ABSTRACT
A typhoid Vi capsular-polysaccharide tetanus toxoid conjugate vaccine (Typbar-TCV®) was recommended by the World Health Organization for use in children >6 months of age. The present post-marketing surveillance study was intended to assess the clinical safety of approximately 11 million doses of TCV sold till 2019 in a diverse age range Indian population. Both active and passive post-marketing surveillance studies were conducted at multiple centers. Active surveillance was performed in two periods, Period-I: February to October 2016, Period-II: April 2017 to October 2018. In Period-II, the Brighton Collaboration Criteria adverse event case definitions were used. Passive surveillance was performed from February 2016 to December 2019 through voluntary reporting by pediatricians across India. During the active surveillance, 1147 adverse events were reported among 4,991 (23.0%) subjects in Period-I, and 596 adverse events among 3898 (21.3%) subjects in Period-II. The most frequent adverse events were fever (9.2% and 12.02%in Periods I and II, respectively), pain at the injection site (8.3% and 7.33%), and swelling (4.0% and 1.93%). No serious adverse events (SAEs) were reported during either Period. Passive surveillance revealed 235 adverse events, including 25 SAEs requiring hospitalization, of which two were due to typhoid fever. All the events mentioned above occurred within one week of vaccination, and all the subjects have recovered from AEs with medications. All reported adverse events resolved with no clinical sequelae. Observations in this study are consistent with the pre-licensure studies with no additional safety signals detected, confirming that Typbar-TCV® is safe.
Abbreviations: AE: Adverse event; LMIC: low- and middle-income countries; PMS: Post-marketing surveillance; SAE: Serious adverse event; TCV: Vi-polysaccharide tetanus –toxoid conjugate vaccine (Typbar-TCV®)
Introduction
Typhoid fever is a major global public health concern, it is estimated between 11 and 21 million cases of febrile illness, and 117,000–161,000 deaths are attributable to the disease each year.Citation1–3 The burden of typhoid is highest in low- and middle-income countries (LMICs) because of increased population, contaminated water, and unhygienic living conditions.Citation4 A recent estimate is 17.8 million cases per year (95% credible interval: 6.9-–48.4 million) in LMICs.Citation3
Vaccination is one of the prerequisite measures for effective prevention and control of typhoid fever, in addition to hygiene measures, including improved sanitation and access to clean potable water. Typbar-TCV® (hereafter referred to as TCV) is a Vi polysaccharide-tetanus toxoid conjugate vaccine that was demonstrated to be safe and efficacious in field trialsCitation5,Citation6 and human challenge model.Citation7 This led to the marketing authorization of TCV in India for use from 6 months of age in 2013. It was pre-qualified in 2017 by WHO, also recommended by the WHO-Strategic Advisory Group of Experts on Immunization (SAGE) for the routine use of a single dose of TCV in children from 6 months of age in typhoid endemic countries and outbreak settings.Citation8
It is essential to perform post-licensure monitoring of the safety of new vaccines, where Adverse Events (AEs) may not be detected until the vaccine is introduced into a real-world scenario with a range of subjects from diverse demographic groups (e.g., age, socioeconomic background), medical history (e.g., immunocompromised host), or multiple medical problems necessitating medication (potential interactions). In its pre-licensure clinical studies, TCV was shown to have an acceptable safety profile leading to licensure.Citation5 The objectives of post-marketing surveillance (PMS) are to identify rare adverse reactions not detected during pre-licensure studies, to identify risk factors or preexisting conditions that may promote reactions, to identify particular vaccine lots with unusually high rates or types of events, and to identify signals of possible adverse reactions which may warrant further study.
The present PMS study was intended to assess the clinical safety of approximately 11 million doses of TCV sold till 2019 in a diverse age range Indian population.
Methods
This was a prospective, multicenter, observational study performed to monitor the safety of TCV using active and passive surveillance methods. Active surveillance was conducted in two periods, from February 2016 to October 2016 (Period I) and April 2017 to October 2018 (Period II), during which AEs were captured and reported in the PMS forms. The passive surveillance was conducted for 47 months, from February 2016 to December 2019, when AEs were reported voluntarily by pediatricians /physicians across India. In this study, all the AEs and SAEs were followed up until they resolved. Most of the subjects were examined for fever using axillary temperatures.
Study vaccines
Safety data was captured from the subjects administered either Typbar-TCV® or Enteroshield® (the trade name of Typbar-TCV® vaccine marketed by Abbott Limited) in the anterolateral thigh in infants and children or the deltoid in teenagers and adults.
Active surveillance – PMS forms
In the active surveillance, AEs were reported by pediatricians/physicians using a specifically designed Post-Marketing Surveillance (PMS) form enclosed as a supplementary document, and no payments were made neither to the subject nor the recruiting physician. A dedicated PMS form was designed in-house and distributed across the country. We have actively coordinated the entire surveillance system with our widely distributed sales and marketing field force along with the in-house Medical Affairs department to collect the duly filled in PMS forms by doctors at periodic intervals for transmission to the central database and analysis. Furthermore, as per Schedule Y, the post-marketing active surveillance studies do not require informed consent by an ethics committee.
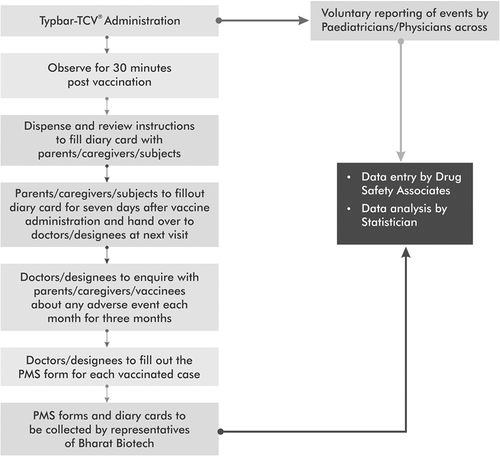
Subjects were observed for 30 minutes post-vaccination and followed up daily for the first seven days and monthly for 3 months. The detailed methodology of PMS is illustrated in . The PMS forms used during Period-I were designed in-house. Following a presentation of the interim data from this Period to the WHO-SAGE and the Global Advisory Committee on Vaccine Safety (GACVS), it was suggested that we revise the case definitions for AEs according to the Brighton Collaboration Criteria (BCC).Citation6 The revised case definitions were used in the PMS forms employed in the Period II surveillance.
Passive surveillance
AE data were obtained through voluntary reporting by pediatricians/physicians across India (). Each reporting physician identified the AEs and SAEs.Citation7
Definitions
An adverse event (AE) was defined as any unfavorable and unintended sign (including an abnormal laboratory finding), symptom, or disease temporally associated with the use of TCV, whether or not considered related to the vaccine.Citation9 Serious adverse events (SAE) were defined as any adverse drug experience occurring at any dose that results in any of the following outcomes: death, life-threatening, hospitalization or prolongation of hospitalization, a congenital anomaly, persistent or significant disability/incapacity, or required intervention to prevent permanent impairment/damage.
Statistical analysis
All values were described as numbers and their respective percentages. P-values to compare between-group differences in the percentages of participants showing AEs were calculated using the chi-square test.
Results
Active surveillance period I
During Period-I, approximately 1.5 million doses of TCV were sold. We monitored the safety in 5,002 vaccine recipients, of whom 11 were excluded from the analysis as they did not meet the minimal reporting criteria (missing vaccination details and suspected reactions, subject lost to follow-up). Period-I covered all zones of India, but most vaccine recipients (44.6%) were from the South, 22.6% from the North, 10.7% from the East, 13.8% from the West, and 8.3% from the Central zone. Mean ages were 8.6 months in the ≥6 months to ≤2 years age cohort and 4.01 years in the ≥2- ≤45 years age cohort. The study comprised 54.6% males and 45.4% females. In Period-I, 196 (3.92%) subjects were <9 months of age, of which 98 (1.96%) were males, and 98 (1.96%) were females.
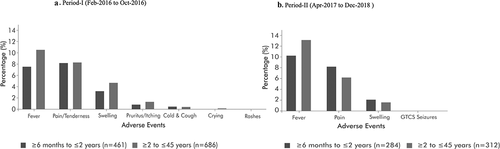
Among the 4,991 analyzed vaccine recipients during Period-I, there were 1,147 (23.0%) AEs, of which 1,106 (96%) were mild in severity. The most frequent systemic AEs were fever (9.20%) and pruritus/itching (1.08%), followed by cough (0.28%), rashes (0.02%), cold (0.10%), and crying (0.06%). Local AEs included injection site pain (8.25%) and swelling (3.99%) ().
Table 1. Age group and severity-wise classification of adverse events (AE) in active surveillance
Profiles of reported AEs were similar in terms of frequency and severity in the younger (≥6 months to ≤2 years) and older (≥2 – ≤45 years) age groups for fever (7.58% and 10.58%) in the younger and older age groups, respectively), pain (8.18% and 8.31%) and swelling (3.17% and 4.67%). Of the 174 fever cases reported in children from ≥6 months to ≤2 years, there were 5 severe cases (102.1°–104°F), two of which resolved within 24 hours and three resolved within 48 hours ( & ). No SAEs were found during the Period-I surveillance.
During Period-I, antipyretics (paracetamol) and non-steroidal anti-inflammatory drugs (NSAIDs) (a combination of ibuprofen and paracetamol or mefenamic acid and paracetamol) were prescribed to 6% of subjects after reports of fever or injection site pain. Further, in Period – I of active surveillance, 20 (0.40%) subjects received concomitant vaccines, apart from TCV. The concomitant vaccines were MMR (n = 9), Hepatitis A (n = 5), Japanese Encephalitis (n = 4), Meningococcal conjugate (n = 1), Varicella zoster (n = 1).
Active surveillance during period-II
In Period-II active surveillance, approximately 2.4 million doses of TCV were sold. Safety was actively monitored in 3923 subjects, of whom 25 were excluded from analysis as they did not meet the minimal reporting criteria. The distribution of gender was 57.36% males and 42.6% females. In Period-II, 168 (4.31%) subjects were <9 months of age, of which 62 (36.9%) were males, and 106 (63.1%) were females.
In 3898 analyzed vaccines, there were 596 (15.29%) AEs, all Level 1 type according to the BCC definitions ( & ). The most frequent AEs in both age groups (≥6 months to ≤2 years and ≥2 – ≤45 years) were fever (7.47% and 9.68%), pain at the injection site (5.98% and 4.59%), and swelling (1.65% and 1.14%). Generalized clonic seizures (GCS) were reported in one subject (0.03%) (). No SAEs were reported during Period-II. The use of concomitant medications such as paracetamol, ibuprofen, and paracetamol plus ibuprofen, in combination, was seen in Period-II. In Period-II of active surveillance, three subjects received concomitant vaccines, in addition to TCV. The concomitant vaccines were DPT+Varicella zoster (n = 1) and MMR (n = 2).
Passive surveillance
During the passive surveillance from February 2016 to December 2019, when approximately 7.3 a million doses of TCV (including as Enteroshield®) were sold, pediatricians/physicians reported a total of 235 AEs, including 25 SAEs. The most frequently reported AEs were fever, pain, and swelling at the injection site. Proportions and types of events in this passive surveillance were similar to the safety profile of pre-licensure clinical study.Citation4
Discussion
Typbar-TCV® is being marketed in India since 2013 after obtaining a marketing license. In 2017, it was recommended by the World Health Organization-Strategic Advisory Group of Experts (SAGE) that TCV, which is the only vaccine available internationally and pre-qualified by WHO as the vaccine of choice for use in children below 2 years and also as a catch-up vaccine to reduce the burden in typhoid endemic countries. SAGE has observed the continuing high burden of typhoid fever and also an alarming increase in antimicrobial resistance of Salmonella Typhi in LMICs. This burden is predominantly seen in sub-Saharan Africa, South and South-East Asia. Most typhoid cases occur in children with age <2 years. Typbar-TCV® has shown longer and higher levels of immunogenicity when compared with injectable Vi polysaccharide vaccine. Immunogenicity data supporting the same up to 5 years are available. SAGE has re-emphasized the importance of systematic use of typhoid vaccine for the control of endemic disease. Typhoid vaccination may be considered in humanitarian emergencies on the basis of risk assessment in the local setting; Data is necessary on co-administration of TCV with other routine childhood vaccines in typhoid endemic countries and PCV, yellow fever, meningococcal A conjugate, and Japanese encephalitis vaccines.Citation10
GACVS reviewed preliminary safety data on Typbar-TCV® from three ongoing trials of effectiveness in the field conducted by Typhoid Vaccine Acceleration Consortium (TyVAC). Data from early public sector use is obtained from both India and Pakistan, and also data from private sector use in India has been reported to the manufacturer. Additional data was presented on passive and active surveillance of adverse events in two mass immunization campaigns with TCV in 2018. Post-licensure safety data for Typbar-TCV® reported is based on approximately 9000 reports received from pediatricians in the private sector in India, some with the inclusion of Brighton Collaboration Criteria case definitions and periodic safety reports, which showed an acceptable safety profile.
On reviewing the available data, GACVS concluded that the safety profile of the Typbar-TCV® vaccine is reassuring, and no signals of serious adverse events were presented. GACVS recommended that countries introduce TCV into their routine immunization schedule and should make every possible effort to ensure robust monitoring of safety so that data can be added on co-administration of TCV with routine childhood vaccines. GACVS will also consider a further review of any safety data as warranted, particularly in special populations.Citation11
Vaccine safety monitoring is a critically important component of immunization practice. Although we examined the safety of Typbar-TCV® in pre-licensure studies, the number of subjects was considerably less in these clinical trials than in the real-world post-license settings and may not detect rare adverse events that can be critically important for the safety of a vaccine. A precedent is the live attenuated rotavirus vaccine, Rotashield®, which was licensed and recommended for use in the United States in 1998, but withdrawn from the market one year later following the detection of rare cases of intussusception in post-marketing surveillance.Citation12 The reported Active PMS study assessed the clinical safety of Typbar-TCV® in Indian infants, children, and adults ranging from ≥6 months to ≤45 years of age.
The study included participants living in four zones in India. Taking into consideration the number of TCV doses sold during this surveillance period, the overall AE observed was reported to be 0.071%, 0.024%, and 0.003% events per million vaccine doses administered during the Period I, Period II, and passive surveillance period, respectively (supporting information is attached herewith). These proportions of AEs are minimal.
In both active and passive surveillance, fever and the local reactions, injection site pain, and swelling constituted the major proportion of all observed AEs ( & 1B). On the basis of safety studies conducted on typhoid vaccines, no AEs were expected.Citation13–15 More importantly, no mortality or any additional new safety signals were detected in this study. A recent study was conducted among 207,000 infants and children of age between 6 months to 10 years who received a single dose of Typbar-TCV® in two XDR (extensively drug-resistant) typhoid affected (outbreak) areas of the Hyderabad city in Sindh, Pakistan reported the adverse events following immunization campaign. The study mentioned that fever, pain or swelling at the injection site, and diarrhea were the commonly reported adverse events during the 14 days study period without any serious adverse events (SAEs).Citation14 The study concluded that a single dose of Typbar-TCV*was safe in an outbreak setting based on the safety report.
Fever is a common post-vaccination solicited systemic reaction,Citation16 and rates observed in this PMS study align with previous studies of other typhoid vaccines.Citation13–15 The proportions of fever cases [Period-I (9.2%) and Period-II (12.02%)] in the current study are much lower than that seen with another typhoid conjugate vaccine, Vi-rEPA (22%).Citation15 In clinical studies, subjects in the younger age group (≥6 months to ≤2 years) are more prone to experience fever after administration of TCV. In our active surveillance study, the incidence of fever was higher in the older age group (≥2 to ≤45 years) compared with the 6 months ≤2 year in Periods I and II. One possible explanation for this unusual and contradictory finding is pediatricians’ regular practice to prophylactically prescribe antipyretics for younger children as a precautionary measure in anticipation of elevated temperature. Such antipyretic may attribute to the low incidence of fever events in this age group.
The act of injecting foreign material into the tissues and the tissues associated with physical irritation can produce an inflammatory response.Citation17 As anticipated in this study, after fever, mainly mild or moderate injection site pain and swelling were the main AEs, all cases resolving within 48 hours of vaccination.
No vaccine is 100% effective, and vaccine failures with the targeted disease occurrence in a vaccinated individual do occur. During the passive surveillance in this study, there were five Widal-positive cases reported. The occurrence of typhoid fever post-TCV administration is an uncommon and rare event. The Widal test is neither sensitive nor specific, and the rate of false-positive results is approximately 14%.Citation18,Citation19 Of the 19 cases, five were Widal positive cases, three had confirmatory blood culture testing for typhoid, five were positive for typhoid test, and seven cases, no tests performed. No antibody titer was measured in these vaccines to show lower levels of antibodies. Therefore, it was impossible to ascribe vaccine failure as the only reason for Widal or typhoid positive tests. Previously, the principal reasons thought to cause vaccine failures after Haemophilus influenzae type b (Hib) glycoconjugate vaccine was the lack of a booster dose or use of acellular pertussis combination vaccine which is known to interfere with the Hib response.Citation20,Citation21,Citation22 In general, vaccine failures may also occur due to errors in administration, cold-chain and storage conditions, and using an expired vaccine, which cannot be accounted for in our study. In this study, the five Widal-positive cases could be due to false-positive diagnosis or infection due to paratyphi A, B, and C, for which TCV does not confer protection.Citation19 Although we attempted to follow up on the 19 reported positive cases for more data, we could not obtain any further information. Nevertheless, considering that approximately 11 million doses were sold either as Typbar-TCV® or Enteroshield® during the study period when only those 19 cases were reported, a potential vaccine-failure rate of 0.00007 per million doses would not be considered to be critical or a failure on the part of the vaccine.
Previously, we assessed the safety and tolerability of TCV under controlled conditions during clinical development and witnessed no major safety concerns.Citation5 In this study, the profile of reported AEs was similar to our observations in those pre-licensure clinical studies. There were three major types of AE – fever and the injection site reactions, pain/tenderness, and swelling in active and passive surveillance. In the phase 3 clinical study, there was a higher incidence of fever (10%) in the ≥6 months to ≤2-year age group compared with active surveillance in Periods I (7.58%) or II (7.47%). Conversely, in the ≥2- ≤45-year age group, there was an increased incidence of fever in Periods I (10.58%) and II (9.68%) compared with the phase 3 clinical study (4.12%). Reported rates of injection site pain/tenderness and swelling in both age groups (≥6 months to≤2 years & ≥2–≤45 years) were higher in Period I than Period II and the phase 3 clinical study.Citation4 Our observations in this safety surveillance study reiterate that TCV is safe and well tolerated across all age groups.Citation5
Although we collected data on concomitant medications in this study, there was minimal data on concomitantly administered vaccines, impacting the causality assessment. In the present study, data were predominantly obtained from pediatricians rather than physicians, as the PMS forms were mainly distributed to the pediatricians to capture AEs from children, which could be a study limitation.
Conclusion
This study is the first of its kind to report the safety findings of a new typhoid conjugate vaccine in an extensive endemic population. The WHO SAGE has recommended it for routine use. Most AEs following TCV vaccination were found to be mild cases of anticipated reactions to the vaccine. All reported SAEs in the passive surveillance were resolved without health complications. There was no mortality and no additional safety signals assessed as part of our surveillance study, while no different design apart from the PMS form was in place, which might pose a study limitation. However, based on the available data from various settings, GACVS concluded that the safety profile of the Typbar-TCV® vaccine is reassuring, and no signals of serious adverse events were presented. In conclusion, our study results suggest that Typbar-TCV® is a safe and well-tolerated vaccine and can be extensively employed to prevent typhoid fever across all ages from ≥6 months to ≤ 45 years.
Disclosure of Potential Conflicts of Interest
All authors are full-time employees of the study sponsor, Bharat Biotech International Limited, with no stock options.
Authors’ contributions
KM and RR contributed to the study’s design and performance, and ER analyzed the data, and ER, BR, and VS prepared the draft manuscript. All authors reviewed and approved the manuscript and agreed to the submission of the publication.
Availability of data and material
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors are grateful to all the doctors who provided data for this study and to Keith Veitch (Keith Veitch Communications, the Netherlands) for editorial help, and Dr Ashwini Maratha to prepare and review the manuscript. We also appreciate Mr Sunil Kumar and Dr Sapan Kumar Behera’s contributions towards data review and N. Sandhya Rani for data collection.
Additional information
Funding
References
- Mogasale V, Maskery B, OchiaiR L, LeeJ S, MogasaleV V, Ramani E, Kim YE, Park JK, Wierzba TF. Burden of typhoid fever in low-income and middle-income countries: a systematic, literature-based update with risk-factor adjustment. Lancet Glob Health. 2014;2(10):e570–7. doi:10.1016/S2214-109X(14)70301-8.
- World Health Organization. Typhoid vaccines: WHO position paper, March 2018 - recommendations. Vaccine. 2019 Jan 7;37(2):214–16. Epub 2018 Apr 13. doi:10.1016/j.vaccine.2018.04.022.
- Antillon M, Warren JL, Crawford FW, Weinberger DM, Kurum E, Pak GD, Marks F, Pitzer VE. The burden of typhoid fever in low- and middle-income countries: a meta-regression approach. PLoS Negl Trop Dis. 2017;27:e0005376: 1–21.
- Waddington CS, Darton TC, Pollard AJ. The challenge of enteric fever. J Infection. 2014;68(Suppl 1):S38–50. doi:10.1016/j.jinf.2013.09.013.
- Mohan VK, Varanasi V, Singh A, Pasetti MF, Levine MM, Venkatesan R, Ella KM. Safety and immunogenicity of a Vi polysaccharide-tetanus toxoid conjugate vaccine (Typbar-TCV) in healthy infants, children, and adults in typhoid endemic areas: a multicenter, 2-cohort, open-label, double-blind, randomized controlled phase 3 study. Clin Infect Dis. 2015;61(3):393–402. doi:10.1093/cid/civ295.
- Shakya M, Colin-Jones R, Theiss-Nyland K, Voysey M, Pant D, Smith N, Liu X, Tonks S, Mazur O, Farooq YG, et al. Phase 3 efficacy analysis of a typhoid conjugate vaccine trial in Nepal. N Engl J Med. 2019;381(23):2209–18. doi:10.1056/NEJMoa1905047.
- Jin C, Gibani MM, Moore M, Juel HB, Jones E, Meiring J, Harris V, Gardner J, Nebykova A, Kerridge SA, et al. Efficacy and immunogenicity of a Vi-tetanus toxoid conjugate vaccine in the prevention of typhoid fever using a controlled human infection model of Salmonella Typhi: a randomised controlled, phase 2b trial. Lancet. 2017;390(10111):2472–80. doi:10.1016/S0140-6736(17)32149-9.
- WHO summary of the October 2017 meeting of the strategic advisory group of experts on immunization. [accessed 2018 Nov 13]. https://www.who.int/immunization/sage/meetings/2017/october/1_Typhoid_SAGE_background_paper_Final_v3B.pdf?ua=1 .
- ICH, clinical safety data management: definitions and standards for expedited reporting E2Aa. [acccessed 2017 Nov 6]. https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E2A/Step4/E2A_Guideline.pdf .
- World Health Organization. Weekly epidemiological record; 1ST December 2017, 92TH year/No. 48. 2017. 729-748. [acccessed 2017 December]. https://www.who.int/wer/2017/wer9248/en/ .
- World Health Organization. Weekly epidemiological record; 25 JANUARY 2019, 94th YEAR/No 4, 2019, 94, 45–52. [acccessed 2019 January]. https://www.who.int/wer/2019/wer9404/en/ .
- Delage G. Rotavirus vaccine withdrawal in the United States; the role of postmarketing surveillance. Can J Infect Dis. 2000;11:10–12.
- Begier EM, Burwen DR, Haber P, Ball R, for the Vaccine Adverse Event Reporting System Working Group. Postmarketing safety surveillance for typhoid fever vaccines from the vaccine adverse event reporting system, July 1990 through June 2002. Clin Infect Dis. 2004;38(6):771–79. doi:10.1086/381548.
- Marcus LC, Froeschle JE, Hill DR, Wolfe MS, Maus D, Connor B, Acosta AM, Rensimer ER, Roberts A, Dardick K. Safety of Typhim Vi vaccine in a postmarketing observational study. J Travel Med. 2007;14(6):386–91. doi:10.1111/j.1708-8305.2007.00158.x.
- Thiem VD, Lin FYC, Canh DG, Son NH, Anh DD, Mao ND, Chu C, Hunt SW, Robbins JB, Schneerson R, et al. The Vi conjugate typhoid vaccine is safe, elicits protective levels of IgG anti-Vi, and is compatible with routine infant vaccines. Clin Vaccine Immunol. 2011;18(5):730–35. doi:10.1128/CVI.00532-10.
- Qamar FN, Yousafzai MT, Khaliq A, Karim S, Memon H, Junejo A, Baig I, Rahman N, Bhurgry S, Afroz H, et al. Adverse events following immunization with typhoid conjugate vaccine in an outbreak setting in Hyderabad, Pakistan. Vaccine. 2020;38(19):3518–23. doi:10.1016/j.vaccine.2020.03.028.
- Lin FY, Ho VA, Khiem HB, Trach DD, Bay PV, Thanh TC, Kossaczka Z, Bryla DA, Shiloach J, RobbinsetJ B, et al. The efficacy of a Salmonella typhi Vi conjugate vaccine in two-to-five-year-old children. N Engl J Med. 2001;344(17):1263–69. doi:10.1056/NEJM200104263441701.
- Kroger A, General recommendations on immunization --- recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR recommendations and reports: morbidity and mortality weekly report recommendations and reports. Atlanta (GA): National Center for Immunization and Respiratory Diseases. 2011;60:1–64. https://www.cdc.gov/mmwr/pdf/rr/rr6002.pdf .
- Parry CM, Hoa NT, Diep TS, Wain J, Chinh NT, Vinh H, Hien TT, White NJ, Farrar JJ. Value of a single-tube widal test in diagnosis of typhoid fever in Vietnam. J Clin Microbiol. 1999;37(9):2882–86. doi:10.1128/JCM.37.9.2882-2886.1999.
- Sen R, Saxena SN. A critical assessment of the conventional Widal test in the diagnosis of typhoid and paratyphoid fevers. Indian J Med Res. 1969;57:1813–19.
- Booy R, Heath PT, Slack MP, Begg N, Moxon ER. Vaccine failures after primary immunisation with Haemophilus influenzae type-b conjugate vaccine without booster. Lancet. 1997;349(9060):1197–202. doi:10.1016/S0140-6736(96)06392-1.
- Kelly DF, Moxon ER, Pollard AJ. Haemophilus influenzae type b conjugate vaccines. Immunology. 2004 Oct;113(2):163–74.doi:10.1111/j.1365-2567.2004.01971.x.