ABSTRACT
In Russia, a universal varicella vaccination (UVV) program has not been implemented, and varicella vaccination coverage is low. We assessed the efficacy, antibody persistence, and safety of one- and two-dose varicella vaccination schedules in Russian children with a ten-year follow-up period, as part of an international phase IIIB, observer-blind, randomized, controlled trial (NCT00226499). Children aged 12–22 months were randomized (3:3:1) to receive two doses of tetravalent measles-mumps-rubella-varicella vaccine (V2 group), one dose trivalent measles-mumps-rubella (MMR) vaccine and one dose of varicella vaccine (V1 group), or two doses of MMR vaccine (V0 [control] group), 42 days apart. Main study outcomes were: vaccine efficacy (VE) against confirmed varicella cases, anti-varicella zoster virus (VZV) seropositivity rates and geometric mean concentrations, and reporting of (serious) adverse events ([S]AEs). The total vaccinated cohort in Russia comprised 1000 children; 900 were followed up until study end (year [Y] 10). VE estimates against confirmed varicella (Y10) were 92.4% in the V2 group and 74.7% in the V1 group. Anti-VZV seropositivity rates remained ≥99.4% in the V2 group and ≥89.7% in the V1 group from day 42 post-vaccination 2 until Y10. Occurrence of (un)solicited AEs and SAEs was similar across groups and confirmed the safety profile of the vaccines. No vaccination-related SAEs or deaths were reported. These results are consistent with the global trial results, i.e., the highest VE estimates observed following the two-dose schedule compared to the one-dose schedule. These data may inform decision-making related to potential implementation of a UVV program.
PLAIN LANGUAGE SUMMARY
What is the context?
Varicella is a common childhood disease caused by the highly contagious varicella zoster virus.
Varicella vaccines have been used for more than three decades.
A large clinical trial conducted in ten countries assessed the efficacy and safety of one dose of monovalent varicella vaccine or two doses of combined varicella vaccine (MMRV). The enrolled children were also followed up for a ten-year period to evaluate the persistence of the immune response and the long-term efficacy of the vaccine.
What is new?
Here, we present the long-term efficacy, immunogenicity, and safety results in the cohort of children enrolled in Russia, as part of the global ten-year follow-up study.
We found that:
The monovalent and combined vaccines reduced the number of varicella cases.
The MMRV two-dose regimen displayed higher efficacy in preventing varicella of all severities compared to the one-dose regimen.
The immune response conferred by the vaccine persisted up to ten years post-vaccination.
No vaccination-related deaths occurred, and no safety concerns were raised.
What is the impact?
Vaccination against varicella resulted in long-term protective efficacy and antibody persistence over ten years post-vaccination in Russian children.
Although one-dose varicella vaccination was effective at protecting against varicella, a two-dose schedule provided a more complete protection. This could inform health policy decisions regarding the implementation of varicella vaccination in routine immunization program in Russia.
Introduction
Varicella zoster virus (VZV) causes varicella (chickenpox) disease, which is highly infectious and affects mostly children. After a first VZV infection, the virus persists in the host’s body and may reactivate later in life to cause herpes zoster (HZ, shingles).Citation1 Although varicella is mostly mild in children, potentially severe complications, such as stroke, encephalitis, and secondary bacterial infections, may occur.Citation1–3 The global burden of varicella disease is high, with approximately 4.2 million complications requiring hospitalization and 4,200 associated deaths, annually.Citation1 It has been estimated that approximately 90% of children living in temperate climates become infected with VZV by the age of 15 years.Citation4,Citation5 In Russia, more than 820,000 varicella cases were reported in 2019, representing an annual incidence of 559.1 cases per 100,000 population. More than 90% of these cases occurred in children, and approximately 70% in one- to six-year-olds.Citation6
The currently available varicella vaccine formulations include monovalent varicella (V) vaccines, and tetravalent vaccines combining antigens against measles, mumps, rubella, and varicella (MMRV).Citation1 Two doses of either formulation have demonstrated >90% efficacy against varicella disease in randomized clinical trials.Citation7–9
Universal varicella vaccination (UVV) with one dose of a monovalent V vaccine was first introduced in the routine childhood immunization program in the United States of America (USA) in 1995.Citation10,Citation11 By 2004, vaccine uptake in the USA had reached approximately 90%, resulting in significant reductions in varicella incidence and associated hospitalization rates.Citation12–14 Similar UVV programs have been subsequently introduced by other countries in other regions of the world, resulting in significant reductions in the varicella disease burden.Citation15,Citation16
The use of a one-dose varicella vaccination schedule has been shown to be associated with vaccine efficacy (VE) estimates of ≥88% in randomized clinical trials.Citation8,Citation17,Citation18 However, this schedule was also associated with breakthrough disease,Citation19–21 with reported incidences ranging from 2.8% to 27.7%.Citation22–26 This observation prompted some countries to introduce a UVV program based on a two-dose schedule.Citation19,Citation27 The World Health Organization (WHO) recommends that the dosing schedule should be determined depending on the programmatic goal: while one dose is adequate for reducing mortality and severe morbidity, two doses further limit disease outbreaks.Citation1 This WHO recommendation is supported by data showing that fewer disease outbreaks occur in countries that implemented a two-dose UVV program.Citation23,Citation27,Citation28
In Russia, two monovalent V vaccines are licensed: Varilrix (GSK) and Varivax (Merck).Citation29,Citation30 The Russian Ministry of Health has approved vaccination with Varilrix according to a two-dose schedule with the first dose administered at the age of 12 months, and the second dose at least six weeks later.Citation29,Citation31 The Union of Pediatricians of Russia recommends the second dose being administered at 6 years of age to allow use of the tetravalent MMRV vaccine in the Russian national calendar of preventive vaccinations and thereby decrease the number of vaccination visits needed throughout childhood.Citation32 While varicella vaccination is included in certain regional immunization programs, to date, there is no UVV program at the country level.Citation31,Citation33 The regional immunization programs may apply universal vaccination, or target immunocompromised patients and/or social and professional risk groups, such as children attending a day care center or military recruits who were not previously vaccinated.Citation31 Regions where a varicella vaccination program is implemented reported a 75% lower varicella incidence than the national average in 2017.Citation34 Nevertheless, vaccination coverage remains low; in 2018, only approximately 5% of children aged 3 to 6 years were vaccinated against varicella.Citation32,Citation35
To further assess varicella vaccination in Europe, we conducted a phase IIIB, randomized, controlled trial in 10 European countries to evaluate the long-term efficacy, immunogenicity, and safety of varicella vaccination.Citation7,Citation9,Citation36 The tetravalent MMRV vaccine was administered as a two-dose schedule, while the monovalent V vaccine was administered as one dose following one dose of the trivalent measles, mumps, and rubella (MMR) vaccine.
The global results of the study have been published.Citation7,Citation9,Citation36,Citation37 After 10 years of follow-up, VE against all varicella was 95.4% in children who received two doses of tetravalent MMRV vaccine and 67.2% in children who received one dose of monovalent V vaccine. VE against moderate to severe varicella was 99.1% and 89.5% for the two-dose VZV-containing vaccine (VCV) and one-dose VCV group, respectively.Citation9 Similar VE estimates were observed after 3 and 6 years of follow-up.Citation7,Citation36 Seropositivity rates across groups receiving a VCV remained high during the study and were ≥98% for the varicella antigen and ≥90% for MMR antigens after 10 years of follow-up.Citation9,Citation37 Additionally, both vaccination schedules showed acceptable reactogenicity and safety profiles.Citation9 Here, we report the long-term efficacy, immunogenicity, and safety of one-dose or two-dose varicella immunization in the cohort of children enrolled in Russia. These data may inform the Russian authorities during decision-making related to potential implementation of a nationwide UVV program.Citation31,Citation38–40
A summary contextualizing the outcomes presented here is displayed in the Plain Language Summary () for the convenience of health-care professionals.
Participants and methods
Study design
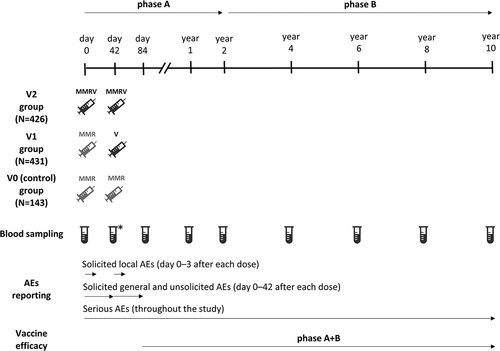
This study was a phase IIIB, observer-blind, randomized, controlled trial conducted between September 2005 and December 2016 in Czech Republic, Greece, Italy, Lithuania, Norway, Poland, Romania, Russia, Slovakia, and Sweden. Children were enrolled and randomly assigned (3:3:1) to receive two doses of tetravalent MMRV vaccine (Priorix-Tetra, GSK; V2 group), one dose of trivalent MMR vaccine (Priorix, GSK) followed by one dose of monovalent V vaccine (Varilrix, GSK; V1 group), or two doses of the MMR vaccine (V0 [control] group), 42 days apart. Here, we present results for Russia, generated in the context of this global study.
The trial consisted of two periods: phase A, which started on the day of first vaccination and lasted until year two; and phase B, which started from year two and lasted until study end (year 10). The phase A + B combined period for efficacy surveillance started 42 days post-vaccination two and extended until study end (year 10) (). At the start of phase B, the study was discontinued in regions where varicella vaccination was introduced in the regional immunization program.
Figure 2. Study design.
The study protocol, protocol amendments, and other study-related documents were reviewed and approved by the national, regional, or investigational center Independent Ethics Committee. The study is registered on ClinicalTrials.gov (NCT00226499) and was conducted in accordance with Good Clinical Practice principles, all applicable regulatory requirements, and the Declaration of Helsinki. All children’s parents or legally acceptable representatives (LARs) provided written informed consent prior to the study procedures.
Participants
Participants were healthy children 12–22 months of age who had at least one sibling who was living at the same place and had no history of varicella disease/vaccination, were attending a day care center or a childminder with at least one child who had no known history of varicella disease/vaccination, or played at least once a week in close physical contact for at least 5 min with a child who had no known history of varicella disease/vaccination. Detailed eligibility criteria were previously published.Citation36,Citation37
Randomization and masking
Randomization has been described previously.Citation36 Administration of the vaccines and efficacy surveillance up to at least two years post-vaccination 2 was conducted in an observer-blind manner. In phase B, Russian children and their parents/LARs in the V1 group were unblinded because the country-recommended national immunization schedule includes a second dose of trivalent MMR vaccine at 6 years of age.Citation33
Study vaccines
Three different lots of the tetravalent MMRV and monovalent V vaccines, and one lot of trivalent MMR vaccine were used. Both VCVs used in this study contain the live attenuated Oka strain.Citation41,Citation42 The detailed composition of the study vaccines was published previously.Citation36 Vaccines were administered subcutaneously in the left deltoid region.
Efficacy assessment
All outcomes reported in the current analysis were secondary and descriptive. The efficacy objectives were to estimate efficacy of one dose of monovalent V (V1 group) or two doses of tetravalent MMRV vaccine (V2 group) in preventing confirmed varicella cases, and to assess the occurrence of complicated varicella cases from vaccination 1 until study end (phase A + B), in all groups.
Assessment of varicella cases is detailed in a previous publication.Citation36 The presence of VZV in samples collected from skin lesions was confirmed by restriction fragment length polymorphism analysis following polymerase chain reaction (PCR) amplification. All cases of varicella-like rash were reviewed in a blinded manner by the independent data monitoring committee (IDMC). Based on the case description, PCR data, photographs of the lesion, and information regarding the child’s contact with an active disease, the IDMC classified each suspected case as: no case, a confirmed varicella case (defined in the previous publicationCitation36), or a probable case. For the latter, the IDMC considered the case in agreement with the Centers for Disease Control and Prevention’s clinical case definition (detailed in supplementary methods), but the case was not PCR-confirmed or epidemiologically linked to a varicella case that was identified as the source of infection.
Immunogenicity assessment
Anti-VZV immune responses were assessed in all children, and immune responses to measles, mumps, and rubella viruses were assessed in a subset of 200 children (subset for MMR testing) from six weeks post-vaccination 2 until year 2.
Blood samples for immunogenicity assessment were obtained from all children prior to administration of the first vaccine (pre-vaccination 1), at 84 days post-vaccination 1, and at years 1, 2, 4, 6, 8, and 10 of the study. An additional sample was obtained from children in the subset for MMR testing at 42 days post-vaccination 1. Anti-VZV (until year 10) and anti-measles, -mumps, and -rubella (until year 2) immunoglobulin G (IgG) antibody levels in serum were measured using a commercial enzyme-linked immunosorbent assay (ELISA) kit (Enzygnost, DiaSorin [formerly Siemens]).
Safety assessment
Serious adverse events (SAEs) were assessed in all children, and solicited local and general adverse events (AEs) and unsolicited AEs were assessed in the subset for MMR testing.
Solicited local AEs were recorded from day 0 to day 3, and solicited general and unsolicited AEs from day 0 to day 42, after each dose. SAEs were reported throughout the study. Solicited general AEs were fever, swelling of the salivary glands, meningism (including febrile seizures), and rash. Solicited AEs were graded 1–3 according to their intensity (grade 1–4 for rash as published previouslyCitation43). The definition of grade 3 and medical events that were considered as SAEs are available in supplementary methods. Severity and causal association of all AEs with study vaccinations were assessed according to the investigator’s clinical judgment.
Statistical analyses
Calculation of the sample size for the primary objectives of the global study was described previously.Citation36 All statistical analyses described here were descriptive. Statistical analyses were performed using the Statistical Analysis System (SAS) version 9.3 (including Proc-StatXact, version 8.1 module).
VE was calculated in the according-to-protocol (ATP) cohort for efficacy which included all children who completed vaccination and fulfilled protocol requirements. For VE calculations, data of children were censored at a varicella event, at the latest date with available data, at study end, or at the date at which mass vaccination with a VCV was implemented. The total time to event was calculated as the sum of the follow-up period expressed in years and censored at first occurrence of an event in each group. The incidence rate per 100 person-years was calculated as the number of children reporting at least one event in each group divided by the total time to event and was reported with its 95% confidence interval (CI). The Cox proportional hazards regression model without adjustmentsCitation44 was used to estimate the hazard ratio (HR) of experiencing a varicella event in the vaccinated group (V2 or V1 group) compared to the control group (V0 group). VE was estimated as 100×(1-HR) and was reported with its 95% CI, calculated in the same regression analysis. To assess robustness of the VE estimate for confirmed varicella cases, a post-hoc sensitivity analysis was done by performing the same calculations for the confirmed and probable varicella cases combined.
VZV immunogenicity outcomes were assessed in the adapted ATP cohort for persistence, which included children who completed vaccination, fulfilled protocol requirements, and complied with all visit intervals up to and including the timepoint considered. Anti-VZV antibody geometric mean concentrations (GMCs) were calculated by taking the anti-log of the mean of the log concentration transformation of all values equal to or above the limit of quantification (40 milli-international units [mIU]/mL) in children who were seronegative (i.e., children who had anti-VZV antibody levels below the assay cutoff [25 mIU/mL]) prior to vaccination. Before log-transformation, values below the cutoff were given an arbitrary value of half the cutoff. Values between 25 mIU/mL and 40 mIU/mL were given the value of 25 mIU/mL. All GMCs were reported with 95% CIs. Seropositivity rates were calculated at each timepoint as the percentage of children with anti-VZV antibody concentrations ≥25 mIU/mL and were reported with 95% CIs.
The levels of anti-measles, -mumps, and -rubella antibodies were measured up to two years post-vaccination 2 in the adapted ATP cohort for persistence in the subset for MMR testing; antibody GMCs were calculated by taking the anti-log of the mean of the log concentration transformation, in children who were seronegative prior to vaccination. Values below the cutoff (150 mIU/mL for anti-measles, 231 U/mL for anti-mumps, and 4 IU/mL for anti-rubella) were given the arbitrary value of half the cutoff. All GMCs were reported with 95% CIs. Seropositivity rates were calculated at each timepoint as the percentage of children with antibody concentrations equal or above the seropositivity thresholds (i.e., the assay cutoff in this study), and were reported with 95% CIs.
SAEs were assessed in the total vaccinated cohort (TVC), which included all children who received at least one dose of a study vaccine. AEs were assessed in the TVC in the subset for MMR testing. All safety endpoints were reported as number and proportion of children who reported the event, with 95% CIs.
Results
Study participants
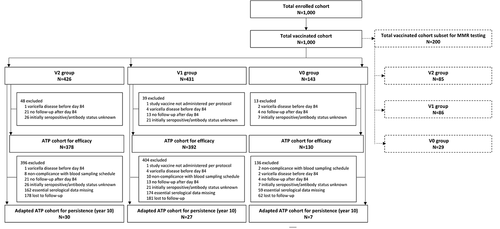
A total of 5,803 children were enrolled in the global study between September 2005 and May 2006; in Russia, enrollment ended in February 2006. Among all children enrolled, 1,000 were included in the Russian TVC, of whom 426 were assigned to the V2 group, 431 to the V1 group, and 143 to the V0 group; 100 children were excluded from the ATP cohort for efficacy (). Sixty-four children in the adapted ATP cohort for persistence completed the ten-year follow-up. The first 200 children enrolled in two pre-specified centers were included in the subset for MMR testing, of whom 85 were assigned to the V2 group, 86 to the V1 group, and 29 to the V0 group.
Figure 3. Participant flow chart.
Demographic characteristics were balanced across the three groups (): the mean age at enrollment was 12.7 months and 47.7% of children were female. Most children (99.4%) were of Caucasian heritage.
Table 1. Demographic characteristics of the study participants (total vaccinated cohort)
Efficacy
The estimated VE against confirmed varicella cases was 92.4% in the V2 group and 74.7% in the V1 group (). A total of 56 varicella cases were confirmed across all groups during the 10 years of follow-up; the percentage of cases was lowest in the V2 group (1.9% [n = 7]), followed by V1 group (6.1% [n = 24]) and V0 group (19.2% [n = 25]) (). Robustness of these data was confirmed by the sensitivity analysis, which included probable varicella cases in addition to confirmed cases.
Table 2. Vaccine efficacy estimates against confirmed varicella cases (according-to-protocol cohort for efficacy)
Immunogenicity
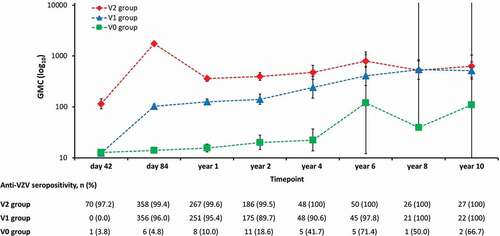
Forty-two days post-vaccination 2 (i.e., day 84 of the study), anti-VZV antibody seropositivity rates were 99.4% in the V2 group and 96.0% in the V1 group (). Anti-VZV antibody seropositivity rates remained high in both groups (≥99.4% in the V2 group and ≥89.7% in the V1 group) from day 42 post-vaccination 2 until year 10. The evolution of GMCs over time paralleled that of seropositivity rates for both groups ().
Figure 4. Anti-varicella zoster virus (VZV) antibody persistence during ten years of follow-up (adapted according-to-protocol cohort for persistence).
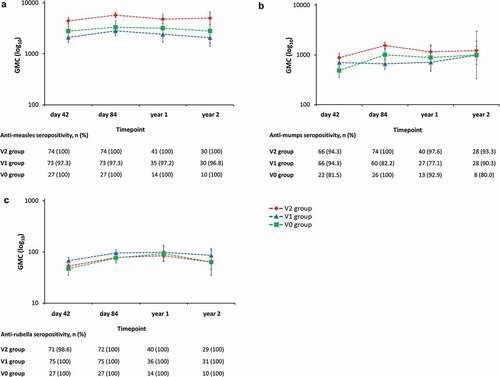
Anti-measles, -mumps, and -rubella antibody seropositivity rates were high in the V2 group (100%, ≥93.3%, and 100%, respectively) and in the V1 group (≥96.8%, ≥77.1%, and 100%, respectively) from day 42 post-vaccination 2 until year 2 (i.e., last sampling for this outcome) (). Throughout the two-year follow-up period, GMCs for anti-measles, -mumps, and -rubella antibodies remained in similar ranges in the V2 group and the V1 group ().
Figure 5. Anti-measles (A), -mumps (B), and -rubella (C) antibody persistence during two years of follow-up (adapted according-to-protocol cohort for persistence, subset for MMR testing).
Safety
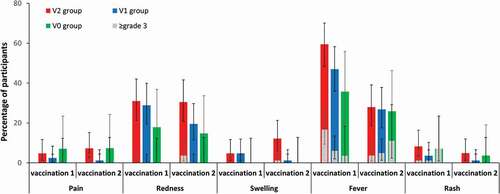
The most frequently reported solicited local AE was redness, reported by 31.0% of children in the V2 group, 28.9% in the V1 group, and 17.9% in the V0 group post-vaccination 1; and by 30.5%, 19.5%, and 14.8%, respectively, post-vaccination 2 (). Fever occurred most frequently after vaccination 1 (in 35.7%–59.5% of children across groups). Grade 3 fever was reported by 3.6%–16.7% of children across groups. The incidence of other grade 3 AEs was limited. Non-varicella-like rash was reported by 3.6%–8.3% of children post-vaccination 1 and by 1.2%–4.9% of participants post-vaccination 2 across groups. Post-vaccination 1, all cases of rash reported by children in the V0 group were categorized as grade ≥3 (). No cases of salivary gland swelling or meningism (including febrile seizures) were reported during the 43 days after each dose.
Figure 6. Incidence of solicited local adverse events (pain, redness, and swelling) from day 0 to day 3, and solicited general symptoms (fever and rash) from day 0 to day 42, after each dose (total vaccinated cohort, subset for MMR testing).
Unsolicited AEs were reported by 27.9%–35.3% of children post-vaccination 1 and by 17.9%–19.3% of children post-vaccination 2 across groups. The most frequently reported unsolicited AEs were: upper respiratory tract infection (n = 20), rhinitis (n = 12), and nasopharyngitis (n = 10) post-vaccination 1; and upper respiratory tract infection (n = 15), viral upper respiratory tract infection (n = 9), and rhinitis (n = 8) post-vaccination 2. Only one grade 3 unsolicited AE was reported, namely an upper respiratory tract infection in the V2 group post-vaccination 1.
A total of 184 SAEs were reported by 137 children throughout the study, among which two deaths occurred: one was caused by asphyxia during a fire and the other was an accidental death at home. None of these SAEs were considered causally related to vaccination. No HZ cases or complicated varicella cases were reported throughout the study.
Discussion
In this trial, we assessed the efficacy, immunogenicity, and safety of two vaccines containing the same live attenuated varicella Oka strain: the monovalent V vaccine, and the tetravalent MMRV vaccine. Vaccination of children according to a one-dose (V1 group) or two-dose (V2 group) VCV schedule allowed to estimate the efficacy of the two varicella vaccination schedules that can be considered for national immunization programs. Importantly, we used an accelerated vaccination schedule with a short interval (i.e., 42 days) between the two vaccinations. Such a shorter interval may improve adherence to complete and timely vaccination.Citation46 Furthermore, the risk of breakthrough varicella disease increases with time between two doses; hence, an accelerated schedule can help ensure that children are fully protected against varicella earlier in life.Citation47 However, a five-year interval between both doses may reduce the number of vaccination visits required, by allowing coadministration of the second dose with the tetanus and diphtheria vaccine at approximately 6 years of age.Citation33,Citation38
The long-term results obtained for children enrolled from Russia presented here may inform Russian authorities regarding the potential implementation of a UVV program at the country level.Citation31,Citation38–40 The results are generally in line with the previously published long-term global study results.Citation9 Our results suggest that the two-dose schedule provided optimal long-term efficacy, as shown by a lower number of breakthrough varicella cases and a higher VE estimate, compared to the one-dose schedule. A superior protection provided by two doses compared to one dose of any VCV was also demonstrated in a meta-analysis which included articles reporting effectiveness of both schedules. A two-dose schedule was shown to additionally reduce varicella disease by 79% (based on three randomized controlled trials), 63% (based on seven cohort studies), and 81% (based on five case-control studies) compared to a one-dose schedule.Citation21 Over the ten-year follow-up period, we observed more than three times more breakthrough varicella cases in children who received one dose VCV compared to children who received two doses of a VCV. Evidence from the global study demonstrated that most one-dose breakthrough cases (n = 469) were of mild or moderate nature except for one case, which was severe (<1%) (Russia-specific data is not available).Citation9 VE estimates against confirmed varicella cases were 74.7% with one dose and 92.4% with two doses of VCV. These estimates are in line with the 67.2% and 95.4% VE estimates, respectively, reported for the global study.Citation9 Additionally, these data are comparable to data from a systematic literature review and meta-analysis of dose-specific, post-licensure vaccine effectiveness estimates in healthy children (81% following one dose and 92% following two doses of any VCV).Citation28
In a previously published meta-analysis which included 14 studies reporting outbreaks after one dose of a VCV, an overall VE of 72.5% was calculated.Citation48 Furthermore, that meta-analysis demonstrated waning of immunity over time post-vaccination. However, in the Russian cohort of the current trial, anti-VZV immune responses persisted in the two-dose and one-dose groups, with all children across both groups being seropositive at year 10. Part of these observed immune responses could be due to the study taking place in a country where varicella is endemic. Immunogenicity was assessed by measuring anti-VZV antibody concentrations using a commercial ELISA kit with cutoff of 25 mIU/mL, in line with previously published studies.Citation49,Citation50 The relevance of this cutoff was recently demonstrated, with study participants having anti-VZV antibody concentrations ≥25 mIU/mL showing a higher level of protection than participants with concentrations <25 mIU/mL.Citation51
Immunogenicity against MMR also remained high in both groups during the period tested, with ≥90.3% children being seropositive for anti-measles, -mumps, and -rubella antibodies at year 2. The seropositivity rates were comparable to those observed in the control group (V0) of children, who were vaccinated with two doses of the MMR vaccine. This suggests that presence of the varicella antigen in the MMRV vaccine does not affect long-term immunogenicity to the vaccine’s MMR antigens. Moreover, the use of the MMRV vaccine decreases the number of vaccinations required to obtain the same level of protection against all four vaccine components. The results of the global study indicated that high seropositivity rates for anti-measles, -mumps, and -rubella antibodies were also observed throughout the study until year 10, regardless of the vaccine schedule administered.Citation37
The safety results reported here for each schedule were comparable to those reported in the global study,Citation36 and support the acceptable safety profiles of both VCVs.Citation5,Citation20,Citation52 Redness at the injection site and fever were the most frequently reported AEs, in line with previous observations for VCVs.Citation52 Post-vaccination 1, there was a trend for higher fever rates in children who received one or two doses of VCV compared to children who received no VCV. Such higher rates of fever post-vaccination 1 have been described in a previously published meta-analysis for tetravalent MMRV vaccine compared to trivalent MMR vaccine. This meta-analysis also reported that these higher rates did not trigger more frequent febrile seizures.Citation52 On the other hand, a large study including more than 400,000 children indicated a higher risk of febrile seizures following vaccination with a tetravalent MMRV vaccine compared to vaccination with the trivalent MMR vaccine together with the monovalent V vaccine.Citation53 Nevertheless, in the Russian cohort of our study, no cases of meningism (including febrile seizures) were reported in children receiving either study vaccine.
While eight SAEs were considered causally related to vaccination in the global study, including four febrile seizures (three in the V2 group and one in the V0 group),Citation36 no vaccination-related SAEs or deaths were reported in Russian children. Additionally, no complicated varicella cases were reported in Russian children, or in the global study.Citation9 Six HZ cases were reported in the global study,Citation9 but none were reported in Russian children.
The strengths of this study include the long-term follow-up in a large number of children and in settings where VZV is endemic. Moreover, the study design was robust and included several vaccination schedules, with different VCVs, and a thorough confirmation of suspected varicella cases involving clinical assessment, PCR testing, and IDMC ascertainment. Together, these factors contributed to the generalizability of the obtained results.
There are some limitations to this study. First, a limited number of children included in the TVC completed the full study and had efficacy and immunogenicity data available for the entire follow-up period. Nevertheless, the VE sensitivity analysis, which was conducted on the confirmed and probable varicella cases, suggested that the data obtained in the main analysis of this study were robust. Second, no data were available for immunogenicity against MMR in Russian children beyond year 2 because the study was discontinued in regions where varicella vaccination was introduced in the regional immunization program in 2006–2007.Citation54 However, the global results of this study have been publishedCitation37 and are likely generalizable to Russian children given the similar demographic characteristics (e.g., ≥98% of children of white Caucasian heritage).
In conclusion, vaccination against varicella resulted in clear reductions in the incidence of varicella disease in Russian children over 10 years of follow-up, with VE estimates in line with the global study. The highest VE estimate and lowest number of breakthrough cases were observed in children who received the two-dose schedule. Anti-VZV antibodies persisted until year 10 of the follow-up in children who received the one-dose or two-dose schedule, and the acceptable safety and tolerability profile of both VCVs was confirmed. Therefore, these data add valuable evidence for authorities who consider implementation of UVV in settings, such as the Russian population. The estimated VE against varicella over 10 years of follow-up supports the use of a two-dose varicella vaccination schedule over a one-dose schedule.
Abbreviations
Disclosure of potential conflicts of interest
G. Casabona, M. A. Habib, M. Povey, E. Shpeer, and M. Scherbakov are employees of the GSK group of companies. M. Scherbakov, M. A. Habib, E. Shpeer, and G. Casabona hold shares in the GSK group of companies as part of their employee remuneration. L. Namazova-Baranova, V. Romanenko, V. Tatochenko, O. Reshetko, Y. Kovshirina, K. Efendieva, M. Fedoseenko, J. Levina, I. Ryzhenkova, I. Sidorenko, Y. Yakovlev, A. Lyamin, O. Fedorova, L. Ogorodova and A. Zhestkov have nothing to disclose. T. Ivleva received personal fees from the GSK group of companies, Sanofi Pasteur, Pfizer, and Merck Sharp & Dohme for performing educational lectures. All authors have no non-financial interest to declare.
Trademark statement
Priorix, Priorix-Tetra, and Varilrix are owned by or licensed to the GSK group of companies. Varivax is a registered trademark of Merck Sharpe & Dohme Corp.
Data sharing statement
The protocol of this study is available at gsk-studyregister.com (ID 100388). GSK makes available anonymized individual participant data and associated documents from interventional clinical studies which evaluate medicines, upon approval of proposals submitted to www.clinicalstudydatarequest.com. To access data for other types of GSK sponsored research, for study documents without patient-level data and for clinical studies not listed, please submit an enquiry via the website.
Supplemental Material
Download MS Word (238.5 KB)Supplemental Material
Download MS Word (66.3 KB)Acknowledgments
The authors acknowledge Dr Nadezhda Snegova and Dr Vitaliy Fomin, who passed away, for their contribution during the conduct of the study. Authors thank Ekaterina Safonova for her involvement in Russia throughout the conduct of the global study. Authors also thank the Modis platform for editorial assistance and manuscript coordination, on behalf of GSK. Lotte Mathé and Urszula Miecielica provided medical writing support and Julie Mellery coordinated the manuscript development and editorial support.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2021.1959148.
Additional information
Funding
References
- World Health Organization. Varicella and herpes zoster vaccines: WHO position paper, June 2014. Week Epidem Rec. 2014;89:265–12.
- Varela FH, Pinto LA, Scotta MC. Global impact of varicella vaccination programs. Hum Vaccin Immunother. 2019;15(3):645–57. doi:10.1080/21645515.2018.1546525.
- Thomas SL, Minassian C, Ganesan V, Langan SM, Smeeth L. Chickenpox and risk of stroke: a self-controlled case series analysis. Clin Infect Dis. 2014;58(1):61–68. doi:10.1093/cid/cit659.
- Warren-Gash C, Forbes H, Breuer J. Varicella and herpes zoster vaccine development: lessons learned. Expert Rev Vaccines. 2017;16(12):1191–201. doi:10.1080/14760584.2017.1394843.
- Gershon AA, Breuer J, Cohen JI, Cohrs RJ, Gershon MD, Gilden D, Grose C, Hambleton S, Kennedy PGE, Oxman MN, et al. Varicella zoster virus infection. Nat Rev Dis Primers. 2015;1(1):15016. doi:10.1097/INF.0000000000002233.
- Federal Service for Supervision of Consumer Rights Protection and Human Well-Being. State report on the state of the sanitary-epidemiological well-being of the population in the Russian Federation in 2019.
- Henry O, Brzostek J, Czajka H, Leviniene G, Reshetko O, Gasparini R, Pazdiora P, Plesca D, Desole MG, Kevalas R, et al. One or two doses of live varicella virus-containing vaccines: efficacy, persistence of immune responses, and safety six years after administration in healthy children during their second year of life. Vaccine. 2018;36(3):381–87. doi:10.1016/j.vaccine.2017.11.081.
- Kuter B, Matthews H, Shinefield H, Black S, Dennehy P, Watson B, Reisinger K, Kim LL, Lupinacci L, Hartzel J, et al. Ten year follow-up of healthy children who received one or two injections of varicella vaccine. Pediatr Infect Dis J. 2004;23(2):132–37. doi:10.1097/01.inf.0000109287.97518.67.
- Povey M, Henry O, Riise Bergsaker MA, Chlibek R, Esposito S, Flodmark C-E, Gothefors L, Man S, Silfverdal S-A, Štefkovičová M, et al. Protection against varicella with two doses of combined measles-mumps-rubella-varicella vaccine or one dose of monovalent varicella vaccine: 10-year follow-up of a phase 3 multicentre, observer-blind, randomised, controlled trial. Lancet Infect Dis. 2019;19(3):287–97. doi:10.1016/s1473-3099(18)30716-3.
- Centers for Disease Control and Prevention. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 1996;45:1–36.
- Marin M, Güris D, Chaves SS, Schmid S, Seward JF. Prevention of varicella - Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2007;56:1–40.
- Seward JF, Watson BM, Peterson CL, Mascola L, Pelosi JW, Zhang JX, Maupin TJ, Goldman GS, Tabony LJ, Brodovicz KG, et al. Varicella disease after introduction of varicella vaccine in the United States, 1995-2000. JAMA. 2002;287:606–11. doi:10.1001/jama.287.5.606.
- Lopez AS, Zhang J, Brown C, Bialek S. Varicella-related hospitalizations in the United States, 2000-2006: the 1-dose varicella vaccination era. Pediatrics. 2011;127(2):238–45. doi:10.1542/peds.2010-0962.
- Davis MM. Successes and remaining challenges after 10 years of varicella vaccination in the USA. Expert Rev Vaccines. 2006;5(2):295–302. doi:10.1586/14760584.5.2.295.
- Quian J, Ruttimann R, Romero C, Dall’Orso P, Cerisola A, Breuer T, Greenberg M, Verstraeten T. Impact of universal varicella vaccination on 1-year-olds in Uruguay: 1997-2005. Arch Dis Child. 2008;93(10):845–50. doi:10.1136/adc.2007.126243.
- Spoulou V, Alain S, Gabutti G, Giaquinto C, Liese J, Martinon-Torres F, Vesikari T. Implementing universal varicella vaccination in Europe: the path forward. Pediatr Infect Dis J. 2019;38(2):181–88. doi:10.1097/INF.0000000000002233.
- Kuter BJ, Weibel RE, Guess HA, Matthews H, Morton DH, Neff BJ, Provost PJ, Watson BA, Starr SE, Plotkin SA. Oka/Merck varicella vaccine in healthy children: final report of a 2-year efficacy study and 7-year follow-up studies. Vaccine. 1991;9(9):643–47. doi:10.1016/0264-410x(91)90189-d.
- Varis T, Vesikari T. Efficacy of high-titer live attenuated varicella vaccine in healthy young children. J Infect Dis. 1996;174(Suppl 3):S330–334. doi:10.1093/infdis/174.supplement_3.s330.
- Marin M, Meer HC, Seward JF. Varicella prevention in the United States: a review of successes and challenges. Pediatrics. 2008;122(3):e744–751. doi:10.1542/peds.2008-0567.
- Wutzler P, Bonanni P, Burgess M, Gershon A, Safadi MA, Casabona G. Varicella vaccination - the global experience. Expert Rev Vaccines. 2017;16(8):833–43. doi:10.1080/14760584.2017.1343669.
- Yin M, Xu X, Liang Y, Ni J. Effectiveness, immunogenicity and safety of one vs. two-dose varicella vaccination: a meta-analysis. Expert Rev Vaccines. 2018;17(4):351–62. doi:10.1080/14760584.2018.1433999.
- Kurugol Z, Gokce S. Outbreak of varicella in preschool children despite one-dose vaccination. Turk J Pediatr. 2018;60:56–62. doi:10.24953/turkjped.2018.01.008.
- Spackova M, Wiese-Posselt M, Dehnert M, Matysiak-Klose D, Heininger U, Siedler A. Comparative varicella vaccine effectiveness during outbreaks in day-care centres. Vaccine. 2010;28(3):686–91. doi:10.1016/j.vaccine.2009.10.086.
- Cheng HY, Chang LY, Lu CY, Huang LM. Epidemiology of breakthrough varicella after the implementation of a universal varicella vaccination program in Taiwan, 2004-2014. Sci Rep. 2018;8(1):17192. doi:10.1038/s41598-018-35451-y.
- Tafuri S, Martinelli D, De Palma M, Germinario C, Prato R. Report of varicella outbreak in a low vaccination coverage group of otherwise healthy children in Italy: the role of breakthrough and the need of a second dose of vaccine. Vaccine. 2010;28(6):1594–97. doi:10.1016/j.vaccine.2009.11.047.
- Kurugol Z, Halicioglu O, Koc F, Koturoglu G, Aksit S. Varicella rates among unvaccinated and one-dose vaccinated healthy children in Izmir, Turkey. Int J Infect Dis. 2011;15(7):e475–480. doi:10.1016/j.ijid.2011.03.016.
- Macartney K. Prevention of varicella: time for two-dose vaccination. Lancet. 2014;383(9925):1276–77. doi:10.1016/s0140-6736(14)60078-7.
- Marin M, Marti M, Kambhampati A, Jeram SM, Seward JF. Global varicella vaccine effectiveness: a meta-analysis. Pediatrics. 2016;137(3):e20153741. doi:10.1542/peds.2015-3741.
- Ministry of Health of the Russian Federation. [Varilrix - Registration certificate] (Russian); 2019 [accessed 2020 June 11]. http://grls.rosminzdrav.ru/Grls_View_v2.aspx?routingGuid=00437fd1-ba5c-4f78-8b06-e8e57dc373f6&t
- State Register of Medicines. [Varivax Registration Certificate] (Russian); 2020 [accessed 2020 September 16]. http://grls.rosminzdrav.ru/Grls_View_v2.aspx?routingGuid=a4bc173d-4610-48cb-9077-126a338414ca&t=
- Ministry of Justice of the Russian Federation. [SP 3.1.3525-18Sanitary rules to prevent varicella & herpes zoster in Russian Federation] (Russian); 2018 [accessed 2020 June 11]. http://docs.cntd.ru/document/557220394
- Vishneva EA, Kostinov MP, Mazankova LN, Malinnikova EY, Namazova-Baranova LS, Plakida AV, Privalova TE, Rtishchev AY, Tatochenko VK, Fedoseenko MV, et al. [Resolution of the expert forum of Russian Federation “Chickenpox: serious infection in Russian Federation, which can be prevented due to vaccination”] (Russian). Curr Pediatrics. 2019;18:491–94.
- World Health Organization. WHO vaccine-preventable diseases: monitoring system. 2019 global summary [accessed 2020 March 17]. http://apps.who.int/immunization_monitoring/globalsummary/countries?countrycriteria%5Bcountry%5D%5B%5D=RUS
- Baryshev MA, Chernyavskaya OP, Saltykova TS. Experience of the Varicella vaccine introduction into regional vaccination Schedule of the Russian Federation. Epidemiol Vacc Prev. 2020;18(6):67–74. doi:10.31631/2073-3046-2019-18-6-67-74.
- Afonina NM, Mikheeva IV. [Efficacy of vaccine prophylaxis of varicella using different tactics for its carrying out] (Russian). Epidemio Infect Dis. 2019;1:29–36.
- Prymula R, Bergsaker MR, Esposito S, Gothefors L, Man S, Snegova N, Štefkovičova M, Usonis V, Wysocki J, Douha M, et al. Protection against varicella with two doses of combined measles-mumps-rubella-varicella vaccine versus one dose of monovalent varicella vaccine: a multicentre, observer-blind, randomised, controlled trial. Lancet. 2014;383(9925):1313–24. doi:10.1016/s0140-6736(12)61461-5.
- Carryn S, Feyssaguet M, Povey M, Di Paolo E. Long-term immunogenicity of measles, mumps and rubella-containing vaccines in healthy young children: a 10-year follow-up. Vaccine. 2019;37(36):5323–31. doi:10.1016/j.vaccine.2019.07.049.
- Ministry of Health of the Russian Federation. [Appendix N 1. National calendar of preventive vaccinations] (Russian); 2014 [accessed 2020 August 17]. https://base.garant.ru/70647158/53f89421bbdaf741eb2d1ecc4ddb4c33/
- [Decree of the President of the Russian Federation of 06.06.2019 N 254 ”On the strategy for the development of healthcare in the Russian Federation for the period until 2025”] (Russian); 2019 [accessed 2021 January 19]. http://www.consultant.ru/document/cons_doc_LAW_326419/
- Forum of Experts of the Russian Federation. [Varicella is a serious infectious threat for Russia which is vaccine-preventable] (Russian). Iss Modern Pediat. 2019;18:491–94.
- GSK. Priorix-Tetra. Summary of product characteristics; 2017 [accessed 2020 March 19]. https://gskpro.com/content/dam/global/hcpportal/en_MT/PDF/Homepage/Products/productlisting/priorix-tetra/Priorix_Tetra_PI_II_078_18_Apr_2017.pdf
- GSK. Varilrix. Summary of product characteristics; 2020 [accessed 2020 March 19]. https://www.medicines.org.uk/emc/medicine/9787
- Vazquez M, LaRussa PS, Gershon AA, Steinberg SP, Freudigman K, Shapiro ED. The effectiveness of the varicella vaccine in clinical practice. N Engl J Med. 2001;344(13):955–60. doi:10.1056/NEJM200103293441302.
- Cox DR. Regression models and life-tables. J Royal Statist Soc Ser B. 1972;34:187–220.
- Gillard P, Povey M, Carryn S. Clinically- versus serologically-identified varicella: a hidden infection burden. A ten-year follow-up from a randomized study in varicella-endemic countries. Hum Vaccin Immunother. 2021:1–10. doi:10.1080/21645515.2021.1932217.
- Bonanni P, Gershon A, Gershon M, Kulcsar A, Papaevangelou V, Rentier B, Sadzot-Delvaux C, Usonis V, Vesikari T, Weil-Olivier C, et al. Primary versus secondary failure after varicella vaccination: implications for interval between 2 doses. Pediatr Infect Dis J. 2013;32(7):e305–313. doi:10.1097/INF.0b013e31828b7def.
- Bonanni P, Breuer J, Gershon A, Gershon M, Hryniewicz W, Papaevangelou V, Rentier B, Rumke H, Sadzot-Delvaux C, Senterre J, et al. Varicella vaccination in Europe – taking the practical approach. BMC Med. 2009;7(1):26. doi:10.1186/1741-7015-7-26.
- Bayer O, Heininger U, Heiligensetzer C, von Kries R. Metaanalysis of vaccine effectiveness in varicella outbreaks. Vaccine. 2007;25(37–38):6655–60. doi:10.1016/j.vaccine.2007.07.010.
- Klein NP, Abu-Elyazeed R, Povey M, Macias Parra M, Diez-Domingo J, Ahonen A, Korhonen T, Tinoco J-C, Weiner L, Marshall GS, et al. Immunogenicity and safety of a measles-mumps-rubella vaccine administered as a first dose to children aged 12 to 15 months: a phase III, randomized, noninferiority, lot-to-lot consistency study. J Pediatric Infect Dis Soc. 2020;9(2):194–201. doi:10.1093/jpids/piz010.
- Prymula R, Simko R, Povey M, Kulcsar A. Varicella vaccine without human serum albumin versus licensed varicella vaccine in children during the second year of life: a randomized, double-blind, non-inferiority trial. BMC Pediatr. 2016;16(1):7. doi:10.1186/s12887-016-0546-5.
- Habib MA, Prymula R, Carryn S, Esposito S, Henry O, Ravault S, Usonis V, Wysocki J, Gillard P, Povey M. Correlation of protection against varicella in a randomized Phase III varicella-containing vaccine efficacy trial in healthy infants. Vaccine. 2021;39(25):3445–54. doi:10.1016/j.vaccine.2021.02.074.
- Ma S-J, Li X, Xiong Y-Q, Yao A-L, Chen Q. Combination measles-mumps-rubella-varicella vaccine in healthy children: a systematic review and meta-analysis of immunogenicity and safety. Medicine. 2015;94(44):e1721. doi:10.1097/MD.0000000000001721.
- Klein NP, Fireman B, Yih WK, Lewis E, Kulldorff M, Ray P, Baxter R, Hambidge S, Nordin J, Naleway A, et al. Measles-mumps-rubella-varicella combination vaccine and the risk of febrile seizures. Pediatrics. 2010;126(1):e1–8. doi:10.1542/peds.2010-0665.
- Shamsheva OV. [Regional immunisation schedules as a step forward in prophylactic work in Russia. Experience of foreign countries.] (Russian). Pediatric Infect J. 2010;4:4–9.