ABSTRACT
This study investigated the implementation status, attitudes, and practices regarding maternal pertussis vaccination in delivery facilities in Kanagawa, Japan. We conducted a questionnaire survey of pertussis vaccination during pregnancy at 125 delivery service facilities, excluding midwifery clinics, in Kanagawa Prefecture from June 18 to July 20, 2020. The questionnaire comprised multiple-choice items, with single or multiple answers depending on the question. Of the 125 facilities surveyed, valid responses were obtained from 72 facilities (58%). There were 41 primary clinics with birthing facilities (57%), 16 general hospitals (not designated perinatal centers) (22%), 10 regional perinatal centers (14%), and five tertiary perinatal centers (7%). None of the facilities administered pertussis vaccination during pregnancy. Forty facilities (56%) reported knowledge of the severity of neonatal and infant pertussis infections. Twenty-three facilities (32%) reported knowledge of the prevention of pertussis infection by vaccination during pregnancy. All facilities reported willingness to consider vaccination if included in the Japanese guidelines. In Japan, the pertussis vaccination during pregnancy is not highly practiced by obstetricians; thus, it is not as popular as in other countries. Further studies are needed to promote strategies to prevent pertussis infection in the neonatal and infant periods.
Pertussis infection tends to be more severe in the neonatal period, with a higher mortality rate in infants under one year of age, than in other age groups.Citation1,Citation2 The pertussis vaccine cannot be administered to infants below two months of age. Therefore, newborns develop their immunity through the mother. Thus, the United States and other countries recommend the administration of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine during pregnancy to transfer antibodies placentally, in a procedure termed maternal immunization.Citation3–7 Maternal immunization for pertussis has been widely recommended in the United States since 2012.Citation3 Promising results of maternal pertussis vaccination have been reported in many countries.Citation8,Citation9 In the U.K., a government-led Tdap vaccination recommendation program was organized in 2012: 60% of all pregnant women were vaccinated within one year, resulting in a reported reduction in pertussis infections of approximately 80% in patients less than three months of age and 60–70% in patients aged three months to one year.Citation9 In Japan, all pertussis infection cases have been registered since 2018, and the number of cases and their backgrounds have been reported. In 2019, there were 16,785 cases reported, with 5% of the cases occurring in patients under six months of age.Citation10 Of those 5%, 72% were unvaccinated, and one death was reported. Therefore, prevention of pertussis infection during the neonatal and infant periods is very important in Japan. However, the Tdap vaccine is not approved in Japan,Citation11 and pertussis vaccination during pregnancy is not mentioned in the guidelines of the relevant Japanese academic societies and organizations. The pertussis vaccines currently approved in Japan are diphtheria, pertussis, tetanus, and inactivated polio (DTaP-IPV) vaccine, a quadruple combination vaccine used for routine immunization, and diphtheria, pertussis, and tetanus (DTaP) vaccine, a triple combination vaccine. Tdap is a vaccine with a reduced antigen level for diphtheria and pertussis that reduces local reactions at the time of vaccination compared to DTaP. The appropriate age for DTaP vaccination is 2–3 months of age or older, including adults. One study showed that maternal immunization by pertussis vaccine is not recognized by the general population in Japan,Citation12 while the actual situation regarding maternal pertussis vaccination in obstetric delivery facilities is not known. In this study, we conducted a questionnaire survey targeting delivery facilities in Kanagawa Prefecture to clarify the status of vaccine delivery and recognition of maternal pertussis vaccination in Japan to promote future maternal pertussis vaccinations.
From June 18 to July 20, 2020, we conducted a questionnaire survey regarding pertussis vaccination during pregnancy at 125 delivery service facilities, excluding midwifery clinics, in Kanagawa Prefecture. In Kanagawa Prefecture, there are 125 delivery facilities: 72 primary clinics, 31 general hospitals (not designated perinatal centers), 17 regional perinatal centers, and five tertiary perinatal centers. The questionnaire was sent by mail or via internet to the administrator of the delivery department, and consent was obtained upon collecting the questionnaire. The questionnaire consisted of multiple-choice items, with single or multiple answers depending on the question. The questionnaire included the following items: type of facility, number of deliveries per year, whether the facility offers pertussis vaccinations during pregnancy, gestational weeks in which vaccinations were received, explanations offered to mothers concerning the vaccinations, percentage of pregnant women who requested vaccination, number of vaccines administered, type of pertussis vaccine, whether the staff recognized the risk of severe illness due to pertussis infection during the neonatal period, whether the staff recognized the preventative effects of vaccination during pregnancy, whether to administer the Tdap vaccine (which is widely used for pregnant women internationally) if approved in Japan, and whether to administer the DTaP vaccine (which has been approved in Japan) if evidence for the prevention of neonatal infection was obtained. Descriptive statistics were calculated for discrete measures using JMP® 15 (SAS Institute Inc., Cary, NC, USA).
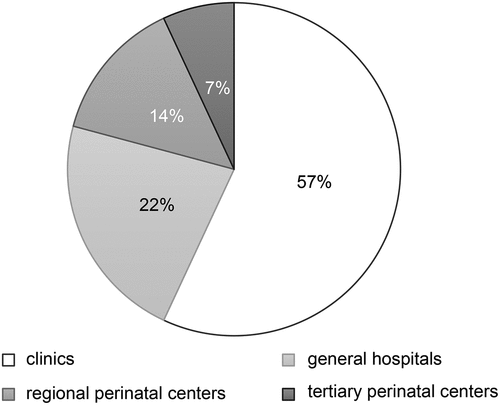
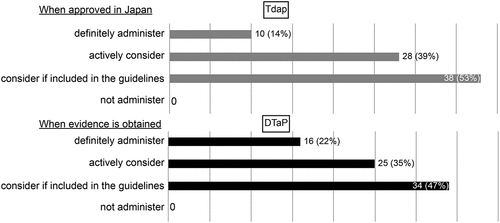
Of the 125 facilities surveyed, 73 responded; one facility was excluded due to a lack of adequate responses to the questionnaire. Appropriate responses to the questionnaire were therefore obtained from 72 facilities (57.6%). The excluded facility was a regional perinatal center, which responded that they did not provide maternal pertussis vaccination. All responses were obtained from administrators of the obstetrics department. The types of facilities that responded to the questionnaire are shown in . There were 41 primary clinics with birthing facilities (57%), 16 general hospitals (not designated perinatal centers) (22%), 10 regional perinatal centers (14%), and five tertiary perinatal centers (7%). The percentages of facilities with respect to the annual number of deliveries in 2019 are shown in . There were <200 annual deliveries in 13 facilities (18%), 200–399 in 15 facilities (21%), 400–599 in 18 facilities (25%), 600–799 in 12 facilities (17%), 800–999 in 8 facilities (11%), and >1000 in 6 facilities (8.3%). None of the facilities administered pertussis vaccines during pregnancy. Responses to each question item are shown in and . Since all facilities responded that they did not offer maternal pertussis vaccination, answers to the following questions regarding vaccination were not available: gestational weeks in which vaccination occurred, explanations offered to mothers concerning the vaccinations, percentage of pregnant women who requested vaccination, number of vaccines administered, and type of pertussis vaccine. More than half of the facilities reported recognizing the severity of neonatal and infant pertussis infections, and 32% reported recognizing the prevention of pertussis infection by vaccination during pregnancy. Ten facilities (14%) responded that they would administer the Tdap vaccine if approved in Japan, 28 facilities (39%) responded that they would actively consider it, and 38 facilities (53%) responded that they would consider it if mentioned in the guidelines. All facilities responded that they would administer Tdap. Sixteen (22%) responded that they would consider the use of DTaP if evidence of its efficacy was demonstrated, 25 (35%) responded that they would actively consider it, and 34 (47%) responded that they would consider it if mentioned in the guidelines. None of the facilities said they would not administer DTaP.
Table 1. Responses to questionnaire items
Figure 2. Percentage distribution of facilities according to the number of annual deliveries in 2019.
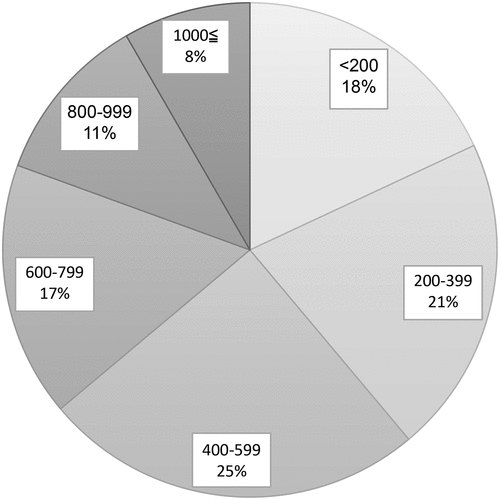
Figure 3. Immunization policies according to facilities if Tdap is approved in Japan and if there is evidence supporting the effectiveness of DTaP (multiple answers).
This questionnaire survey revealed that none of the facilities in Kanagawa Prefecture administered the vaccine (). Many delivery facilities reported that they would consider pertussis vaccination during pregnancy if approved and included in the vaccine guidelines of Japan.
In contrast to the global trend mentioned in the introduction, a previous questionnaire survey that questioned pregnant women and mothers who had visited pediatricians in Japan showed that only 4.6% of the respondents recognized maternal immunization for pertussis.Citation12 In the current study, we found many obstetricians do not recognize maternal immunization for pertussis and that pertussis vaccination during pregnancy is not practiced in Kanagawa Prefecture. To control pertussis infection among neonates and infants in Japan in the future, it is important to provide information about pertussis immunization during pregnancy. In addition, improving the recognition rate of the pertussis vaccine among obstetricians and making pertussis vaccination available during pregnancy in Japan, as in other countries, is also important.
One strategy to promote pertussis vaccination for pregnant women in Japan is to accumulate evidence for the administration of DTaP or advocate for the approval of Tdap. Endorsements from governments and academic societies were major factors for increasing the use of maternal pertussis vaccines in other countries. In the U.K., an administration-led advisory on pertussis vaccination for pregnant women was conducted in 2012; a year later, the rate of pregnant women receiving the vaccine rose from less than 10% to 60%.Citation9 In Japan, the Japan Society of Obstetrics and Gynecology has published obstetric guidelines since 2008, and the rate of compliance with these guidelines is high.Citation13 In this study, many facilities responded that they would positively consider pertussis vaccination if included in the guidelines. Therefore, recommendations in the guidelines could sufficiently change the pertussis vaccination rate for pregnant women in Japan.
Limitations of this study include the risk of selection bias and small sample size. Since the survey was limited to Kanagawa Prefecture and thus comprised a relatively small sample size, the results may differ depending on the area due to differences in the prevalence of infection and community education levels. In addition, because the present study only included facilities with birthing facilities, the status of facilities that do not provide delivery services is unknown. However, this study is valuable because it is the first report in Japan to clarify pertussis vaccination during pregnancy is not popular in Japan, unlike in other countries. To promote strategies to prevent pertussis infection in the neonatal and infant periods, further studies on the validity of pertussis vaccination for pregnant women in Japan are needed.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplemental Material
Download MS Word (17.1 KB)Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2021.1958609
References
- Masseria C, Martin CK, Krishnarajah G, Becker LK, Buikema A, Tan TQ. Incidence and burden of pertussis among infants less than 1 year of age. Pediatr Infect Dis J. 2017;36(3):e54–61. doi:10.1097/INF.0000000000001440.
- Skoff TH, Hadler S, Hariri S. The epidemiology of nationally reported pertussis in the United States, 2000–2016. Clin Infect Dis. 2019;68:1634–40. doi:10.1093/cid/ciy757.
- Liang JL, Tiwari T, Moro P, Messonnier NE, Reingold A, Sawyer M, Clark TA. Prevention of pertussis, tetanus, and diphtheria with vaccines in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2018;67:1–44.
- World Health Organization. Pertussis vaccines: WHO position paper—September 2015. Wkly Epidemiol Rec. 2015;90:433–58.
- Gov.UK. Guidance vaccination against pertussis (whooping cough) for pregnant women. [accessed 2021 May 31]. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/897039/Pertussis_vaccination_for_pregnant_women_2020.pdf.
- Government of Canada. An Advisory Committee Statement (ACS). National Advisory Committee on Immunization (NACI): update on immunization in pregnancy with tetanus toxoid, reduced diphtheria toxoid and reduced acellular pertussis (Tdap) vaccine. [accessed 2021 Mar 30]. https://www.canada.ca/en/public-health/services/publications/healthy-living/update-immunization-pregnancy-tdap-vaccine.html.
- Australian Government. Department of health. The Australian Immunisation Handbook: Pertussis (whooping cough). [accessed 2021 Mar 30]. https://immunisationhandbook.health.gov.au/vaccine-preventable-diseases/pertussis-whooping-cough.
- Walls T, Graham P, Petousis-Harris H, Hill L, Austin N. Infant outcomes after exposure to Tdap vaccine in pregnancy: an observational study. BMJ Open. 2016;6(1):e009536. doi:10.1136/bmjopen-2015-009536.
- Amirthalingam G, Andrews N, Campbell H, Ribeiro S, Kara E, Donegan K, Fry NK, Miller E, Ramsay M. Effectiveness of maternal pertussis vaccination in England: an observational study. Lancet. 2014;384(9953):1521–28. doi:10.1016/S0140-6736(14)60686-3.
- National Institute of Infectious Diseases, Center for Epidemiology of Infectious Diseases and Bacteria Division II. Epidemiology of pertussis-reported cases in Japan based on overall reported surveillance (updated information). Epidemiology Week 1–52. 2019 [accessed 2021 Mar 30]. https://www.niid.gchapo.jp/niid/ja/pertussis-m/pertussis-idwrs/9463-pertussis-20200306.html. (in Japanese).
- Ujiie M, Tsuzuki S, Suzuki M, Ota M, Suzuki T, Nomoto H, Yamamoto K, Saito S, Kokaze A, Kinoshita N, et al. Safety of diphtheria and tetanus toxoids and acellular pertussis (DTaP) vaccine in adults in Japan. Jpn J Infect Dis. 2021 Jan 29. doi:10.7883/yoken.JJID.2020.947. Online ahead of print.
- Kitano T, Onishi T, Takeyama M, Shima M. Questionnaire survey on maternal pertussis vaccination for pregnant women and mothers in Nara prefecture, Japan. Hum Vaccin Immunother. 2020;16(2):335–39. doi:10.1080/21645515.2019.1651000.
- Ohkuchi A, Baba Y, Matsubara S. Maternal medical checkup system in the region: effect of obstetrical practice guideline 2008 Japan on several obstetrical practice. J Jpn Soc Perinatal Neonatal Med. 2010;46:1155–56. (in Japanese).