ABSTRACT
The vagina is an excellent site for topical passive immunization, as access is relatively easy, and it is an enclosed space that has been shown to retain bioactive antibodies for several hours. A number of sexually transmitted infections could potentially be prevented by delivery of specific monoclonal antibodies to the vagina. Furthermore, our group is developing antisperm antibodies for vaginally delivered on-demand topical contraception. In this article, we describe physical features of the vagina that could play a role in antibody deployment, and antibody modifications that could affect mAb retention and function in the female reproductive tract. We also review results of recent Phase 1 clinical trials of vaginal passive immunization with antibodies against sexually transmitted pathogens, and describe our current studies on the use of anti-sperm mAbs for contraception.
Passive immunization, the transfer of antibodies to an unprotected individual for the prevention or treatment of diseases, has been used in humans for over a century, but has only recently become accepted as a highly reliable clinical procedure. This medical breakthrough is attributable to advances in monoclonal antibody (mAb) technology which can now produce reagent grade mAb reagents. Just in the past 10 years, over 100 mAbs have been approved for clinical use. The majority of clinical applications entail systemic administration of antibodies, but topical antibody applications are increasingly being explored, especially for mucosal surfaces that may not be adequately accessed by systemically administered antibodies or antibodies elicited by active systemic immunization. Topical passive immunization has the advantage of delivering mAbs in high concentrations to desired target surfaces. A number of groups are investigating passive immunization of the human vagina to prevent sexually transmitted infections (STIs), particularly the transmission of pathogenic viruses such as HIV-1 and HSV-2. In addition, vaginal application of antisperm antibodies, under development in our laboratory, could provide a mechanism for on-demand contraception. In this article we describe physical features of the vagina that could affect the efficacy of passive immunization, and antibody modifications that could affect mAb retention and function in the female reproductive tract. We also review results of recent Phase 1 clinical trials of vaginal passive immunization with antibodies against sexually transmitted pathogens, and describe our current studies on the use of anti-sperm mAbs for contraception.
Physical characteristics of the human vagina that may influence passive immunization
The human vagina is a tube-shaped structure extending from the introitus (vaginal opening) to the cervical os; it is usually a potential space with anterior and posterior walls in apposition. There are few published reports on the dimensions of the human vagina. In one study that used MRI to image the contours of vaginas of 28 reproductive aged women, the average length was determined to be 6.2 cm (range: 4.0–9.5 cm), and average width 3.25 cm (range: 1.5–3.6 at midvagina, and 2.6–8.3 at the fornix).Citation1 This group also demonstrated that the radiopaque gel used for imaging ascended from the vagina into the endocervical canal. In another study of 62 women that were administered vinyl polysiloxane casts, the surface area of the vagina was determined to range from 65 to 107 cm2.Citation2 Factors affecting vaginal shape and size included age, height, weight, race, and parity.Citation1,Citation3 In addition, the vaginal wall contains many rugae (folds) which allow it to distend during sexual intercourse and childbirth. As a consequence, the surface area and volume of the human vagina can be highly variable. Furthermore, the volume of secretions in the vagina varies between individuals and is affected by age, menstrual cycle stage, intercourse, and other factors. The volume of vaginal secretions ranged from 300 µl to 700 µl in reproductive-aged women from Africa and the US.Citation4,Citation5 Sexual excitation can increase blood flow to the vagina, resulting in serum exudation, and stimulate the release of secretions from the Skene’s and Bartholin’s glands, located near the introitus;Citation6 semen can add up to 10 ml of volume to vaginal secretions after intercourse.Citation7 All of these factors could affect the distribution and final concentration of passively administered antibodies in the vagina.
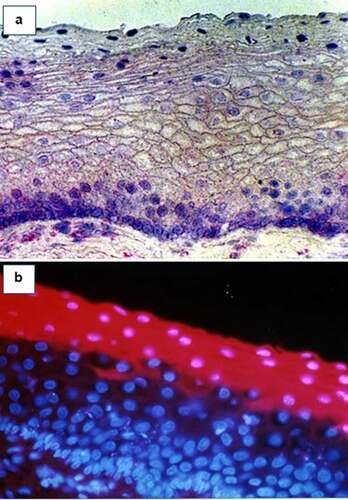
The vaginal wall is comprised of a stratified squamous epithelium, approximately 30 cell layers thick, that transitions to a simple columnar epithelium (single-cell layer) at the endocervix. Basal epithelial cells in the vaginal mucosa express the immunoglobulin (Ig) transport molecule FcRn which transports IgG from the basal compartment into the lumen, but probably not in the other directionCitation8 (). The epithelial cells in the topmost layer of the vaginal mucosa, the stratum corneum, absorb and retain Igs until they are exfoliated, at which time the Igs are releasedCitation9 ().
Figure 1. (a) FcRn (IgG transport molecule) expression by basal epithelial cells in the human vaginal epithelium, as visualized by immunohistology. FcRn-positive cells appear purple. (b) IgG uptake by apical epithelial cells in the stratum corneum of the human vaginal epithelium. Cy3-labeled (red) IgG, which had been added to the apical surface of vaginal tissue, was visualized by fluorescence microscopy in the apical cells.
Mucin glycoproteins constitute a large proportion of the apical glycocalyx that covers the vaginal surface. The vaginal mucosa expresses membrane-bound mucins (e.g., MUC 1, MUC 4), but does not contain glands that secrete high MW gel-forming mucins. On the other hand, the endocervix contains numerous glands that secrete high MW mucins (e.g., MUC2, MUC5AC, MUC5B, and MUC6) that flow into the vaginal cavity.Citation10 At least one of these, MUC5B, is under hormonal control and is secreted in abundance at midcycle.Citation11 Vaginal and endocervical mucins may play an important role in vaginal passive immunization. Various studies have shown that mucins can interact with Igs to impede the penetration of antibody-coated pathogens and sperm.Citation12–14
The vaginal epithelium contains innate immune cells that express Fc receptors (FcR); these receptors bind to the Fc region of immunoglobulins and confer a variety of antibody-dependent protective functions on the immune cells.Citation15 In particular, macrophages and neutrophils in the FRT express FcRγ and FcRα, respectively, conferring the ability to phagocytose pathogens coated with IgG and IgA.Citation16 FcR functions of innate immune cells in the FRT have been implicated in the protective effects of antibodies following the administration of HIV vaccines,Citation17 and in animal studies of passive immunization to prevent HIV-1 transmission (described below).
The vagina is profoundly affected by its rich and varied microbiome. A majority of women in the US have a predominance of “healthy” lactobacillus species which create an acidic environment and support a healthy vaginal mucus. Antibodies function well and are relatively stable under these conditions.Citation18 However, many women worldwide have a more complex vaginal microflora, often referred to as dysbiosis or bacterial vaginosis (BV).Citation19,Citation20 This condition is associated with an elevation in vaginal pH, and degradation of vaginal mucus which adversely affects antibody trapping defense mechanisms.Citation21 Other sexually transmitted pathogens such as trichomonas vaginalis also produce glycosidases and other enzymes that degrade vaginal mucins.Citation22 The enzymes associated with abnormal vaginal flora (e.g. glycosidases, sialydases, sulphatases and proteases) could also directly affect the activity and stability of antibodies in the vagina.
Engineering antibodies to improve efficacy in the vagina
The overwhelming majority of FDA-approved mAbs for clinical use are dimeric IgG1 antibodies. This is the most common antibody type found in blood and vaginal secretions, and has a number of immunological functions including viral neutralization, mucus trapping, complement fixation, and other FcR-mediated effects. However, there is increasing interest in using molecularly engineered antibodies for passive immunization to enhance retention and function. Common modifications entail engineering the Fab (antigen binding) region, engineering the Fc region (FcR-dependent mechanisms), and creating multivalent antibodies and antibody fragments.
Fab alterations are a common way to increase affinity for a target to improve binding and pathogen neutralization. An example of this is VRC07-523, a clonal relative of the anti-HIV mAb VRC01, engineered to have increased affinity; this antibody protected non-human primates from SHIV challenge at a 5-fold lower concentration compared to VRC01.Citation23 Increasing the valence of an antibody is another method to increase avidity and breadth. Techniques are currently being developed for the manufacture of s-IgA and IgM multivalent mAbs. In addition, bispecific antibodies potently and broadly neutralize HIV due to their ability to bind multiple epitopes.Citation24 The trispecific antibody VRC01/PGDM1400-10E8v4 also has broad specificity and neutralization potential.Citation25
Another approach to antibody engineering entails alteration of the Fc region. The LALA-PG variant is comprised of a series of point mutations (L234A,/L235A/P329G) that inhibit mAb binding to FcγRs and complement to limit effector functions and possible inflammation.Citation26 An anti-HIV antibody engineered with these mutations, b12-LALA-PG, was shown to be less effective than wild type b12 in protecting rhesus macaques from low-dose repeated vaginal SHIV challenge.Citation27 On the other hand, the GASDALIE variant (G236A/S239D/A330L/I332E) confers enhanced Fc function, and anti-HIV antibodies engineered with these mutations were more protective in SHIV mucosal challenge studies.Citation28,Citation29 In humanized mice, anti-HIV antibodies with the GASDALIE mutations also demonstrated enhanced protection against viral challenge.Citation30 These studies provide evidence that FcR-mediated immune functions play an important protective role in the vagina.
LS point mutations in the Fc region (M428L/N434S) increase antibody affinity for the FcRn receptor and increases serum half-life of systemically administered mAbs. In a recent clinical trial, VRC0-LS had an average half-life of 71 days in serum, about four times longer than wild-type VRC01 (15 days).Citation31 It is unknown whether the LS mutation would enhance mAb half-life in the vagina, as FcRn is only expressed on the basolateral side of the epithelium and appears to only transport IgG into the lumen.Citation32
Alterations to glycosylation in the Fc region can also improve certain antibody functions. IgG molecules without the core fucose residue have an increased affinity for FcγRIIIa and enhanced antibody-dependent cellular cytotoxicity (ADCC);Citation33 nonfucosylated HIV antibodies have also demonstrated these effects.Citation29 Furthermore, a recent study demonstrated that nonfucosylated antibodies interact better with MUC16, a mucus glycoprotein found in the FRT, and therefore could enhance mucus trapping protective functions.Citation12
Antibody fragments that still bind to antigens but do not have Fc regions may have enhanced pharmacokinetics and penetration into tissues due to their smaller size47. Common antibody fragments are Fab and F(ab’)2 regions, single-chain variable fragments (scFv), and single-domain antibodies. It is currently unknown whether the use of these antibody fragments would confer an advantage in the FRT.
Clinical trials demonstrating passive vaginal immunization for protection against STIs in women
A number of vaginal passive immunization studies have been conducted in nonhuman primates and mice using mAbs against HIV-1 and HSV-2. They have for the most part demonstrated efficacy of the passive immunization approach in preventing HIV/SHIV and HSV-2 infections (reviewed in Anderson et al.Citation34). However to date only 3 Phase 1 clinical trials have been reported that describe the safety of mAbs delivered to the human vagina. They also provide data on pharmacokinetics and efficacy of the mAbs. We will review these reports in depth as they provide important information about the feasibility of vaginal passive immunization in women.
MABGEL is a vaginal microbicide developed by the European Microbicides Programme, containing three broadly neutralizing HIV antibodies: 4E10, 2F5, and 2G12.Citation35 The antibodies were formulated into 20 mg/mL high dose and 10 mg/mL low-dose gels which were applied vaginally for 12 consecutive days. No serious adverse effects were reported by the women in the study and effective concentrations of the antibodies were detected in cervicovaginal secretions up to 8 hours after application. Antibody activity was not assessed. There also appeared to be no systemic uptake of the antibodies. A trial by another European consortium conducted a first-in-human clinical trial of vaginal application of an anti-HIV antibody, 2G12, manufactured in Nicotiana tobacum.Citation36 In this trial, 11 participants were randomized into mAb vs. placebo groups and received either a single dose of 28 mg of 2G12 mAb in 1 ml of saline, or saline alone. None of the women reported serious adverse events, and no systemic absorption was observed. Pharmacokinetic data were not reported for this study.
Our group recently reported the results of a Phase 1 clinical trial that tested the safety, acceptability, pharmacokinetics, and ex vivo efficacy of MB66, a vaginal film containing 10 mg each of VRCO1 (anti-HIV mAb) and HSV8 (anti-HSV-1 and −2 mAb). The mAbs were produced by transfection into Nicotiana benthamina (a species of tobacco plant). Women received one dose of the film on one day only (n = 9), or daily for 7 days (n = 14 placebo film, n = 15 active film). The product was generally safe and well-tolerated with no serious adverse events recorded. Acceptability and willingness to use the product were high in post-use interviews. Antibody levels peaked 1 hour post dosing with active film (median concentration 1,008 µg/ml in the midvagina), and remained significantly elevated at 24 hours after film use (median concentration 88ug/ml in the midvagina). Importantly, vaginal samples collected 24 hrs after MB66 insertion neutralized both HIV an HSV2 ex vivo providing evidence that antibodies remain stable and active in the vaginal environment for at least 24 hours. This study provides further data to confirm the safety and acceptability of vaginal passive immunization in women, and is the first study to demonstrate detailed pharmacokinetics and antibody activity of a mAb-based multipurpose prevention technology (MPT) product.Citation37
Topical administration of antisperm mAbs for contraception
Our research team has produced an antisperm mAb in Nicotiana that shows excellent potential for topical contraception. The Human Contraception Antibody (HCA) was derived from an IgM antisperm mAb made by fusing lymphocytes from an infertile woman with mouse hybidoma cells.Citation38 We combined the variable sequence of this antibody with invariant IgG1 sequences to produce an IgG1 mAb for potential clinical use. Preclinical testing indicates that this mAb has potent sperm agglutination activity, and immobilizes sperm in the presence of complement.Citation39 Studies are underway to determine whether HCA traps sperm in cervical mucus and affects other sperm functions such as the acrosome reaction and oocyte fertilization. IND enabling studies including tissue cross reactivity, rabbit vaginal irritation and rat toxicology tests were successfully completed, and an IND for HCA film (ZB-06) was recently approved. A Phase I Clinical Trial testing ZB-06 for safety and efficacy in postcoital tests is underway.
General comments
Pharmacokinetic data from the MB66 clinical trial described above and presented in detail in provide important information on mAb concentrations in vaginal secretions achieved through passive topical immunization. Antibody concentrations at the one-hour time point following administration of 10 mg of VRC01 IgG1 were comparable to levels of natural IgG found in vaginal secretions of normally cycling women, which range from 70 to 200 ug/ml,Citation40 and levels of specific IgG antibodies found in female genital secretions following systemic immunization with tetanus toxoid (up to 59 ug/ml)Citation41 or HIV vaccines (range: 10 to 1,000ug/ml; median 100ug/ml).Citation42 A number of studies have compared HPV antibody levels in serum to those in cervicovaginal secretions following parenteral immunization with HPV vaccines.Citation43 Overall, levels of HPV-specific IgG and IgA antibodies in cervicovaginal secretions were much lower than those in serum (1–2%), but were correlated indicating that genital antibodies are transudated from the serum. Antibodies were detectable in genital secretions for at least 2 years after HPV immunization.Citation44 Clearly, in certain cases such as the HPV vaccine, a durable and effective antibody response can be elicited in the genital tract with parenteral vaccination that is superior to topical passive immunization. However, apart from HPV and hepatitis B vaccines, efforts to produce vaccines against other leading STI pathogens such as Chlamydia trachomatis, Neisseria gonnorhea, HIV-1, and Herpes simplex virus, have been unsuccessful. Until such vaccines are available, passive immunization with mAbs at the time of intercourse is a promising option to prevent STI transmission.
Table 1. MAb pharmacokinetic data from the MB66 trial.
The data from the MB66 trial indicate that on average approximately one-tenth of the applied antibody remained in vaginal secretions after 1 hr, and 1/100 of the antibody remained after 24 hours. This information, while preliminary, begins to provide guidance for determining doses of antibodies required for passive vaginal immunization in future clinical trials. For example, since median antibody concentrations in midvaginal secretions were approximately 1/10 of the starting dose one hour after film insertion, one could multiply the target antibody concentration 10-fold to approximate the starting dose needed to achieve an effective concentration after one hour. However, there are two important caveats to applying this approach. First, antibody concentrations differed according to sample site, with highest concentrations detected in midvaginal secretions, and lowest concentrations detected at the cervical os and distal vagina (vaginal opening). Therefore, the proposed site of action of the mAbs must be taken into account when calculating the effective dose. Second, there was considerable interindividual variation in the amount of antibody present in vaginal secretions after film use. For example, midvaginal secretions contained the highest antibody concentrations of all the sites tested after 1 hour (median: 1,000ug/ml), but VRC01 antibody concentrations ranged from 15 to 3,174 ug/ml at this site amongst the 15 women in the active film group at this time point. Variables such as film placement location, vaginal wetness and other factors could affect mAb concentrations in vaginal fluid after film insertion and should be further explored.
Future directions
Multivalent variants of HCA are being developed that could reduce the amount of mAb needed for sperm immobilization.Citation45 More potent antibodies may make it feasible to deliver mAbs to the vagina via vaginal rings or other devices for long-term protection.
It may be possible to deliver mAbs to the penis to deliver contraception and/or protection against STIs in men. The mAbs could potentially be applied to the penis as a lubricant or fast drying film. This is technically feasible because the lubricant/film can contain up to 20 mg/ml of mAb, and mAbs effectively inactivate viruses and sperm at concentrations below 10 ug/ml. The penile product could be billed as a male contraceptive or microbicide, and would protect both partners.
We hope to eventually combine antisperm mAb(s) with HIV and HSV-2 mAbs in a single product to deliver mAb-based multipurpose technology for protection of women, and potentially men, against both STIs and unplanned pregnancies.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The research described in this report was funded by NIH grants U19 AI096398 and P50 HD096957. The author acknowledges contributions from the entire research team, Drs. Richard Cone, Kevin Whaley, Larry Zeitlin, Thomas Moench, Francois Villinger, Jeffrey Pudney and Joseph Politch, who have been a steady source of inspiration, and without whom this research would not have been possible.
Additional information
Funding
References
- Barnhart KT, Izquierdo A, Pretorius ES, Shera DM, Shabbout M, Shaunik A. Baseline dimensions of the human vagina. Hum Reprod. 2006 Jun;21(6):1–6. doi:https://doi.org/10.1093/humrep/del022.
- Pendergrass PB, Reeves CA, Belovicz MW. A technique for vaginal casting utilizing vinyl polysiloxane dental impression material. Gynecol Obstet Invest. 1991;32(2):121–22. doi:https://doi.org/10.1159/000293010.
- Pendergrass PB, Reeves CA, Belovicz MW, Molter DJ, White JH. Comparison of vaginal shapes in Afro-American, caucasian and hispanic women as seen with vinyl polysiloxane casting. Gynecol Obstet Invest. 2000;50(1):54–59. doi:https://doi.org/10.1159/000010281.
- Belec L, Meillet D, Levy M, Georges A, Tevi-Benissan C, Pillot J. Dilution assessment of cervicovaginal secretions obtained by vaginal washing for immunological assays. Clin Diagn Lab Immunol. 1995 Jan;2(1):57–61. doi:https://doi.org/10.1128/cdli.2.1.57-61.1995.
- Mitchell C, Paul K, Agnew K, Gaussman R, Coombs RW, Hitti J. Estimating volume of cervicovaginal secretions in cervicovaginal lavage fluid collected for measurement of genital HIV-1 RNA levels in women. J Clin Microbiol. 2011 Feb;49(2):735–36. doi:https://doi.org/10.1128/JCM.00991-10.
- Pastor Z, Chmel R. Differential diagnostics of female “sexual” fluids: a narrative review. Int Urogynecol J. 2018 May;29(5):621–29. doi:https://doi.org/10.1007/s00192-017-3527-9.
- WHO laboratory manual for the examination and processing of human semen. Geneva (Switzerland)o: World Health Organization; 2010.
- Li Z, Palaniyandi S, Zeng R, Tuo W, Roopenian DC, Zhu X. Transfer of IgG in the female genital tract by MHC class I-related neonatal Fc receptor (FcRn) confers protective immunity to vaginal infection. Proceedings of the National Academy of Sciences of the United States of America. 2011 Mar 15;108(11):4388–93. doi:https://doi.org/10.1073/pnas.1012861108.
- Anderson DJ, Marathe J, Pudney J. The structure of the human vaginal stratum corneum and its role in immune defense. Am J Reprod Immunol. 2014 Jun;71(6):618–23. doi:https://doi.org/10.1111/aji.12230.
- Gipson IK, Ho SB, Spurr-Michaud SJ, Tisdale AS, Zhan Q, Torlakovic E, Pudney J, Anderson DJ, Toribara NW, Hill JA, et al. Mucin genes expressed by human female reproductive tract epithelia. Biol Reprod. 1997 Apr;56(4):999–1011. doi:https://doi.org/10.1095/biolreprod56.4.999.
- Gipson IK, Moccia R, Spurr-Michaud S, et al. The Amount of MUC5B mucin in cervical mucus peaks at midcycle. J Clin Endocrinol Metab. 2001 Feb;86(2):594–600.
- Gunn BM, Schneider JR, Shansab M, Bastian AR, Fahrbach KM, Smith AD, Mahan AE, Karim MM, Licht AF, Zvonar I, et al. Enhanced binding of antibodies generated during chronic HIV infection to mucus component MUC16. Mucosal Immunol. 2016 Mar 9;9(6):1549–58. doi:https://doi.org/10.1038/mi.2016.8.
- Kremer J, Jager S. The significance of antisperm antibodies for sperm-cervical mucus interaction. Hum Reprod. 1992 Jul;7(6):781–84. doi:https://doi.org/10.1093/oxfordjournals.humrep.a137737.
- Schroeder HA, Nunn KL, Schaefer A, Henry CE, Lam F, Pauly MH, Whaley KJ, Zeitlin L, Humphrys MS, Ravel J, et al. Herpes simplex virus-binding IgG traps HSV in human cervicovaginal mucus across the menstrual cycle and diverse vaginal microbial composition. Mucosal Immunol. 2018 Sep;11(5):1477–86. doi:https://doi.org/10.1038/s41385-018-0054-z.
- Bournazos S, Gupta A, Ravetch JV. The role of IgG Fc receptors in antibody-dependent enhancement. Nat Rev Immunol. 2020 Oct;20(10):633–43. doi:https://doi.org/10.1038/s41577-020-00410-0.
- Sips M, Krykbaeva M, Diefenbach TJ, Ghebremichael M, Bowman BA, Dugast A-S, Boesch AW, Streeck H, Kwon DS, Ackerman ME, et al. Fc receptor-mediated phagocytosis in tissues as a potent mechanism for preventive and therapeutic HIV vaccine strategies. Mucosal Immunol. 2016 Nov;9(6):1584–95. doi:https://doi.org/10.1038/mi.2016.12.
- Cocklin SL, Schmitz JE. The role of Fc receptors in HIV infection and vaccine efficacy. Curr Opin HIV AIDS. 2014 May;9(3):257–62. doi:https://doi.org/10.1097/COH.0000000000000051.
- Castle PE, Karp DA, Zeitlin L, Garcı́a-Moreno E B, Moench TR, Whaley KJ, Cone RA. Human monoclonal antibody stability and activity at vaginal pH. J Reprod Immunol. 2002 Jul-Aug;56(1–2):61–76. doi:https://doi.org/10.1016/S0165-0378(02)00013-X.
- Bayigga L, Kateete DP, Anderson DJ, Sekikubo M, Nakanjako D. Diversity of vaginal microbiota in sub-Saharan Africa and its effects on HIV transmission and prevention. Am J Obstet Gynecol. 2019 Feb;220(2):155–66. doi:https://doi.org/10.1016/j.ajog.2018.10.014.
- Koumans EH, Sternberg M, Bruce C, McQuillan G, Kendrick J, Sutton M, Markowitz LE. The prevalence of bacterial vaginosis in the United States, 2001-2004; associations with symptoms, sexual behaviors, and reproductive health. Sex Transm Dis. 2007 Nov;34(11):864–69. doi:https://doi.org/10.1097/OLQ.0b013e318074e565.
- Hoang T, Toler E, DeLong K, Mafunda NA, Bloom SM, Zierden HC, Moench TR, Coleman JS, Hanes J, Kwon DS, et al. The cervicovaginal mucus barrier to HIV-1 is diminished in bacterial vaginosis. PLoS Pathog. 2020 Jan;16(1):e1008236. doi:https://doi.org/10.1371/journal.ppat.1008236.
- Wiggins R, Hicks SJ, Soothill PW, Millar MR, Corfield AP. Mucinases and sialidases: their role in the pathogenesis of sexually transmitted infections in the female genital tract. Sex Transm Infect. 2001 Dec;77(6):402–08. doi:https://doi.org/10.1136/sti.77.6.402.
- Rudicell RS, Kwon YD, Ko S-Y, Pegu A, Louder MK, Georgiev IS, Wu X, Zhu J, Boyington JC, Chen X, et al. Enhanced potency of a broadly neutralizing HIV-1 antibody in vitro improves protection against lentiviral infection in vivo. Journal of Virology. 2014 Nov;88(21):12669–82. doi:https://doi.org/10.1128/JVI.02213-14.
- Huang Y, Yu J, Lanzi A, Yao X, Andrews CD, Tsai L, Gajjar MR, Sun M, Seaman MS, Padte NN, et al. Engineered bispecific antibodies with exquisite HIV-1-neutralizing activity. Cell. 2016 June 16;165(7):1621–31. doi:https://doi.org/10.1016/j.cell.2016.05.024.
- Xu L, Pegu A, Rao E, Doria-Rose N, Beninga J, McKee K, Lord DM, Wei RR, Deng G, Louder M, et al. Trispecific broadly neutralizing HIV antibodies mediate potent SHIV protection in macaques. Science (New York, N.Y.) 2017 Oct 6;358(6359):85–90. doi:https://doi.org/10.1126/science.aan8630.
- Hessell AJ, Hangartner L, Hunter M, Havenith CEG, Beurskens FJ, Bakker JM, Lanigan CMS, Landucci G, Forthal DN, Parren PWHI, et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007 September 6;449(7158):101–04. doi:https://doi.org/10.1038/nature06106.
- Hessell AJ, Poignard P, Hunter M, Hangartner L, Tehrani DM, Bleeker WK, Parren PWHI, Marx PA, Burton DR. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nature Medicine. 2009 Aug;15(8):951–54. doi:https://doi.org/10.1038/nm.1974.
- Ko S-Y, Pegu A, Rudicell RS, Yang Z-Y, Joyce MG, Chen X, Wang K, Bao S, Kraemer TD, Rath T, et al. Enhanced neonatal Fc receptor function improves protection against primate SHIV infection. Nature. 2014 August 13;514(7524):642–45. doi:https://doi.org/10.1038/nature13612.
- Moldt B, Shibata-Koyama M, Rakasz EG, Schultz N, Kanda Y, Dunlop DC, Finstad SL, Jin C, Landucci G, Alpert MD, et al. A nonfucosylated variant of the anti-HIV-1 monoclonal antibody b12 has enhanced FcgammaRIIIa-mediated antiviral activity in vitro but does not improve protection against mucosal SHIV challenge in macaques. Journal of Virology. 2012 Jun;86(11):6189–96. doi:https://doi.org/10.1128/JVI.00491-12.
- Bournazos S, Klein F, Pietzsch J, Seaman MS, Nussenzweig MC, Ravetch JV. Broadly neutralizing anti-HIV-1 antibodies require Fc effector functions for in vivo activity. Cell. 2014 Sep 11;158(6):1243–53. doi:https://doi.org/10.1016/j.cell.2014.08.023.
- Gaudinski MR, Coates EE, Houser KV, Chen GL, Yamshchikov G, Saunders JG, Holman LA, Gordon I, Plummer S, Hendel CS, et al. Safety and pharmacokinetics of the Fc-modified HIV-1 human monoclonal antibody VRC01LS: a Phase 1 open-label clinical trial in healthy adults. PLoS Med. 2018 Jan;15(1):e1002493. doi:https://doi.org/10.1371/journal.pmed.1002493.
- Gupta S, Gach JS, Becerra JC, Phan TB, Pudney J, Joseph SB, Landucci G, Supnet MJ, Ping L-H, et al. The Neonatal Fc receptor (FcRn) enhances human immunodeficiency virus type 1 (HIV-1) transcytosis across epithelial cells. PLoS Pathog. 2013;9(11):e1003776. doi:https://doi.org/10.1371/journal.ppat.1003776.
- Ferrara C, Grau S, Jager C, Sondermann P, Brunker P, Waldhauer I, Hennig M, Ruf A, Rufer AC, Stihle M, et al. Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcgammaRIII and antibodies lacking core fucose. Proceedings of the National Academy of Sciences of the United States of America. 2011 Aug 2;108(31):12669–74. doi:https://doi.org/10.1073/pnas.1108455108.
- Anderson DJ, Politch JA, Zeitlin L, Hiatt A, KadaMoldoveanusia K, Mayer KH, Ruprecht RM, Villinger F, Whaley KJ. Systemic and topical use of monoclonal antibodies to prevent the sexual transmission of HIV. AIDS. 2017 Jul 17;31(11):1505–17. doi:https://doi.org/10.1097/QAD.0000000000001521.
- Morris GC, Wiggins RC, Woodhall SC, Bland JM, Taylor CR, Jespers V, Vcelar BA, Lacey CJ. MABGEL 1: first phase 1 trial of the anti-HIV-1 monoclonal antibodies 2F5, 4E10 and 2G12 as a vaginal microbicide. PloS One. 2014;9(12):e116153. doi:https://doi.org/10.1371/journal.pone.0116153.
- Ma JK, Drossard J, Lewis D, Altmann F, Boyle J, Christou P, Cole T, Dale P, Van Dolleweerd CJ, Isitt V, et al. Regulatory approval and a first-in-human phase I clinical trial of a monoclonal antibody produced in transgenic tobacco plants. Plant Biotechnology Journal. 2015 Oct;13(8):1106–20. doi:https://doi.org/10.1111/pbi.12416.
- Politch JA, Cu-Uvin S, Moench TR, Tashima KT, Marathe JG, Guthrie KM, Cabral H, Nyhuis T, Brennan M, Zeitlin L, et al. Safety, acceptability, and pharmacokinetics of a monoclonal antibody-based vaginal multipurpose prevention film (MB66): a Phase I randomized trial. PLoS Med. 2021 Feb;18(2):e1003495. doi:https://doi.org/10.1371/journal.pmed.1003495.
- Isojima S, Kameda K, Tsuji Y, Shigeta M, Ikeda Y, Koyama K. Establishment and characterization of a human hybridoma secreting monoclonal antibody with high titers of sperm immobilizing and agglutinating activities against human seminal plasma. J Reprod Immunol. 1987 Jan;10(1):67–78. doi:https://doi.org/10.1016/0165-0378(87)90051-9.
- Baldeon-Vaca G, Marathe JG, Politch JA, et al. Production and characterization of a human antisperm monoclonal antibody against CD52g for topical contraception in women. EBioMedicine. 2021;69: 103478.
- Franklin RD, Kutteh WH. Characterization of immunoglobulins and cytokines in human cervical mucus: influence of exogenous and endogenous hormones. J Reprod Immunol. 1999 Mar;42(2):93–106. doi:https://doi.org/10.1016/S0165-0378(98)00086-2.
- Bouvet JP, Belec L, Pires R, Pillot J. Immunoglobulin G antibodies in human vaginal secretions after parenteral vaccination. Infect Immun. 1994 Sep;62(9):3957–61. doi:https://doi.org/10.1128/iai.62.9.3957-3961.1994.
- Seaton KE, Deal A, Han X, Li SS, Clayton A, Heptinstall J, Duerr A, Allen MA, Shen X, Sawant S, et al. Meta-analysis of HIV-1 vaccine elicited mucosal antibodies in humans. NPJ Vaccines. 2021 Apr 15;6(1):56. doi:https://doi.org/10.1038/s41541-021-00305-8.
- Pattyn J, Van Keer S, Tjalma W, Matheeussen V, Van Damme P, Vorsters A. Infection and vaccine-induced HPV-specific antibodies in cervicovaginal secretions. A review of the literature. Papillomavirus Res. 2019 Dec;8:100185. doi:https://doi.org/10.1016/j.pvr.2019.100185.
- Scherpenisse M, Mollers M, Schepp RM, Meijer CJLM, de Melker HE, Berbers GAM, van der Klis FRM. Detection of systemic and mucosal HPV-specific IgG and IgA antibodies in adolescent girls one and two years after HPV vaccination. Hum Vaccin Immunother. 2013 Feb;9(2):314–21. doi:https://doi.org/10.4161/hv.22693.
- Anderson DJ, Politch JA, Cone RA, Zeitlin L, Lai SK, Santangelo PJ, Moench TR, Whaley KJ. Engineering monoclonal antibody-based contraception and multipurpose prevention technologiesdagger. Biol Reprod. 2020 Aug 4;103(2):275–85. doi:https://doi.org/10.1093/biolre/ioaa096.
