 912 babies aged 1–5 years were conversely affected by LBW.Citation22 There was no difference between the group of babies weighted 1000–2000 g and babies with a weight more than 2000 g at the time of vaccination, in terms of anti‑HBs antibody levels (). In contrast with the previous studies, we suggest that even VLBW newborns could generate as much antibody response as in term newborns. This contradiction can be explained by the following reasons: previous studies were conducted before the recent innovations in vaccine technology, or the control time of antibody levels as 1 month later the first dose of the vaccine. Besides, racial-regional differences, the high rate of exclusively breastfed infants or possible immune-stimulating effect of current treatments for diseases of preterm infants can be contributing factors to our results.
Various factors, such as postpartum corticosteroid exposure or blood transfusions in the neonatal period, hyperbilirubinemia, sepsis, and presence of specific antibodies transmitted by the mother through the placenta, have been investigated for the deficiency of antibody response after administering HBV vaccine in premature LBW infants.Citation21 Present study, revealed that the anti-HBs levels were similar or even better than those in the control group, although betamethasone was administered to mothers to reduce complications related to premature birth.
A recent meta-analysis showed that preterm birth was associated with an impaired immune response to the HBV vaccine.Citation23 However, no statistically significant relationship was found between LBW and inadequate immune response to the HBV vaccine. Since the studies used a standard definition of <2500 g for LBW, it was thought that the results might be different, if another cutoff value of birth weight was chosen for comparison.Citation23
To analyze this situation in a more accurate way, it is necessary to evaluate the antibody levels by classifying the infants into subgroups according to the weight at the time of vaccination such as 1000–1500 g and 1500–2000 g, as in this study (). The results of this study suggested that the practice of delaying HBV vaccine in VLBW infants should be reconsidered. Therefore, the recommendation to wait for HBV vaccination in ˂2000 g newborns might cause a delay in immunization against HBV.
Limitations
This study had some limitations. First, the antibody level, including the first dose of HBV vaccine, was evaluated in the first postnatal month; it was not medically possible to create a control group with a similar gestational week and weight. Second, the study area was only a NICU. Finally, the study did not provide long-term results, and the case number was limited. Today, HBV vaccination is performed routinely. One of the strengths of the study was that the mothers included in the study had neither HBV vaccine nor HBV infection.
Conclusion
Antibody titers were high sufficient in preterm newborns with VLBW after a single dose of HBV vaccine. The results of prospective studies in which the vaccine series are completed, can make significant contributions to the literature in the future. Further studies with a wide number of participants are needed to confirm the efficacy and efficiency of postponement of HBV vaccination in babies with a birth weight of <2000 g.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Etiler N. Vaccination guide for primary health care workers. Ankara/Turkey: Turkish Medical Association Publications; 2018 April. Ankara/Turkey.
- Gomella TL. Gomella’s neonatology management, procedures, on-call problems, diseases, and drugs. Section VII, infection disease. 8th ed. The United States. McGraw-Hill Education; 2020. p. 1131.
- Bharati P, Pal M, Bandyopadhyay M, Bhakta A, Chakraborty S, Bharati P. Prevalence and causes of low birth weight in India. Malays J Nutr. 2011;17(3):301–13. Epub 2012/ 06/05. PubMed PMID: 22655452.
- Zeleke BM, Zelalem M, Mohammed N. Incidence and correlates of low birth weight at a referral hospital in Northwest Ethiopia. Pan Afr Med J. 2012;12:4. Epub 2012/ 07/25. PubMed PMID: 22826729; PubMed Central PMCID: PMCPMC3396870.
- Darling RD, Atav AS. Risk factors for low birth weight in New York state counties. Policy Polit Nurs Pract. 2012;13(1):17–26. Epub 2012/ 05/16. PubMed PMID: 22585673. doi:10.1177/1527154412442391.
- Ebadi F, Ghashghaee A, Bragazzi NL, Martini M, Sepehrian R, Ghaemmohamadi MS, Saeedi Shahri SS, Behzadifar M, Aryankhesal A, Behzadifar M, et al. Low birth weight in Iran: implications from a systematic review of the literature and meta-analysis in the period 1999–2017. Med J Islam Repub Iran. 2018;32(13). Epub 2018/ 08/31. PubMed PMID: 30159264; PubMed Central PMCID: PMCPMC6108244. doi:10.14196/mjiri.32.13.
- Ozgen DS. Characteristics of the mothers of low birth weight babies. J Hum Rhythm. 2016;2:72–77.
- Cavlin A. Turkey demographic and health survey 2018. Ankara/Turkey: Hacettepe University Institute of Population Studies; 2019.
- Kirmani KI, Lofthus G, Pichichero ME, Voloshen T, D’Angio CT. Seven-year follow-up of vaccine response in extremely premature infants. Pediatrics. 2002;109(3):498–504. Epub 2002/ 03/05. PubMed PMID: 11875147. doi:10.1542/peds.109.3.498.
- Jack AD, Hall AJ, Maine N, Mendy M, Whittle HC. What level of hepatitis B antibody is protective? J Infect Dis. 1999;179(2):489–92. Epub 1999/ 01/07. PubMed PMID: 9878036. doi:10.1086/314578.
- Mast EE, Margolis HS, Fiore AE, Brink EW, Goldstein ST, Wang SA, Moyer LA, Bell BP, Alter MJ. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) part 1: immunization of infants, children, and adolescents. MMWR Recomm Rep. 2005;54(RR–16):1–31. Epub 2005/ 12/24. PubMed PMID: 16371945.
- Kesler K, Nasenbeny J, Wainwright R, McMahon B, Bulkow L. Immune responses of prematurely born infants to hepatitis B vaccination: results through three years of age. Pediatr Infect Dis J. 1998;17(2):116–19. PubMed PMID: 9493806. Epub 1998/ 03/11. doi:10.1097/00006454-199802000-00007.
- Arora NK, Ganguly S, Agadi SN, Irshad M, Kohli R, Deo M, Paul VK, Deorari AK, Chellani H, Prasad MS, et al. Hepatitis B immunization in low birthweight infants: do they need an additional dose? Acta Paediatr. 2002;91(9):995–1001. Epub 2002/ 11/05. PubMed PMID: 12412879. doi:10.1080/080352502760272722.
- Gagneur A, Pinquier D, Quach C. Immunization of preterm infants. Hum Vaccin Immunother. 2015;11(11):2556–63. Epub 2015/ 08/21. PubMed PMID: 26291883; PubMed Central PMCID: PMCPMC4685684. doi:10.1080/21645515.2015.1074358.
- Hipgrave DB, Tran TN, Huong VM, Dat DT, Nga NT, Long HT, Van NT, Maynard JE, Biggs BA. Immunogenicity of a locally produced hepatitis B vaccine with the birth dose stored outside the cold chain in rural Vietnam. Am J Trop Med Hyg. 2006;74(2):255–60. Epub 2006/ 02/14. PubMed PMID: 16474080. doi:10.4269/ajtmh.2006.74.255.
- Committee on Infectious Diseases. Update on timing of hepatitis B vaccination for premature infants and for children with lapsed immunization. Pediatrics. 1994;94(3):403–04. Epub 1994/ 09/01. PubMed PMID: 8065872.
- van Steenbergen JE, Leentvaar-Kuijpers A, Baayen D, Dukers HT, Van Doornum GJ, van den Hoek JA, Coutinho RA. Evaluation of the hepatitis B antenatal screening and neonatal immunization program in Amsterdam, 1993–1998. Vaccine. 2001;20(1–2):7–11. Epub 2001/ 09/25. PubMed PMID: 11567738. doi:10.1016/s0264-410x(01)00315-2.
- Sadeck LSR RJ, Ramos JLA. Immune response of preterm infants to hepatitis B vaccine administered within 24 hours after birth. J Pediatr (Rio J). 2004;80(2):113–18. doi:10.2223/1149.
- Huang FY, Lee PI, Lee CY, Huang LM, Chang LY, Liu SC. Hepatitis B vaccination in preterm infants. Arch Dis Child Fetal Neonatal Ed. 1997;77(2):F135–8. Epub 1998/ 02/12. PubMed PMID: 9377137; PubMed Central PMCID: PMCPMC1720692. doi:10.1136/fn.77.2.f135.
- Sood A, Singh D, Mehta S, Midha V, Kumar R. Response to hepatitis B vaccine in preterm babies. Indian J Gastroenterol. 2002;21(2):52–54. Epub 2002/ 05/07. PubMed PMID: 11990326.
- Kashyap BDP, Kaur T, Kashyap A, Faridi M, Kaur IR. Effect of birth weight on immunogenicity of hepatitis B vaccine among Neonatal Intensive Care Unit graduates. Trop J Med Res. 2017;20:31–35. doi:10.4103/1119-0388.198110.
- Suleyman A, Gokcay G, Badur S, Aykin S, Kilic G, Tamay Z, Unüvar E, Güler N. Evaluation of serological status of children following hepatitis B vaccination during infancy. Mikrobiyol Bul. 2012;46(1):47–56. Epub 2012/ 03/09. PubMed PMID: 22399171.
- Fan W, Zhang M, Zhu YM, Zheng YJ. Immunogenicity of hepatitis B vaccine in preterm or low birth weight infants: a meta-analysis. Am J Prev Med. 2020;59(2):278–87. Epub 2020/ 06/23. PubMed PMID: 32564973. doi:10.1016/j.amepre.2020.03.009.
