ABSTRACT
Children with hematological malignancies are at increased risk of hepatitis B virus infection. This study assessed the immunogenicity and safety profile of HBV vaccination in pediatric hemato-oncological children. A nonrandomized interventional study was conducted from January 2017 to February 2020 in Shanghai, China. Seventy-three pediatric hemato-oncological children with hepatitis B surface antibody (anti-HBs) titers <10 mIU/ml were recruited. The participants received three doses of recombinant HBV vaccine according to the 0-, 1-, and 6- month immunization schedule. Adverse events following immunization and anti-HBs titers (at baseline, 1 month, and 6 months after inoculation) were recorded. Forty-three males and thirty females with median ages of 9.12 and 9.60 years, respectively, were included. The mean anti-HBs titer was 4.88 ± 2.61 mIU/ml, 893.12 ± 274.12 mIU/ml, and 711.45 ± 337.88 mIU/ml at baseline, one month, and six months after inoculation, respectively (P< .001). A total of fourteen adverse events following immunization were reported, and among them, 5 (6.85%), 5 (6.85%), and 4 (5.48%) events were reported after the first, second, and third inoculation, respectively (P= .927). In conclusions, the HBV vaccine is immunogenic and safe in children with hematological malignancies. It is worth noting that the anti-HBs titer was decreased at the 6-month follow-up, and periodic monitoring of the anti-HBs titer accompanied by timely booster vaccination should be carefully considered.
Abbreviations: AEFI: Adverse events following immunization; HBV: Hepatitis B virus; Anti-HBs: Antibody against hepatitis B surface antigen; HBsAg: Hepatitis B surface antigen; APC: Antigen-presenting cell; HSCT: Hemopoietic stem cell transplantation; COVID-19: Corona Virus Disease 2019
Introduction
Hepatitis B virus (HBV) infection is one of the major causes of liver disease. In China, the Centers for Disease Control and Prevention recorded approximately 78 million hepatitis B virus carriers and nearly 300,000 deaths from hepatitis B related diseases annually.Citation1,Citation2 Since the launch of a universal neonatal hepatitis B vaccination program in 1992, vaccination coverage had increased from 22.2% to 95.6% in 2015.Citation3,Citation4 The main component of the recombinant HBV vaccine is the hepatitis B surface antigen (HBsAg). HBsAg is a thymus-dependent antigen mainly produced by cellular immunity under the combined action of many immune regulatory factors. Clinical studies have shown that it takes at least six months after the end of chemotherapy for the body’s immune system to gradually recover to produce a sufficient immune response to the vaccine in children with hematological malignancies.Citation5–7 The immune response induced by the HBV vaccine mainly occurs through the main component, hepatitis B surface antigen. Upon inoculation, HBsAg is taken up by an antigen-presenting cell (APC) and degraded into various peptides on the surface of APCs as a peptide-MHC (p-MHC) complex. p-MHC integrates with the TCR-CD3 complex on the surface of HBsAg-specific CD4 + T cells, activates MHC class II-restricted T cells, and promotes T cell proliferation, activation, and differentiation toward Th1 cells, thereby mediating cellular immune responses. Cytokines activate HBsAg-specific B lymphocytes, induce them to differentiate into plasma cells that secrete anti-HBs, and generate immunity toward HBV.Citation8 In recent years, HBV reactivation in immunosuppressive therapy settings and chemotherapy has been a major contributing factor to delayed treatment, liver failure, and even death, thereby attracting increasing research attention.Citation8–11
Children with hematological malignancies are susceptible to infectious diseases due to the various degrees of immune deficiency, which results from the nature of the disease and immunosuppressive treatments.Citation12,Citation13 The risk of HBV infection is exceptionally high in Chinese children with hematological malignancies due to the regional HBV epidemic.Citation14 Moreover, due to tumor therapy and frequent blood transfusion, the risk of HBV infection for children with hematological malignancies is 6.5-fold higher than that for healthy children.Citation15,Citation16 However, due to the lack of standardized vaccination guidelines for children with hematological malignancies in China, the low recognition of the necessity of HBV vaccination among pediatric patients’ parents has led to a low rate of vaccination in this group of children.Citation17 Furthermore, there are currently only a few studies investigating the immune effect of hepatitis B vaccination in children with hematological malignancies. There is a lack of seroprotective monitoring of the antibody against hepatitis B surface antigen (anti-HBs).Citation16,Citation18
The present study investigates the compliance, safety, and immunogenicity efficacy of HBV vaccinations in children with hematological malignancies. The compliance of the subjects was evaluated by performing three anti-HBs tests after the completion of HBV vaccination. Children prospectively included in this study were inoculated with 3 doses of HBV vaccine at baseline, 1 month, and 6 months. They were subjected to 6-month follow-ups of anti-HBs titer. We also assessed the adverse events following immunization (AEFI) after each vaccination. With this information, the risks and benefits of booster HBV immunization in children with hematological malignancies can be assessed.
Materials and methods
Study design and objectives
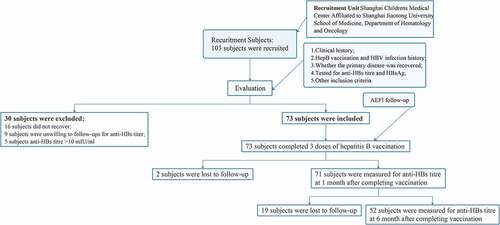
In this voluntary inoculation, a nonrandomized, interventional study was performed in China. Children aged 0 to 18 years who were clinically diagnosed with hematological malignancies were prospectively recruited from January 2017 to February 2020. Written informed consent was obtained from all legal guardians of the participants. This study was approved by the Ethics Committee of Shanghai Children’s Medical Center, School of Medicine, Shanghai Jiaotong University (Number: SCMCIRB-K2019076-2). The study was registered at www.clinicaltrials.gov (NCT03373656). One-hundred-and-three subjects were recruited from Shanghai Children’s Medical Center Affiliated with Shanghai Jiaotong University School of Medicine in this study. After screening by clinical evaluation and the inclusion criteria, 73 subjects were successfully included, and 30 subjects were excluded due to reasons such as recovering from diseases, unwillingness to provide blood, and anti-HBs titer >10 mIU/ml ().
Inclusion and exclusion criteria
Subjects were comprehensively evaluated by experienced pediatrics doctors for clinical history, prognosis and duration of immunosuppression. The inclusion criteria included the following: 1) aged 0 to 18 years old; 2) clinically diagnosed hematological malignancies; 3) completed the China national neonatal hepatitis B vaccination program; 4) completed antitumor treatment, such as chemotherapy for at least 12 months; 5) adequate immune profile (platelet count > 150 × 109/ μL, neutrophil count > 1.40 × 109/L; 6) anti-HBs titer <10 mIU/ml; 7) negative HBsAg index (<0.050 IU/ml); and 8) consented to participate.
The exclusion criteria were as follows: 1) history of HBV infection; 2) received antibody treatment, especially antitumor necrosis factor-related preparations; 3) immunosuppressant treatment within the past 5 months; 4) history of blood transfusion within the past 5 months; and 5) unwillingness to consent to timely completion of inoculation and follow-ups for anti-HBs titer.
Vaccine and serological analysis
Recombinant HBV vaccine (01190526A, Hissen Vaccine, Dalian, China) was used throughout the study period of 2017–2020 and was given as three doses of 10 μg at 0, 1 and 6 months. Anti-HBs titers were measured by collecting 3 to 5 ml of venous blood from the study subjects at 1 month and 6 months after the completion and were detected by enzyme-linked immunosorbent assay (anti-HBs ELISA kit, Shanghai Zheke Biological Engineering Center, Shanghai, China) with the EVO automatic enzyme immunoassay system (Tecan Group Ltd., Zürich, Switzerland). The detection limit was 0 mIU/mL, and the range of the anti-HBs quantification assay for the ELISA was 0–1000 mIU/mL.
Adverse events
Adverse events following immunization were collected from medical records and self-report questionnaires designed according to presurvey reliability and validity,Citation19 as shown in Supplementary 1.
Statistical analysis
Data analysis was performed using R3.4 statistical software (R3.4 formerly AT&T, now Lucent Technologies, New Jersey, USA). Continuous data are expressed as the mean ± SD. Comparisons between the two groups were tested by Student’s t-test. The rank sum test was used in the case of a failed normality assumption or homogeneity of variance test. Comparisons were made using the chi-square test or Fisher’s exact probability method. P< .05 was considered statistically significant.
Results
Clinical characteristics
A total of 73 study subjects were successfully included in this study (). Among the study subjects, 43 were male (58.90%), and 30 were female (41.10%). Among the patients with a history of hepatitis B vaccination, 68 (93.2%) and 5 (6.8%) patients have the complete history of hepatitis B vaccination and unknown, respectively. Among the patients with a history of previous clinical treatment for hematological malignancies, 32 (74.4%) and 11 (25.6%) male patients received chemotherapy and transplantation, respectively. Among female patients, 21 (70.0%), and 9 (30.0%) received chemotherapy and transplantation. There was no statistically significant difference in the composition of male and female ratios for clinical treatment (P> .05), suggesting that the confounding bias of sex can be excluded.
Table 1. Analysis of general characteristics of subjects
Analysis of the immunogenic effect of the HBV vaccine
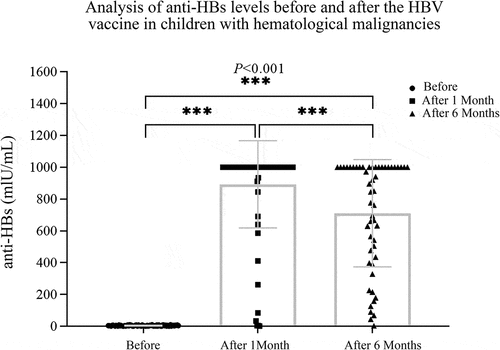
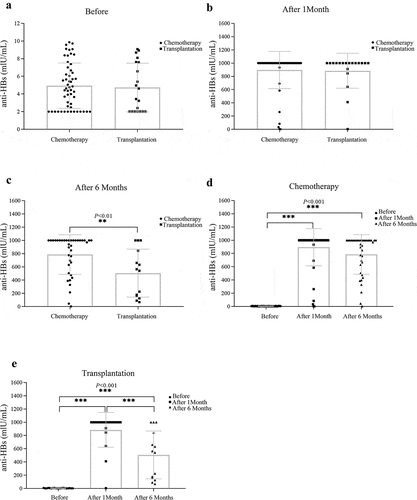
The anti-HBs titers in children with hematological malignancies tumors at baseline and 1 month and 6 months after completing the three-dose inoculation were 4.88 ± 2.61, 893.12 ± 274.12, and 711.45 ± 337.88, respectively (). A significant difference was observed at 1 month and 6 months in the anti-HBs titer compared with baseline (P< .001). However, a statistically significant drop in the anti-HBs titer was observed at 6 months compared to that at 1 month (P< .01), suggesting that the immune effect of children with hematological malignancies was excellent after HBV vaccination and that the persistence of the antibody was appropriate.
Age-based subgroup analysis of the immunogenic effect of the HBV vaccine
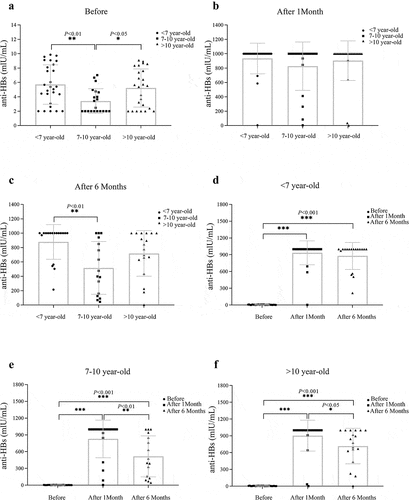
We performed a subgroup analysis of the anti-HBs titer in different age groups. At the 1-month follow-up, the anti-HBs titers were 933.95 ± 213.88 mIU/ml, 827.07 ± 336.35 mIU/ml, and 903.49 ± 276.40 mIU/ml in the <7-year-old, 7–10-year-old, and >10-year-old age groups, respectively (). A statistically significant difference was found between the <7-year-old group and the 7–10-year-old groups at the one-month follow up (P< .01). At the 6-month follow-up, the anti-HBs titer differed significantly among the three groups (P< .05) and was 877.75 ± 241.44 mIU/ml, 516.55 ± 366.87 mIU/ml, and 718.4 ± 316.78 mIU/ml in the <7-year-old, 7–10-year-old, and >10-year-old age groups, respectively (). The immunization effect of the HBV vaccine in the <7-year-old age group was better than that in the other two groups, and the hepatitis B antibody levels of children in the 7–10-year-old and >10-year-old age groups were decreased faster than in the <7-year-old group (). These results suggested that the age of children was related to the duration of HBV vaccine antibody persistence, and the younger the children were, the better the immunogenic effect.
Diagnosis-based subgroup analysis of the immunogenic effect of the HBV vaccine
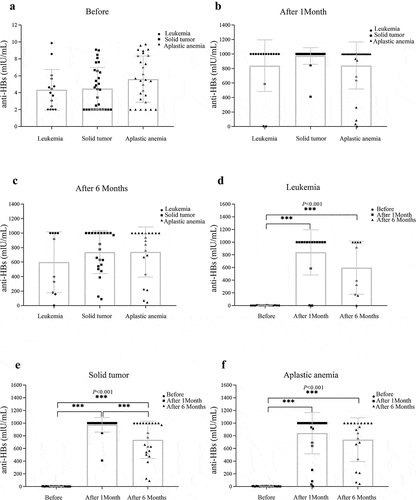
After vaccination completion, the anti-HBs titer increased in children in the diagnosis-based subgroup. However, there were no statistically significant differences in the anti-HBs titer between the 1-month and 6-month follow-ups (). At baseline, the anti-HBs titer in children with leukemia was 4.34 ± 2.41 mIU/ml, and it was 839.54 ± 355.74 mIU/ml and 598.66 ± 419.5 mIU/ml at the 1-month and 6-month follow-ups, respectively (). Compared with baseline, a significant increase in the anti-HBs titer was observed at the 1-month and 6-month follow-ups (P< .001 and P< .01, respectively). There was also a statistically significant difference between the 1-month and 6-month hepatitis B anti-HBs titers after vaccination in children with solid tumors () (P< .001). These results suggest that the hepatitis B vaccine antibody persistence of children with solid tumors was worse than that of children with leukemia and aplastic anemia.
Analysis of the effect of the hepatitis B vaccine in children with different types of anticancer treatment
The differences of in anti-HBs titers in children with different types of anticancer treatment at baseline and 1 month were not statistically significant (). However, statistically significant differences in anti-HBs titers in children at 6 months (). We further analyzed the immunogenic effect of the HBV vaccine in children with hematological malignancies according to past antitumor treatment. In the post-chemotherapy group, the anti-HBs titer was 4.94 ± 2.57 mIU/ml, 896.15 ± 280.42 mIU/ml, and 786.93 ± 299.22 mIU/ml at baseline, 1 month, and 6 months, respectively (). Compared with baseline, a significant increase in the anti-HBs titer was observed at the 1-month and 6-month follow-ups (P< .001 and P< .05, respectively) in children who had previously received chemotherapy. In the post-transplantation group, the anti-HBs titer was 4.73 ± 2.77 mIU/ml, 884.81 ± 263.28 mIU/ml, and 506.57 ± 362.19 mIU/ml at baseline () month, and 6 months, respectively. Compared with baseline, a significant increase in the anti-HBs titer was observed at the 1-month and 6-month follow-ups (P< .001 and P< .05, respectively) in children who had previously received transplantation therapy. Together, these results suggest that the persistence of hepatitis B vaccine antibodies in children after chemotherapy was better than that in children after transplantation.
Analysis of the anti-HBs positive conversion rate of HBV vaccine in children with hematological malignancies
At baseline, all the patients recruited had anti-HBs titers of <10 mIU/ml. One month after the three-dose inoculations, 68 (95.77%) were had anti-HBs titers of >10 mIU/ml (range 34.18–1000.00 mIU/mL, 932.36 ± 203.76 mIU/mL), while three cases (4.23%) remained at anti-HBs titers of <10 mIU/ml (range 2.00–4.49 mIU/mL, 3.65 ± 1.43 mIU/mL) (). A statistically significant difference in the anti-HBs positive conversion rate was found compared to baseline (P < .001). At 6 months after vaccination, among the 52 patients who completed serological follow-up, 51 patients (98.08%) had anti-HBs titers of >10 mIU/ml (range 44.61–1000.00 mIU/mL, 725.34 ± 325.92 mIU/mL), while one patient (1.92%) remained at anti-HBs titers of <10 mIU/ml. Together, these results suggest that children with hematological malignancies after vaccination with the hepatitis B vaccine have a relatively high anti-HBs positive conversion rate and can maintain a high level within six months (). There were no reports of hepatitis B virus infection report among all the children followed.
Table 2. Analysis of anti-HBs positive conversion rate in children with hematological malignancies before and after 3-dose HBV vaccinations
Analysis of adverse events following immunization
A total of 14 suspected AEFI incidents occurred throughout the vaccinations timeframe in the present study. The number of adverse events reported after the first, second, and third inoculations was 5 (6.85%), 5 (6.85%), and 4 (5.48%), respectively (). The number of local swelling events and redness of ≤ 2.5 cm in diameter reported after the first, second, and third inoculations were 1 (1.37%), 2 (2.74%), and 1 (1.37%), respectively (P> .05). Together, these results suggest that the incidence of AEFIs in children with hematological malignancies after vaccination with the hepatitis B vaccine was low. It was safe for this group of children to receive the HBV vaccinations.
Table 3. Analysis of AEFI of HBV vaccination in children with hematological malignancies
Discussion
In this study, children received three doses of the HBV vaccine and were serologically followed up at 1 month and 6 months, and the anti-HBs titers were 893.12 ± 274.12 mIU/ml and 711.45 ± 337.88 mIU/ml, respectively. Our results were similar to those reported by Keskin et al.,Citation20 indicating that the immune effect of HBV vaccines in children with hematological malignancies is similar to that in healthy children.
Children with hematological malignancies often have a history of chemotherapy or hemopoietic stem cell transplantation (HSCT). For children after HSCT, the recovery of the number and function of peripheral blood lymphocytes is relatively slow. A previous study reported that for patients without a history of chronic graft-versus-host disease (GVHD), CD4 + T lymphocytes recovery takes at least one year.Citation21 In contrast, in those with chronic GVHD, immune reconstitution may take several years. Up to date, there were not national vaccination guidelines for children with hematological malignancies in China.Citation22 The vaccination program of the healthy children who were anti-HBs and HBsAg double negative, with HBV vaccination history in China and the published expert consensus both suggest that the HBV vaccination program was to revaccinate with 3 doses of hepatitis B vaccine for children with hematological malignancies.Citation23 In the present study, the anti-HBs conversion rate one month after the three-dose vaccination protocol in children previously treated with chemotherapy or transplantation was 95.77%, similar to that in healthy children, which may be related to the immune memory response of anti-HBs. In addition, the study further showed that the three-dose vaccination protocol in children with cancer was more effective than a single dose of HBV vaccine booster.Citation24 However, there were three nonresponse cases who have complete history of hepatitis B vaccination, which may be associated with interleukins involved in Th1 functioning, the vaccination dose, and other factors.Citation25
The study by Ghosh N et al. evaluated the effect of vaccination with 40 µg of hepatitis B vaccine for children with hematological malignancies. The results of this study were consistent with previous studies; however, the protection rate of HBV of Ghosh N et al. was lower than that in our study.Citation17 Compared with the study by Ghosh N et al., the vaccination procedure and recruitment subjects of this study were different. The recruitment subjects of their study were children in the maintenance phase of chemotherapy, and they were not previously vaccinated against the hepatitis B vaccine. Chemotherapy not only reduces the immune function of children, but also significantly reduces anti-HBs titers in children, resulting in a reduction in the effective immune response of the hepatitis B vaccine.Citation24,Citation26 On the one hand, because the recruitment subjects have no history of hepatitis B vaccination compared with our study, the subjects lack the immune memory response of anti-HBs, which leads to a low level of vaccine response. On the other hand, the hepatitis B vaccination program by the study by Ghosh N et al. was 0, 1 and 2 months, which was different from this study. The hepatitis B vaccine is an inactivated vaccine. If the interval between the second and third doses is short, effective booster immunization may not be achieved.
Common adverse reactions after hepatitis B vaccination included local reactions, systemic reactions, and rare adverse reactions with varying degrees of severity, from local skin redness, swelling, fever, vomiting and diarrhea to potentially life-threatening reactions such as anaphylactic shock. No severe adverse reactions were reported among the reported adverse reactions, while fever, needle insertion-related local redness, and swelling were reported. These results suggested that the safety profile of HBV vaccination in children with hematological malignancies is similar to that of the healthy children, as reported by previous studies.Citation24,Citation26 Together, it may be interpreted that the revaccination of the HBV vaccine in children with hematological malignancies is safe.
It is worth noting that in children with hematological malignancies, the anti-HBs titer will gradually decrease after HBV vaccination. We noticed a decline in the anti-HBs titer at the 6-month follow-up. Although the anti-HBs level remains within the effective range at 6 months, the drop in the anti-HBs level seems to be slightly faster than that of healthy children, and the anti-HBs level of transplant patients declines faster than that of chemotherapy patients.Citation27 This phenomenon may be partially due to the damaged innate immune system due to diseases and clinical treatments. Meanwhile, the development of immune function and surveillance in young children may weaken the antigen presentation ability following vaccination and further reduce the immunogenicity effect of immune memory cells.Citation8 However, due to the COVID-19 pandemic, our present study lacks a 12-month follow-up after vaccination completion, and approximately 28.77% of the patients (6 months after inoculation/before vaccination = 73/52) were lost to follow-up 6 months after vaccination. In future studies, the duration of protective antibodies in children with hematological malignancies should be further observed to determine the interval between subsequent hepatitis B vaccine revaccination.
There are some shortcomings in the study. First, the sample size of the study was relatively small. Second, potential underreporting may arise from the self-reporting questionnaire upon monitoring for adverse reactions. Third, there were missing data for the 6-month follow-up for some participants due to the COVID-19 pandemic, and the anti-HBs tests could not be performed.Citation28,Citation29 Therefore, the present study’s data should be interpreted with caution and further large-scaled clinical observational studies using objective adverse reaction monitoring designs and evaluating antibody levels are warranted to improve the accuracy and validity of the study.
Conclusions
After the child completes chemotherapy or transplantation, hepatitis B vaccine revaccination is safer, and the vaccine response rate is similarly to that of healthy children. Periodic monitoring of anti-HBs titers accompanied by timely booster vaccination is warranted.
Authors’ contribution statement
The study protocol was designed by DPF, FY and GYJ. Data collection was done by YT, ZH, ZF, YDD and WWP. Data analysis was completed by DPF, XCY and GYJ. DPF, YT and GYJ prepared the manuscript which was reviewed by all authors.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Provenance and peer review
Not commissioned; externally peer reviewed.
Supplemental Material
Download PDF (96.7 KB)Acknowledgments
The authors appreciate the assistance from the Department of Hematology and Oncology Shanghai Children’s Medical Center affiliated with Shanghai Jiaotong University School of Medicine, Shanghai, China.
Supplemental Material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2021.1953303.
Additional information
Funding
References
- Su H, Shao Z, Pu Z, Wang Y, Zhang L, Zhang W, Wang B, Wang A, Ji Z, Yan Y, et al. Overt and occult hepatitis B virus infection among community children in Northwest China. J Viral Hepat. 2017;24(9):797–803. doi:10.1111/jvh.12709.
- Zhang S, Wang F, Zhang Z. Current advances in the elimination of hepatitis B in China by 2030. Front Med. 2017;11(4):490–501. doi:10.1007/s11684-017-0598-4.
- Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, Zhang Y, Liu J, Gong X, Chen Y, et al. Epidemiological serosurvey of hepatitis B in China--declining HBV prevalence due to hepatitis B vaccination. Vaccine. 2009;27(47):6550–57. doi:10.1016/j.vaccine.2009.08.048.
- Cui J, Cao L, Zheng JS, Cao LS, Yuan P, Li L. Coverage analysis of national immunization program vaccines reported in China,2013. Chin J Vacc Imm. 2015;21(3):289–94. doi:CNKI:SUN:ZGJM..
- Alanko S, Pelliniemi TT, Salmi TT. Recovery of blood B-lymphocytes and serum immunoglobulins after chemotherapy for childhood acute lymphoblastic leukemia. Cancer. 1992;69(6):1481–86. doi:10.1002/1097-0142(19920315)69:6<481::aid-cncr2820690628>3.0.co;2-l.
- Kovacs GT, Barany O, Schlick B, Csoka M, Gado J, Ponyi A, Müller J, Nemeth J, Hauser P, Erdelyi DJ, et al. Late immune recovery in children treated for malignant diseases. Pathol Oncol Res. 2008;14(4):391–97. doi:10.1007/s12253-008-9073-5.
- Esposito S, Cecinati V, Brescia L, Principi N. Vaccinations in children with cancer. Vaccine. 2010;28(19):3278–84. doi:10.1016/j.vaccine.2010.02.096.
- Moser M, Leo O. Key concepts in immunology. Vaccine 2010;28(Suppl 3):C2–13. doi:10.1016/j.vaccine.2010.07.022.
- Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50(3):661–62. doi:10.1002/hep.23190.
- Shouval D, Shibolet O. Immunosuppression and HBV reactivation. Semin Liver Dis. 2013;33(2):167–77. doi:10.1055/s-0033-1345722.
- Perrillo RP, Martin P, Lok AS. Preventing hepatitis B reactivation due to immunosuppressive drug treatments. JAMA. 2015;313(16):1617–18. doi:10.1001/jama.2015.2571.
- Ozkurt ZN, Suyani E, Haznedar R, Yağcı M. A randomized study comparing the efficacy of three hepatitis B vaccine induction regimens in adult patients with hematological malignancies. Turk J Haematol. 2016;33(3):231–35. doi:10.4274/tjh.2015.0079.
- Bonaventure A, Harewood R, Stiller CA, Gatta G, Clavel J, Stefan DC, Carreira H, Spika D, Marcos-Gragera R, Peris-Bonet R, et al. Worldwide comparison of survival from childhood leukaemia for 1995-2009, by subtype, age, and sex (CONCORD-2): a population-based study of individual data for 89 828 children from 198 registries in 53 countries. Lancet Haematol. 2017;4(5):e202–e217. doi:10.1016/S2352-3026(17)30052-2.
- Zhang Z, Zhang Y, Xu N, Huang C, Li X, Li J. High risk of occult hepatitis B virus infection in leukemia patients from China. Arch Virol. 2017;162(2):349–57. doi:10.1007/s00705-016-3111-5.
- Akimkin VG, Ledin EV, Skvortsov SV, Rukavitsyn OA. Incidence of hepatitis B virus infection in patients with blood disease. Ter Arkh. 2007;79(11):28–31.
- Kibria CH, Islam KA, Islam MA, Rahman A, Biswash AK. Hepatitis B antibody level falls after initial phage of chemotherapy in childhood haematological malignancy. Mymensingh Med J. 2019;28(2):418–22.
- Ghosh N, Mannan MA, Monjur F, Rizwan F, Salim AF. Escalated regimen of hepatitis B vaccine in childhood hematological malignancies while on chemotherapy. Southeast Asian J Trop Med Public Health. 2010;41(3):555–61.
- Elkady A, Aboulfotuh S, Ali EM, Sayed D, Abdel-Aziz NM, Ali AM, Murakami S, Lijima S, Tanaka Y. Incidence and characteristics of HBV reactivation in hematological malignant patients in south Egypt. World J Gastroenterol. 2013;19(37):6214–20. doi:10.3748/wjg.v19.i37.6214.
- Inoue T, Tanaka Y. Cross-protection of hepatitis B vaccination among different genotypes. Vaccines (Basel). 2020 Aug 16;8(3). doi:10.3390/vaccines8030456.
- Keskin YZ, Buyukavci M. Assessment of humoral immunity to hepatitis B, measles, rubella, and mumps in children after chemotherapy. J Pediatr Hematol Oncol. 2018;40(2):e99–e102. doi:10.1097/mph.0000000000001072.
- Conrad A, Alcazer V, Valour F, Ader F. Vaccination post-allogeneic hematopoietic stem cell transplantation: what is feasible? Expert Rev Vaccines. 2018;17(4):299–309. doi:10.1080/14760584.2018.1449649.
- Xiang G, Chou Jing XS. Application of expert consensus to guide the vaccination of children with special health status. Chin J Prevent Med. 2021;55(2):284–87. doi:10.3760/cma.j.cn112150-20201013-01275.
- YI W. Expert consensus on immunization in children with special health state (ⅩⅩIII) ——Allo-HSCT and immunization. Chin J Pract Pediatr. 2019;34(7):538–40. doi:10.19538/j.ek2019070602.
- Fayea NY, Kandil SM, Boujettif K, Fouda AE. Assessment of hepatitis B virus antibody titers in childhood cancer survivors. Eur J Pediatr. 2017;176(9):1269–73. doi:10.1007/s00431-017-2970-4.
- Mormile R. Hepatitis B vaccine non response: a predictor of latent autoimmunity? Med Hypotheses. 2017 Jul;104:45–47. doi:10.1016/j.mehy.2017.05.020.
- Viana SS, Araujo GS, Faro GB, Cruz-Silva LLD, Araújo-Melo CA, Cipolotti R. Antibody responses to Hepatitis B and measles-mumps-rubella vaccines in children who received chemotherapy for acute lymphoblastic leukemia. Rev Bras Hematol Hemoter. 2012;34(4):275–79. doi:10.5581/1516-8484.20120071.
- Velu V, Nandakumar S, Shanmugam S, Shankar EM, Thangavel S, Kulkarni PS, Thyagarajan SP. Comparative efficacy of two dosages of recombinant hepatitis B vaccine in healthy adolescents in India. Pediatr Infect Dis J. 2007;26(11):1038–41. doi:10.1097/inf.0b013e3181342887.
- Nicola M, Alsafi Z, Sohrabi C, Kerwan A, Al-Jabir A, Iosifidis C, Agha M, Agha R. The socio-economic implications of the coronavirus pandemic (COVID-19): a review. Int J Surg. 2020;78:185–93. doi:10.1016/j.ijsu.2020.04.018.
- Peng F, Tu L, Yang Y, Hu P, Wang R, Hu Q, Cao F, Jiang T, Sun J, Xu G, et al. Management and Treatment of COVID-19: the Chinese Experience. Can J Cardiol. 2020;36(6):915–30. doi:10.1016/j.cjca.2020.04.010.