ABSTRACT
This was a phase I/II study testing the feasibility of a vaccine by autologous leukemic apoptotic corpse-pulsed dendritic cells (DC) in elderly acute myeloid leukemia (AML) patients in first or second complete remission. Pulsed DC were administered at doses of 9 × 106 subcutaneously (1 mL) and 1 × 106 intra-dermally (0.1 mL). Five doses of vaccine were planned on days +1 + 7 + 14 + 21 and +35. Five DC-vaccines were produced and injected for all five patients included in the study. No severe adverse event was documented. Larger Phase 2 studies are now required to precise the role of DC-vaccines with leukemic apoptotic bodies in older as well as younger AML populations. (Clinicaltrials.gov NCT01146262)
Elderly acute myeloid leukemia (AML) patients still have very poor outcome. Only a third of older patients (defined generally as individuals older than 60–65 years of age) with AML received definitive therapy for their disease due to concerns about their overall fitness and potential treatment-related mortality.Citation1,Citation2 However, individuals up to the age of 80 years benefit from AML therapy in order to obtain a complete remission (CR), as reported for example by a retrospective study, where the most significant differences between survival in treated versus untreated patients were noted in individuals between the ages of 65 and 69 years (10 vs 4 months, p < .01) and 70–74 years of age (8 vs 3 months, p < .01).Citation3 But still, even with currently available agents median survival durations do not exceed 12 months and a 5-year overall survival of 5 to 10%.Citation1,Citation2
Among the approaches explored to prolong CR, such immune-strategies as dendritic cells (DC) vaccination could be used to trigger the immune system of the patient against residual leukemic cells.Citation4 Although the latter can be transformed into DC, this involves injection of tumor cells. The transformation of autologous/allogeneic monocytes into immature DC, subsequently loaded with tumor antigen during maturation, appears more ethical and perhaps more efficient.Citation5Using autologous apoptotic leukemic cells to pulse such DC is particularly interesting since it does not require to identify tumor-associated antigens. Moreover, this is likely to yield both major histocompatibility complex (MHC) class I- and MHC class II-restricted epitopes and trigger both CD8 and CD4 T-cells. We have previously demonstrated in vitro that non-leukemic mature DC could cross-present epitopes from apoptotic leukemic blasts and induce leukemia-specific cytotoxic immune responses.Citation6 Here we report on a phase I/II trial using such an autologous DC-vaccine in AML patients.
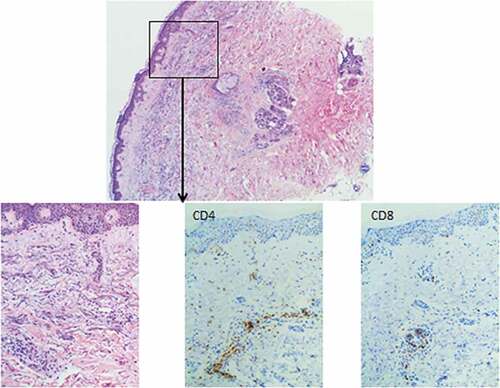
This prospective monocentric study (Clinicaltrials.gov NCT01146262) was conducted at Nantes University Hospital for elderly AML patients in first or second complete remission (CR), to test the feasibility of DC-vaccination and appreciate its potential toxicity. AML patients could be included if older than 60 years of age, not eligible for allogeneic transplantation or another trial, with an ECOG ≤2, at least 50% of leukemic blasts in the bone marrow (BM) or peripheral blood at diagnosis and no contra-indication to apheresis. Vaccines were produced by the Nantes cell-therapy unit (UTCG) according to good manufacturing practices and to standards of the French Drug Agency. All patients provided informed consent and the study was approved by the Comité de Protection des Personnes at the CHU of Tours (Tours Ethical committee) and the cellular therapy committee of the “Agence Française de Sécurité Sanitaire des Produits de Santé” (AFSSAPS), currently ANSM. Patients were pre-included at diagnosis or first relapse. Leukemic cells (≥2.4×108 required) were collected over 1 or 2 days prior to chemotherapy. Blast apoptosis was induced by thermal shock (1 h at 42°C) followed 3 hours later by UVB irradiation (100 kJ/m2) and at least 20 hours of culture. Apoptotic blasts (>99% ToPro-3 fluorescent cells) were frozen at −196°C without DMSO. Patients were definitively included when in CR. Peripheral monocytes were collected by apheresis and cultured for 6 days. Immature DC (iDC) were then generated by addition of GM-CSF (1000 UI/mL) and IL-4 (200 UI/mL). After another 6 days, iDC were pulsed with thawed irradiated (100 grays) autologous apoptotic blasts (ratio: 1/1 to 1/2) and matured for 24 hours with GM-CSF (1000 UI/mL), IL-4 (200 UI/mL), keyhole limpet hemocyanin (10 µg/mL), TNFa (1000 UI/mL), IL-1 (10 ng/mL) and Prostaglandin E2 (1 µg/mL). Pulsed mature DC (mDC) were frozen in DMSO at 5 × 106 cells/mL after checking sterility. The safety and quality of the vaccine was controlled on a thawed sample for each patient assessing that both viability and purity were >70%. DC maturation was confirmed by the expression by over 50%, 70%, and 70% of the cells of i) CD83 at a mean fluorescence intensity (MFI) ratio higher than 5 compared to control, ii) CD40 at a MFI ratio≥40, and iii) CD86 at a MFI ratio ≥10. Cell viability (>70%) was controlled on the thawed DC-vaccine before conditioning in two syringes. Pulsed mDC were administered at doses of 9 × 106 subcutaneously (1 mL) and 1 × 106 intra-dermally (0.1 mL). Five doses of vaccine were planned on days +1 + 7 + 14 + 21 and +35 ± 2. No chemotherapy was allowed after vaccination. On day +17, for each patient, a skin biopsy was taken under local anesthesia from the site of the third injection. Immunohistochemistry was used to investigate for lymphocyte infiltration in favor of a local immune response.
Between November 2009 and March 2015, 23 patients were pre-included. Leukemic blasts could not be collected for two patients (one rapid progression and one pancytopenia). Overall, 21 elderly AML patients (male n = 14; median age: 74 years-old [range: 65–84], secondary AML n = 8) were pre-enrolled at diagnosis (n = 14 including 3 primary refractory) or at first relapse (n = 7, including 3 refractory after salvage regimen). The median percentage of BM blasts was 63% (range: 20–92), including 3 and 4 cases with less than 40% and between 40% and 49%, respectively (protocol deviation). A median of 470 × 106 (range: 91–3400 × 106) blasts was collected. For one patient with >50% BM blasts, the target of 2.4 × 108 collected cells could not be reached despite 2 days of collection. Conversely, this target was reached for all patients between 40% and 49% BM blasts (after 2 days of collection for only 1). Collection was insufficient for 2 out of 3 patients with <40% BM blasts despite 2 days of collection. The median number of apoptotic blasts frozen was 490 106 (range: 260–1100 × 106). Among the 21 pre-included patients, 19 received non-intensive chemotherapy including 5ʹazacytidine (n = 2) and low-dose Ara-C (n = 2) alone or in combination with 1 day of idarubicin 8 mg/m2 (Ida/Ara-C n = 11) or with gemtuzumab ozogamicin 3 mg/m2 (GO/AraC n = 4). One patient received intensive chemotherapy with daunorubicin and Ara-C and one progressive patient was not treated. Only 5/21 (24%) patients achieved CR, a rate that was expected for this very old population (). All 5 could proceed to apheresis after 2 (n = 4) or 4 (n = 1) courses of non-intensive consolidation (Ida/Ara-C n = 3; GO/Ara-C n = 1, 5ʹ azacytidine n = 1) and 5 DC-vaccines were produced and injected. All quality criteria were met except for one DC-vaccine with a CD86 MFI ratio of 9 instead of 10 (minor deviation) that did not preclude injection. The first infusion was performed at a median of 25 days (range: 20–28) from apheresis. A median of 27 vaccines (range: 8–85) could have been produced from collected cells, indicating the possibility of achieving a longer maintenance in the future.
Table 1. Characteristics of the 5 AML patients who received a DC-vaccine after achieving complete remission
No severe adverse event was documented. Dermal CD4 and CD8 T-cell infiltrates were documented for each patient (). The median duration of response from CR and from first vaccine was 157 (range: 125–264) and 132 days (range: 38–165), respectively. Two patients relapsed before day+60. At this time, the three other patients were in persistent CR with undetectable minimal residual disease evaluated by flow cytometry. The median overall survival (OS) from CR was 472 days (range: 169–973). Although all patients died of relapse, the median OS from pre-inclusion was 147,5 days (range: 27–730) for patients not achieving CR (n = 16) and 509 days (range: 271–1003) for the 5 vaccinated CR patients (p = 0,03).
Figure 1. Skin biopsy at the injection site after the third DC vaccine in patient 1. Immunohistochemistry on formaldehyde-fixed paraffin embedded tissue sections. CD4+ and CD8 + T-cell infiltrates can be seen in the dermis around capillaries (x 100 magnification)
Although the study size is too small to draw definitive conclusions, the latter results are encouraging since, as already mentioned, for an AML population between 70/75 years, median overall survival with currently available treatment is around 8 monthsCitation3 while here it was almost 16 months for patients receiving the vaccine.
Several trials using various strategies of tumor antigen loading have been published recently with promising results.Citation7–10 There is thus now strong evidence in the literature that DC-vaccines can prevent or delay relapse in AML patients, especially in a situation of low tumor burden. Our approach may have the advantage to be more immunogenicCitation4 and adapted to every patient, independently of a particular or specific tumor antigen. It also allows to produce a large quantity of vaccine, a condition that may help to maintain a durable anti-leukemic response by repeated injections, more than the five times performed here. DC-vaccine production was feasible in all cases, large, reproducible and compliant for off-the-shelf clinical use. In order to improve the efficacy of DC-vaccine, next step could be to improve the immunogenicity of our DC for example using a new cytokine cocktail containing a synthetic Toll-like receptor (TLR)7/8 agonist as already reported,Citation11 or to combine DC-vaccine in combination with hypomethylating agentsCitation12 or immune checkpoint inhibitors.Citation13 Larger Phase 2 studies are definitely required to precise the role of DC-vaccines with leukemic apoptotic bodies in older as well as younger AML populations.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Authorships and disclosures
PC and MG designed, performed, and coordinated the research, collected, analyzed, interpreted the data, and wrote the manuscript.
SS, SB, and BD produced the vaccines.
IG and the EFS of Nantes performed the cytapheresis for DC production.
CB performed immunostaining of skin biopsies.
All authors, including VD, TG, PP, YLB, NJM, MCB, contributed data and commented on the manuscript.
Acknowledgments
We want also to thank data managers and the DRC of Nantes (especially Mrs Laetitia Biron).
Additional information
Funding
References
- Wang ES. Treating acute myeloid leukemia in older adults. Hematology Am Soc Hematol Educ Program. 2014 May;2014(1):14–20. doi:10.1182/asheducation-2014.1.14.
- Pettit K, Odenike O. Defining and treating older adults with acute myeloid leukemia who are ineligible for intensive therapies. Front Oncol. 2015;5:280. doi:10.3389/fonc.2015.00280.
- Oran B, Weisdorf DJ. Survival for older patients with acute myeloid leukemia: a population-based study. Haematologica. 2012 Dec;97(12):1916–24. doi:10.3324/haematol.2012.066100.
- Van Acker HH, Versteven M, Lichtenegger FS, Roex G, Campillo-Davo D, Lion E, Subklewe M, Van Tendeloo VF, Berneman ZN, Anguille S, et al. Dendritic cell-based immunotherapy of acute myeloid leukemia. J Clin Med. 2019;8(5):579. doi:10.3390/jcm8050579.
- Spisek R, Chevallier P, Morineau N, Milpied N, Avet-Loiseau H, Harousseau JL, Meflah K, Gregoire M. Induction of leukemia-specific cytotoxic response by cross-presentation of late-apoptotic leukemic blasts by autologous dendritic cells of nonleukemic origin. Cancer Res. 2002;62:2861–68.
- O’Brien LJ, Guillerey C, Radford KJ. Can dendritic cell vaccination prevent leukemia relapse? Cancers (Basel). 2019;11(6):875. doi:10.3390/cancers11060875.
- Rosenblatt J, Stone RM, Uhl L, Neuberg D, Joyce R, Levine JD, Arnason J, McMasters M, Luptakova K, Jain S, et al. Individualized vaccination of AML patients in remission is associated with induction of antileukemia immunity and prolonged remissions. Sci Transl Med. 2016;8:368ra171. doi:10.1126/scitranslmed.aag1298.
- Anguille S, Van de Velde AL, Smits EL, Van Tendeloo VF, Juliusson G, Cools N, Nijs G, Stein B, Lion E, Van Driessche A, et al. Dendritic cell vaccination as postremission treatment to prevent or delay relapse in acute myeloid leukemia. Blood. 2017;130(15):1713–21. doi:10.1182/blood-2017-04-780155.
- Wang D, Huang XF, Hong B, Song XT, Hu L, Jiang M, Zhang B, Ning H, Li Y, Xu C, et al. Efficacy of intracellular immune checkpoint-silenced DC vaccine. JCI Insight. 2018;3(3):e98368. doi:10.1172/jci.insight.98368.
- van de Loosdrecht A, van Wetering S, Santegoets SJ, Singh SK, Eeltink CM, den Hartog Y, Koppes M, Kaspers J, Ossenkoppele GJ, Kruisbeek AM, et al. A novel allogeneic off-the-shelf dendritic cell vaccine for post-remission treatment of elderly patients with acute myeloid leukemia. Cancer Immunol Immunother. 2018;67(10):1505–151. doi:10.1007/s00262-018-2198-9.
- Subklewe M, Geiger C, Lichtenegger FS, Javorovic M, Kvalheim G, Schendel DJ, Bigalke I. New generation dendritic cell vaccine for immunotherapy of acute myeloid leukemia. Cancer Immunol Immunother. 2014 Oct;63(10):1093–103. doi:10.1007/s00262-014-1600-5.
- Lichtenegger FS, Schnorfeil FM, Rothe M, Deiser K, Altmann T, Bücklein VL, Köhnke T, Augsberger C, Konstandin NP, Spiekermann K et al. Toll-like receptor 7/8-matured RNA-transduced dendritic cells as post-remission therapy in acute myeloid leukaemia: results of a phase I trial. Clin Transl Immunol. 2020 Mar 3;9(3):e1117. doi:10.1002/cti2.1117.
- Versteven M, Van den Bergh JMJ, Marcq E, Smits ELJ, Van Tendeloo VFI, Hobo W, Lion E. Dendritic cells and programmed death-1 blockade: a joint venture to combat cancer. Front Immunol. 2018 Mar 1;9:394. doi:10.3389/fimmu.2018.00394.
