ABSTRACT
Neisseria meningitidis is a bacterial pathogen capable of causing rapidly progressing illness from nonspecific symptoms to end-organ failure or death in a matter of hours to days. Despite the availability of meningococcal vaccines, there remains a notable disease incidence peak among individuals aged 18–19 years, with college students at increased risk for disease relative to non-college students. Between 2007 and 2017, as many as one in five colleges in the United States experienced an outbreak of meningococcal disease at their own or a nearby institution. Evidence-based strategies to promote meningococcal vaccination among students can be adapted for the college setting, but barriers exist that limit widespread implementation of these strategies by colleges. In this article, we review meningococcal disease characteristics and epidemiology among US college students, vaccination indications and coverage levels among US college students, as well as college vaccination policies and practices that can impact students’ vaccine uptake.
Introduction
Neisseria meningitidis is among the most common causes of bacterial meningitis in the United States (US) with one relative peak incidence of the disease occurring among persons 18 to 19 years of age.Citation1,Citation2 Students at 2- or 4-year colleges or universities (hereafter referred to collectively as colleges) are at increased risk for invasive N. meningitidis compared to non-college students.Citation2 Over 40% of the US population ages 18 to 24 years are enrolled in college.Citation3 This figure includes 17.8 million undergraduate students and 4.3 million graduate students.Citation4 Their increased risk of meningococcal disease is associated with their greater likelihood of living in college dormitories and engaging in social behaviors that facilitate the transmission of respiratory pathogens.Citation5–10
The first conjugate vaccine recommended to protect against N. meningitidis was licensed in the US in 2005.Citation11 The overall incidence of meningococcal disease has been declining over the last few decades, assisted by increasing vaccination prevalence. Nonetheless, sporadic outbreaks continue to occur, and college-associated outbreaks account for approximately 20% of all meningococcal outbreaks in the US.Citation12 Between 2007 and 2017, as many as one in five US colleges experienced an outbreak of meningococcal disease at their institution or a nearby institution according to a recent survey.Citation13 In that survey, 53% of the student health representatives reported that their college required any meningococcal vaccination among their student body. Individual colleges’ vaccination mandates likely have contributed to the observed higher meningococcal vaccination coverage level among college students overall compared with non-college students.Citation14–17 Nonetheless, the survey suggests approximately half of all US colleges still have the opportunity to implement policies to render their students and their students’ contacts considerably less vulnerable to meningococcal disease.Citation13 Past college-associated outbreaks have been estimated to cost hundreds of thousands of dollars in outbreak containment measures related to contact identification, post-exposure prophylaxis, mass vaccination, and educational campaigns.Citation18 These costs to colleges and public health institutions do not consider the financial and psychological costs to infected individuals and society associated with meningococcal disease treatment and sequelae.Citation19
Meningococcal vaccination is the foundation for the prevention of invasive meningococcal disease within and beyond the college setting. In this article, we review meningococcal disease characteristics and epidemiology among college students, vaccination indications and coverage levels among college students, as well as college vaccination policies and practices that can impact students’ vaccine uptake.
Meningococcal disease characteristics
Most individuals exposed to N. meningitidis do not manifest symptoms of disease but are asymptomatic bacterial carriers capable of transmitting infection to others.Citation20 Nonetheless, N. meningitidis is a fearsome pathogen that can cause rapidly progressing illness from nonspecific, “flu-like” symptoms to fulminant disease in a matter of hours or days.Citation20 The Gram-negative bacterium is transmitted by contact with large droplet respiratory secretions from ill individuals and asymptomatic carriers.Citation21 Disease occurs when the pathogen invades via the nasopharynx, with patients most often presenting for clinical care with meningitis, septicemia, or both.Citation21 Less commonly, patients first present with arthritis, conjunctivitis, myocarditis, pericarditis, pneumonia, or urethritis, which can progress to fulminant disease.Citation20,Citation21 Among individuals with invasive disease, the mortality rate is 10–20%,Citation1,Citation21–24 and among disease survivors, the rate of long-term sequelae is 10–20%.Citation19,Citation21,Citation22,Citation24 Sequelae of invasive meningococcal disease include hearing loss, limb amputation, seizure disorder, cognitive dysfunction, and loss of independent daily functions.Citation19–22,Citation24
Twelve serogroups of N. meningitidis have been described by the structure of their polysaccharide capsule.Citation21,Citation25 The polysaccharide capsule is a key determinant of the bacteriums’ invasive potential. Serogroups A, B, C, W, X, and Y meningococci are responsible for most cases of invasive disease globally, but incidence by serogroup varies widely with geography.Citation21,Citation26 In the US, serogroups B, C, and Y meningococci have been the most common causes of sporadic and outbreak-associated invasive disease over the past two decades when considering cases for which the serogroup has been identifiable.Citation1,Citation23,Citation26
Meningococcal vaccines
There are currently five Food and Drug Administration (FDA)-approved meningococcal vaccines available in the US (). Three of these vaccines are quadrivalent meningococcal conjugate vaccines that confer protection against serogroups A, C, W, and Y meningococci (MenACWY vaccines). These vaccines function by presenting the recipients’ immune systems with serogroups A, C, W, and Y meningococcal capsular polysaccharide structures coupled to protein carrier molecules to induce a T-cell mediated response to enhance immune response and memory.Citation26 The remaining two vaccines protect against serogroup B meningococcus (MenB vaccines). These vaccines function by presenting the recipients’ immune systems with recombinant, meningococcal subcapsular lipoproteins found in prevalent serogroup B (as well as other) meningococcal strains to induce the production of bactericidal antibodies.Citation21,Citation27–29
Table 1. Meningococcal vaccines currently licensed in the United States.Citation21
Epidemiology of meningococcal disease on college campuses
The incidence of meningococcal disease in the US has been declining among all age groups since the 1990s;Citation1,Citation16,Citation21,Citation30 however, there remain notable incidence peaks at three distinct life stages: infancy, late adolescence to early adulthood, and late adulthood ().Citation1,Citation30 Among individuals ages 18 to 24 years old, the average US annual incidence was 0.17 cases per 100,000 population between 2014 and 2016, with the highest incidence occurring in individuals ages 18 to 19 years old (0.29 cases per 100,000 population).Citation2 Among individuals ages 18 to 24 years old in the US, the incidence of invasive meningococcal disease for persons attending college was nearly double the incidence for persons not attending college between 2015 and 2018, suggesting that college attendance is a risk factor for invasive meningococcal disease ().Citation14–17 Serogroup B meningococcus was responsible for the majority of meningococcal cases among all individuals ages 18 to 24 years in the US between 2014 and 2018 (), with a relative risk for serogroup B meningococcal disease among college students of 3.54 compared to non-college students.Citation2,Citation14–17,Citation31
Figure 1. US incidence of meningococcal disease by age group, 2006–2015.Citation1
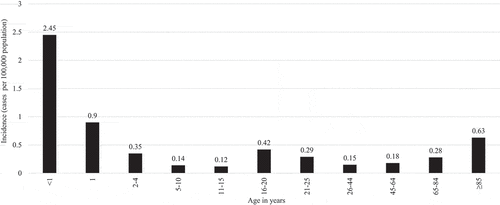
Figure 2. Mean US incidence of meningococcal disease per serogroup among individuals aged 18–24 years per 100,000 population, 2015–2018.Citation14–17*.
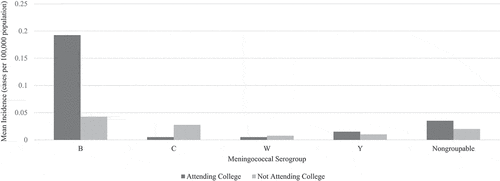
Table 2. Meningococcal disease incidence in US individuals aged 18–24 years per 100,000 population by college attendance.Citation14–17
While the incidence of sporadic meningococcal disease has been declining, the incidence of outbreak-associated disease has remained relatively stable over time, resulting in the proportion of cases attributable to an outbreak increasing by almost 2% from 2009 (4.5%) to 2013 (6.3%).Citation12 Between 2009 and 2013, one in five (22%) meningococcal outbreaks in the US occurred in college populations, with 75% of the college-related outbreaks attributable to serogroup B meningococcus.Citation2 In the 2010s, a rapid succession of serogroup B meningococcal disease outbreaks on 10 college campuses led to the development of outbreak containment planning guides and resources to aid in rapid mobilization of control and containment measures.Citation24 These outbreaks lasted from 0 to 376 days (median of 34 days) and were associated with 39 cases and two deaths (5%).Citation24
Several studies have attempted to identify risk factors for meningococcal disease among college students. Evidence from the 1990s suggests that being a first-year student living in a college dormitory, having a recent upper respiratory tract infection, consuming alcohol, patronizing bars, and smoking cigarettes are associated with increased odds of meningococcal disease among college students.Citation6,Citation7 A more recent study adds participating in college fraternities and sororities and having more than one kissing partner as additional risk factors.Citation8
Given that many students cross state and international borders to attend college, outbreaks of N. meningitidis initially identified on college campuses have been observed to propagate far beyond institutional borders to students’ families and community contacts.Citation15 When outbreaks that begin on a college campus extend to the broader community, containment efforts are complicated by the additional challenges of identifying and reaching at-risk individuals for education, post-exposure chemoprophylaxis, and vaccination.Citation20
Between 2015 and 2018, 90% to 100% of US college students with a known case of meningococcal disease had previously received MenACWY vaccination, and 0% to 14% had previously received MenB vaccination.Citation14–17 In comparison, 38% to 75% of non-college students with a known case had previously received MenACWY vaccination, and 0% had previously received MenB vaccination.Citation14–17 As a consequence of the high MenACWY vaccination coverage level among college students, relatively few college-associated cases of invasive meningococcal disease were attributable to serogroup A, C, W or Y meningococcus; as a result of the lower MenACWY vaccination coverage among non-college students, a higher proportion of cases in this population was attributable to serogroup A, C, W or Y meningococcus.Citation24,Citation31 Limited uptake of MenB vaccination among both college and non-college students likely explains the relatively high proportion of cases attributable to serogroup B meningococcus in both populations.Citation18,Citation31
Epidemiology of N. meningitidis carriage among college students
Asymptomatic nasopharyngeal carriage of N. meningitidis is believed to contribute to the population burden of meningococcal disease.Citation32,Citation33 Maiden et al. demonstrated that the prevalence of meningococcal serogroup C carriage was reduced among children who had received a conjugate meningococcal vaccine with serogroup C coverage,Citation34 and this reduction in carriage among vaccinated children was also associated with decreased disease incidence in unvaccinated children in the population.Citation32 These findings demonstrate indirect protection of unvaccinated individuals and thereby suggest that high levels of population MenACWY vaccination may contribute to herd protection, although this has not been demonstrated with MenB vaccination.Citation32 This is consistent with the observed effect of other conjugate vaccines that have been associated with reduced bacterial carriage among vaccine recipients and population-level herd protection.Citation33
In a 2015 cross-sectional survey of students at a US college that had not had experienced a recent outbreak, the prevalence of all-serogroup N. meningitidis nasopharyngeal carriage was 12.7% to 14.6%.Citation10 In a separate study that longitudinally surveyed US students four times during the 12 months after a campus-based serogroup B meningococcus outbreak, the prevalence of all-serogroup meningococcal carriage remained relatively stable at 11% to 17% over the course of the study.Citation9
Similar behavioral factors associated with invasive disease have been associated with carriage of N. meningitidis including consuming alcohol, patronizing bars, attending parties, and smoking.Citation5,Citation9,Citation10, A dose response was identified with alcohol consumption in one study with greater alcohol consumption associated with higher odds of carriage.Citation5 Additional risk factors for carriage include male sex and recent upper respiratory tract infection.Citation9,Citation10 Recent antibiotic use has been shown to be inversely correlated with N. meningitidis carriage.Citation9,Citation10
Efficacy of meningococcal vaccines in college-age students
Knowledge of meningococcal vaccines’ clinical efficacy is relatively limited, as FDA licensure for all MenACWY and MenB vaccines has been granted based on immunogenicity and safety data due to the low incidence of meningococcal disease in the US.Citation21,Citation35 For the purposes of licensure, immunogenicity studies assessed candidate vaccines’ ability to induce complement-dependent bactericidal activity in recipients.Citation21 Candidate vaccines demonstrated sufficient immunogenicity by exceeding predefined serum bactericidal activity titer thresholds among a set minimum proportion of study participants.Citation21
Studies have demonstrated that all three MenACWY vaccines currently licensed in the US induce overall high levels of immunity in adolescents and adults with some degree of variability in their level of protection against each of the four vaccine-included serogroups.Citation36–41 Additional studies have demonstrated that immunity subsequently wanes as soon as 12–22 months after primary vaccination with MenACWY-D or MenACWY-CRM, though the timing of waning antibody titers appears to vary between studies.Citation42,Citation43Antibody persistence after primary vaccination with MenACWY-TT vaccine has not yet been studied. In a study of adolescents who received a MenACWY-D vaccine 4 to 6 years after primary vaccination, the booster dose induced a robust amnestic response to all four included serogroups that persisted four years later.Citation44,Citation45Similar studies in adolescents and adults in the US and Puerto Rico demonstrated robust responses to booster doses of MenACWY-CRM and MenACWY-TT vaccines after primary vaccination with MenACWY-D or MenACWY-CRM at least four years earlier.Citation46,Citation47
Clinical effectiveness data exist for MenACWY-D vaccine but not for MenACWY-CRM or MenACWY-TT vaccines. The overall effectiveness of MenACWY-D vaccine against meningococcal disease caused by serogroups A, C, W, and Y meningococci in adolescents is approximately 69% (95% confidence interval (CI) of 51% to 80%) up to 8 years after receipt of a single dose.Citation21 While decreasing prevalence of the serogroups C, W, and Y meningococcal carrier state among adolescents and young adults has coincided with increasing population coverage with the currently licensed MenACWY vaccines, there is limited evidence demonstrating a causal link between MenACWY vaccination and reduced likelihood of becoming a meningococcal carrier.Citation9,Citation10,Citation32,Citation33,Citation48
The two MenB vaccines licensed in the US have also demonstrated high levels of immunogenicity in adolescents and adults when administered in accordance with the series recommendations, though it is important to note that available data reflect immunogenicity in a global population.Citation49,Citation50 In a study conducted in Australia, Canada, and Chile, antibody titers in recipients of MenB-4C vaccine waned 4 and 7.5 years after receipt of the primary series, but a robust immune response was demonstrated when a booster dose was administered 4 and 7.5 years after the primary vaccination series.Citation51 Similarly, in a multi-country study that included US-based sites, antibody titers in recipients of MenB-fHbp vaccine waned 12 months after completion of the primary series, but vaccines demonstrated a robust immune response when a booster dose was administered 4 years after the primary vaccine series.Citation52 MenB-4C clinical effectiveness among individuals aged less than 21 years in Canada has been estimated at 79% (95% CI of -231% to 99%) four years post-vaccination, but the point estimate is difficult to interpret since the confidence interval is wide and contains the null value due to the low incidence of serogroup B meningococcal disease.Citation53 Clinical effectiveness data is not available for MenB-fHbp.
Multiple studies have failed to demonstrate an association of vaccination with either of the currently approved MenB vaccines and acquisition of carriage.Citation9,Citation28,Citation35,Citation48,Citation54,Citation55 The apparent inability of MenB vaccines to prevent acquisition of carriage suggests that MenB vaccines are unable to impact herd protection, and thus, the objective of MenB vaccination is the protection of individual vaccinees.Citation28,Citation56
Safety of meningococcal vaccines in college-age students
The US-licensed serogroup A, C, W, and Y meningococcal vaccines have demonstrated acceptable safety profiles in adolescents and young adults.Citation21 Among these vaccines, the most common local reactions among recipients include injection site pain, erythema, induration, and/or swelling, whereas the most common systemic reactions include headache, fatigue, malaise, and myalgia.Citation36–39,Citation41 Symptoms are generally mild to moderate, and, when reported, generally resolve within three days.Citation36–39,Citation41 Concurrent administration with other vaccines, such as human papillomavirus (HPV) and/or tetanus-diphtheria-pertussis vaccines, generally leads to increases in reported systemic symptoms, though immunogenicity is not significantly altered.Citation38,Citation57,Citation58 Post-licensure review of MenACWY-D vaccine considered a possible link with Guillain Barré Syndrome (GBS), but the risk of GBS post-vaccination was not significantly increased from the risk in the general population.Citation59,Citation60 Similarly, with the MenACWY-CRM vaccine, post-licensure review revealed a temporal association of vaccination with Bell’s palsy, but there were several possible confounders, including medical comorbidities and concomitant vaccination of some study participants, and further investigation is required to determine causality.Citation61 To date, no post-licensure data has become available for MenACWY-TT vaccine since its approval in 2020.
Similar to the MenACWY vaccines in adolescents and young adults, the most common local reactions in the US-licensed serogroup B meningococcal vaccines include injection site pain, erythema, and induration, and the most common systemic reactions include headache, fatigue, myalgia, and arthralgia.Citation49,Citation50,Citation62 Previous studies demonstrated non-inferiority of MenB-fHbp when concurrently administered with tetanus-diphtheria-pertussis, MenACWY-D, and HPV vaccines; however, no data exist to date regarding concurrent administration of MenB-4C with other vaccines.Citation63,Citation64 Post-licensure review of MenB-4C demonstrated an association with syncopal events, but no other safety concerns for MenB-4C or MenB-fHbp.Citation21,Citation65 There is a theoretical possibility of inducing Factor H autoantibodies in recipients of either MenB vaccine products since both contain fHbp, but the clinical relevance of this possibility is unknown.Citation21
Recommended meningococcal vaccinations for college-aged individuals
There are an estimated 1,500 student health centers on college campuses across the US.Citation66 The American College Health Association (ACHA) is the largest professional organization for US-based college health practitioners with members representing over 700 colleges. The ACHA’s Vaccine-Preventable Diseases Advisory Committee annually updates its Immunization Recommendations for College Student which are designed to assist colleges in setting comprehensive vaccination policies including pre-matriculation requirements and criteria for school exclusion of unvaccinated students during vaccine-preventable disease outbreaks.Citation67 The ACHA vaccination guidelines are aligned with the immunization schedule recommended by the Centers for Disease Control and Prevention‘s (CDC’s) Advisory Committee on Immunization Practices (ACIP) which updates its vaccination recommendations three times per year (www.cdc.gov/vaccines/schedules/index.html).
The ACIP recommends routine administration of a first dose of MenACWY vaccine to children at ages 11 to 12 years, and a second dose at age 16 years ().Citation21 Individuals of typical college age may require catch-up dosing if they did not receive MenACWY vaccination per these recommendations. Others may require booster dosing prior to starting college if they received MenACWY vaccination at a younger age than the routine recommendation. MenACWY vaccination is indicated for individuals as young as 2 months who are at increased risk for meningococcal disease for reasons including anatomic or functional asplenia including sickle cell disease, complement component deficiency including complement inhibitor medication use, infection with human immunodeficiency virus (HIV), residence in a locality experiencing an outbreak, and travel to a country where meningococcal disease is hyperendemic or epidemic. It is recommended that incoming first-year college students who will reside in a dormitory have a history of at least one dose of MenACWY vaccine received at age 16 years or older and within 5 years prior to matriculation. Incoming students who do not meet these recommendations should obtain one booster dose before starting.
Table 3. Meningococcal vaccination recommendations for US college students.Citation21,Citation67*
The ACIP recommends routine administration of MenB vaccine for individuals ages 10 years and older who are at increased risk for meningococcal disease due to anatomic or functional asplenia including sickle cell disease, complement component deficiency including complement inhibitor medication use, or high potential for meningococcal exposure such as residing in a locality experiencing an outbreak ().Citation27,Citation68 For individuals ages 16 through 23 years who are not at increased risk of meningococcal disease, the ACIP suggests MenB vaccination may be administered on the basis of shared clinical decision-making between patients and their healthcare practitioner. Patients who opt for vaccination should receive 2 doses of MenB vaccine administered 1 or 6 months apart depending on which product is used (MenB-4C vaccine versus MenB-fHbp vaccine, respectively). The two MenB vaccine formulations are not interchangeable, and the same product should be used for both doses.
Meningococcal vaccination precautions and contraindications
Healthcare providers should consider a 15-minute observation period for syncope after MenACWY or MenB vaccination especially for adolescent patients.Citation21 If syncope occurs, healthcare providers should continue to monitor patients until it resolves. Severe allergic reaction to a previous dose of meningococcal vaccine or any component of the vaccine is a contraindication to future receipt of that vaccine. In addition, severe allergic reaction to any vaccine containing diphtheria toxoid or CRM197 is a contraindication to receiving MenACWY-D and MenACWY-CRM vaccines. Severe allergic reaction to a tetanus toxoid-containing vaccine is a contraindication to receiving MenACWY-TT vaccine. Previous history of Guillain-Barré syndrome is a precaution for receiving MenACWY-D vaccine. Because the tip caps of prefilled MenB-4C vaccine syringes contain latex, latex sensitivity is a precaution for receiving MenB-4C vaccine. Women who are pregnant or breastfeeding should receive MenACWY vaccination if indicated but should defer MenB vaccination due to lack of sufficient safety data in pregnant and breastfeeding women. Per the CDC, healthcare providers should engage pregnant or breastfeeding women at increased risk of serogroup B meningococcal disease in shared clinical decision-making to help them decide whether or not to receive MenB vaccination.Citation21
Engaging patients in shared clinical decision-making for MenB vaccination
In practice, the shared clinical decision-making recommendation for MenB vaccination means healthcare providers can decide whether or not to stock MenB vaccines, and whether or not to initiate a discussion about MenB vaccination with patients who lack disease risk factors.Citation69,Citation70 In contrast to its universal recommendation for MenACWY vaccination, the ACIP does not suggest healthcare providers assume the default position of encouraging MenB vaccination for all eligible individuals. Instead, the CDC offers these points for possible discussion:
the high severity of meningococcal disease;
the overall low incidence of serogroup B meningococcal disease with higher incidence among college students;
the broad efficacy of MenB vaccines against most strains of serogroup B meningococcus;
the limited duration (approximately 1–2 years) of full protection induced by MenB vaccination;
the evidence to date suggests that MenB vaccination has no effect on meningococcal carriage.Citation21
The goal of the conversation is for healthcare providers to help patients weigh potential benefits of MenB vaccination based on personal risk factors and preferences regarding risk tolerance. According to the CDC, shared decision-making can be initiated by anyone who orders or administers vaccines including physicians, nurse practitioners, registered nurses, and pharmacists. This is relevant to college students studying outside of their hometown who may have lower access to their primary care provider than to other vaccine providers located within their college student health center or at nearby pharmacies and public health clinics.
Considering mass MenB vaccination of college students in outbreak settings
The current ACIP recommendations for MenB vaccination reflect cost-effectiveness data that suggest high cost and uncertain population-level benefit associated with implementation of a universal recommendation for vaccination of all college students. According to one study estimate, an universal MenB vaccination program for college students would require 305,000 individuals to be vaccinated to prevent 11 cases of meningococcal disease, and 2,765,000 individuals to be vaccinated to prevent 1 death.Citation31 Depending on model assumptions, the incremental cost per quality-adjusted life year (QALY) gained from implementing an universal MenB vaccination program for US college students may range from $748,129 to $13.9 million.Citation71,Citation72 By comparison, the current US MenACWY vaccination program is estimated to cost $212,000 per QALY gained.Citation11 Thus, while MenB vaccination provides some protective benefit to individual recipients, implementing a universal MenB vaccination recommendation for all eligible college students is not a cost-effective strategy for impacting population health.Citation69
Mass vaccination is a recommended containment strategy during meningococcal outbreaks due to a vaccine-included serogroup.Citation73 One modeling study varied the probability of outbreak and found that if a serogroup B meningococcus outbreak was highly probable on a college campus, mass vaccination of incoming students to that campus would be both life-saving and cost-saving.Citation72 This led the study authors to suggest research into predictors of an imminent outbreak so colleges could anticipate when a mass MenB vaccination program for students would be worthwhile.
The role of state mandates in supporting college students’ meningococcal vaccination
US national data are not available for meningococcal vaccination coverage levels specifically among college students. The 2019 National Immunization Survey found that only 53.7% of US adolescents aged 17 years had received 2 or more doses and 88.9% 1 or more doses of MenACWY vaccine.Citation74 In the same survey, only 21.8% of individuals aged 17 years had received at least 1 dose of MenB vaccine, suggesting many adolescents reach college age inadequately vaccinated against N. meningitidis.
Multiple studies have demonstrated that state-mandated vaccination for school attendance increases population vaccination coverage levels and decreases vaccine-preventable disease incidence.Citation75–78 Twenty-four US states require at least a subset of college students to have received MenACWY vaccination.Citation79 Details of state mandates are heterogeneous with some limiting the requirement only to students who are: attending publicly funded institutions, attending 4-year institutions, younger than 21 or 22 years old, newly matriculated, attending in-person classes, residing on campus, or enrolled in a minimum number of class hours. The other 26 US states and the District of Columbia have no MenACWY vaccination mandate for college students, and no state currently requires MenB vaccination.Citation80
The role of colleges in supporting college students’ meningococcal vaccination
All colleges have a legal obligation to meet their state’s meningococcal vaccination policy requirements and have an ethical obligation to take steps to reduce risk of a meningococcal disease outbreak. Colleges have varying tools at their disposal to fulfill these obligations (). For instance, all colleges may request students’ meningococcal vaccination records and instate vaccination requirements with reasonable penalties for noncompliance. Colleges may also help motivate students to comply with vaccination mandates by engaging campus leaders in meningococcal education and promotion activities. Finally, colleges may help students receive MenACWY and MenB vaccinations either by administering them at their student health center or referring students to community-based vaccination providers. Several reports suggest colleges can increase vaccination coverage among students through implementation of multimodal vaccination promotion and delivery interventions.Citation83–85 Nonetheless, colleges typically face challenges to doing so. For instance, the mix of staff and administrators who contribute to student health on a campus depends on the college’s available resources. Some 33% to 45% of colleges do not have a full-time physician on staff within their student health center, and smaller colleges and nonresidential colleges may not have a student health center at all.Citation86,Citation87 Even on campuses with a student health center, limited financial resources and personnel may preclude staff from initiating vaccination quality improvement initiatives themselves, although not necessarily from participating in initiatives organized by external groups such as professional organizations or college student health insurers.Citation85,Citation88
Table 4. Strategies to increase student body meningococcal vaccination coverage levels.Citation18,Citation81,Citation82
In addition to promoting routine meningococcal vaccination among students, college health administrators should consider their level of preparedness for mass meningococcal vaccination in the event of a campus outbreak. According to the CDC, considerations for launching a mass vaccination campaign in the event of a meningococcal outbreak (not specific to the college setting) include the number of index cases, population size, ability to identify potential vaccinees, likelihood of ongoing transmission, feasibility, and likely lag time between identification of index cases and vaccination in earnest.Citation73 In a survey of ACHA members, fewer than half of the respondents reported that their college had a plan that included administration of MenACWY or MenB vaccines to students in the event of a campus meningococcal outbreak.Citation89 Lessons learned from the Coronavirus Disease 2019 (COVID-19) pandemic, as well as college-specific guidance from the CDC and ACHA on pandemic preparedness, should be used by college health authorities and public health officials to plan for a campus outbreak of meningococcus with protocols that include the potential administration of meningococcal vaccines to students.Citation90,Citation91
Financial obstacles to high meningococcal vaccine coverage levels among college students
The limited investment by some colleges in student health has been described in lay publications and is not necessarily inappropriate, especially at smaller institutions, given their primary mission of education. Nonetheless, the situation presents a barrier to some colleges’ ability to intervene to achieve a high meningococcal vaccination coverage level among their students. A 2020 examination by the Washington Post of 1500 4-year, residential colleges with at least 500 students enrolled revealed that 16.7% did not have a student health center, although some without a campus health center did offer students medical services via telehealth or partnership with off-campus healthcare providers.Citation87 According to the Washington Post, the average amount 200 colleges with a health center spent per student on health center services in 2017 was only $185. With such a limited budget, vaccine promotion and administration may pose a strain on the resources of some student health centers. The net effect of the COVID-19 pandemic on colleges’ future investment in student health is unclear. In an ACHA survey of its membership from August 2020, 43.5% of 207 respondents anticipated a reduction to their student health center budget in 2021.Citation92
In 2021, the manufacturer contract price per dose in the US ranged from approximately $135 to $140 for MenACWY vaccine and from $157 to $192 for MenB vaccine.Citation93 Thus, even at colleges with student health centers that have the capacity to stock and administer vaccines, cost may restrict access to meningococcal vaccinations. In a recent survey of 147 college health administrators and clinicians across the US, the most frequently cited barriers to being able to offer students MenB vaccination services were financial in nature.Citation81 Over half of respondents felt that the limited ability of their health center to pay up front to stock the vaccine and of students to pay the out-of-pocket costs to receive the vaccine were major barriers to having a MenB vaccination program at their college.Citation81 This is in contrast to findings from a separate survey of US community-based healthcare providers in which financial burden was reportedly the most impactful parameter in less than 10% of their decisions not to prescribe MenACWY or MenB vaccination for their eligible patients.Citation94
In terms of meningococcal vaccination reimbursement, most college student health centers do not have the infrastructure required to support broad health insurance billing.Citation95 As a result, many derive their operational budgets from an annual health fee assessed to students which covers basic services with additional services rendered at additional charge. Student health centers reliant on the health fee model may be unable to include meningococcal vaccinations as fully covered services, and students may be unwilling or unable to pay more than a nominal cost to be vaccinated. Low student body demand for meningococcal vaccination may induce low levels of enthusiasm among health center administrators for purchasing vaccine supply since there is risk that stock will expire before it can be administered.
Some college student health centers that do not operate under the health fee model instead accept a limited number of insurance plans designed exclusively for students.Citation95 These student health insurance plans (SHIPs) may be funded directly by the school or through an unaffiliated insurer. The ACHA standards call on these SHIPs to include all ACIP-recommended immunizations, including MenACWY and MenB vaccinations, as fully covered services.Citation96 Even so, the ACHA also suggests that short-term, limited duration insurance (STLDI) plans for students “could be appropriate in limited circumstances.”Citation97 Such plans typically do not cover vaccinations.
Individuals covered by a school-funded SHIP may be limited in the healthcare they can receive outside of the college health center setting or may lose coverage over the summer if they do not meet minimum course enrollment criteria or transfer to a different college.Citation98 Passage of the Affordable Care Act (ACA) by the US Congress in 2010 has permitted a greater number of students to maintain their childhood health plans through their college years since the law specifies that dependents may remain on their parents’ or guardians’ insurance plan until age 26 years.Citation99 Additionally, the ACA requires health plans (with a few exceptions such as STLDI plans) to cover all ACIP-recommended vaccinations. Nonetheless, students that attend college outside of the state in which they are insured under a parent’s plan may have difficulty finding a vaccination provider in their college town that participates in their out-of-state health plan.
Meningococcal vaccination delivery and promotion best practices for colleges
The US National Vaccine Action Coalition (NVAC) has published Standards for Adult Immunization Practice designed to enhance vaccine uptake.Citation82 These standards call on healthcare providers to engage in the following practices, regardless of whether or not they administer vaccinations:
ASSESS the immunization status of all patients at every healthcare visit;
Strongly RECOMMEND indicated vaccinations to patients;
ADMINISTER all indicated vaccinations if able, or REFER patients to an outside vaccinator if not;
DOCUMENT vaccinations received by patients.
In addition to NVAC standards, immunization experts have compiled evidence-based strategies likely to enhance vaccination coverage levels on college campuses.Citation18,Citation81 These strategies are consistent with NVAC standards (). To the best of their ability, colleges should endeavor to implement these standards and strategies in their meningococcal vaccination practices and policies, regardless of whether they can administer meningococcal vaccinations to students.
Assessing college students’ meningococcal vaccination status
Colleges should assess their students’ meningococcal vaccination status at a minimum on an annual basis, prior to the start of the academic year. This is a formidable task given the influx of new students admitted each year. In one survey involving 147 colleges, 77.3% and 46.9% of the respondents reported requesting records of all students for MenACWY and MenB vaccination, respectively.Citation81 At colleges with limited or no healthcare staff and where verification of students’ MenACWY vaccination is state mandated, data collection may be completed by nonclinical administrators with little to no experience interpreting the validity of vaccinations. Besides collection of student-submitted records, vaccination assessments should also include searches of the local or state immunization information system for additional student vaccination records.Citation18 Searches of the local or state immunization information system may be more fruitful for colleges with a high percentage of in-state students.
At colleges with student health centers, students’ vaccination status should be assessed at each healthcare encounter, as well. Physical vaccination records obtained from students upon matriculation should be entered into the student health center’s record system to permit healthcare providers to easily determine whether students presenting for care should opportunistically be offered or referred for meningococcal vaccinations that day.
Giving a strong MenACWY vaccination recommendation to college students
In the college setting, providing a strong vaccination recommendation includes having a robust school policy supportive of vaccination. While states rarely require students to have received all ACIP-recommended vaccinations, 45% to 65.3% of colleges require students to have received at least one additional vaccination beyond the minimum mandated by their state.Citation100,Citation101 Surveys of colleges conducted from 2014 to 2018 reveal MenACWY vaccination to be among the top three and MenB vaccination among the bottom two most commonly required vaccinations.Citation81,Citation100,Citation101 In these surveys, 51.9% to 73.7% of colleges reported requiring MenACWY vaccination, and 0.93% to 10.5% reported requiring MenB vaccination.Citation81,Citation89,Citation100,Citation101 Various college characteristics have been associated with having a student MenACWY vaccination requirement including being located in a state with a vaccination mandate, having a smaller study body, and being privately funded.Citation89,Citation101
Most colleges with a meningococcal vaccination mandate require students to submit proof of vaccination, such as a physician-signed vaccination record or a written request for an exemption from the vaccination requirement.Citation89 The conditions under which an exemption is granted vary. In one survey, almost a quarter of colleges (23.7%) required students who desired an exemption to receive meningococcal vaccination counseling by a healthcare provider first.Citation89 In a survey conducted from 2014 to 2015, 98% of the colleges permitted students to be exempted from vaccination requirements in general based on their religious beliefs and 54% based on their philosophical beliefs.Citation101
After a grace period, colleges may apply various penalties to students who are not in compliance with their vaccination policy including preventing course registration, imposing fees, barring access to campus housing, restricting class attendance, preventing participation in organized sports, and excluding them during an outbreak.Citation100–102 Preventing course registration is the most common consequence applied, with 56% to 89% of colleges choosing this enforcement strategy.Citation100–102
For colleges that have a student health center, healthcare providers should convey the importance of MenACWY vaccination to all students and engage them a discussion about MenB vaccination. In a qualitative study involving 33 college students, participants expressed low knowledge about vaccines in general, and low perceived risk for vaccine-preventable disease outbreaks.Citation103 Participants also expressed a high degree of trust in the advice of medical professionals with respect to vaccine decision-making. This suggests the value of student healthcare providers taking the time to educate students about meningococcal disease and the benefits of vaccination since students may have little preexisting knowledge.
Administering meningococcal vaccination or referring students for off-campus vaccination
Student health center providers should broach meningococcal vaccination with all eligible students regardless of their presenting complaint since only 9% of student health center visits are made for the primary purpose of vaccination.Citation66 After discussion and obtaining consent, health center providers should either administer MenACWY and/or MenB vaccinations on site or refer students to community-based vaccine providers.
Among colleges with a student health center, approximately half stock MenACWY vaccine, and 25% to 37% stock MenB vaccine.Citation81,Citation89 Surveys suggest health center providers may not use every opportunity to offer meningococcal vaccinations to students even when the vaccines are available; in one survey, approximately 23% of respondents did not order and administer MenACWY vaccine to students even though it was stocked, and in another, slightly under half did not routinely do so at each healthcare encounter.Citation81,Citation89 In addition, not all student health center providers give students information about how to receive meningococcal vaccination in the community; in surveys, 59.9% of student health center providers reported referring students to community-based providers for MenACWY vaccine, and 55.1% to 62.1% for MenB vaccine.Citation81,Citation89
Students value convenience in receiving vaccinations.Citation104 As such, colleges should maximize the accessibility of their vaccination services. One survey suggests a minority of colleges ever hold outreach events to administer MenACWY (21.5%) or MenB vaccine (8.4%) to students at high-traffic locations across campus.Citation81 Greater percentages of colleges permit their students to walk into their student health center to receive a MenACWY or MenB vaccination without an appointment (64.9% and 57.7%, respectively).Citation81
Documenting vaccinations administered to students
Meningococcal vaccinations administered in the student health center or by community-based vaccine providers should be documented in the student’s health center record with a copy given to the student. Ideally, the health center’s electronic medical record system interfaces with the local or state immunization information system for bidirectional data exchange. The electronic health record systems used most frequently by college health centers are designed specifically for the college health setting. The ease with which health center providers can enter and access vaccination data, as well as customize vaccination data entry pages, varies across systems. Smaller student health centers may lack sufficient technological support to optimize their vaccination entry pages.Citation86 In a survey, 56.7% of respondents reported documenting their students’ MenACWY vaccination status, and 42.5% their MenB vaccination status at each health center encounter.Citation81
Conclusions and prospects
Colleges are uniquely positioned to promote meningococcal vaccine uptake among young adults, a population at higher risk for meningococcal disease than other age groups. Colleges with or without student health centers have many options for adopting meningococcal vaccine-friendly policies that are consistent with NVAC recommendations. Nonetheless, their engagement in promoting meningococcal vaccination to students is often limited by motivational, logistical, and financial barriers. Motivational barriers could be reduced if more states were to pass legislation requiring meningococcal vaccination of college students, especially if the mandates were to restrict or prohibit religious and philosophical exemptions. Logistical barriers could be reduced if the federal government were to deploy a national vaccination registry to facilitate colleges’ access to students’ vaccination records regardless of students’ home state. Additional logistical barriers could be reduced if health insurers were to offer college student health centers the ability to participate in organized learning collaboratives to assist them with implementing meningococcal vaccine delivery best practices. Finally, financial barriers could be reduced if national legislation were passed requiring commercial insurance plans and SHIPs to cover all vaccination costs for college students regardless of whether care was delivered by an in-network healthcare provider.
Abbreviations
Disclosure of potential conflicts of interest
In accordance with Taylor & Francis policy and our ethical obligation as researchers, we are reporting the following interests: Sarah Schaffer DeRoo is a consultant to Pfizer, Inc. Linda Y. Fu has received research support from Pfizer, Inc., which has also provided salary support for Rachel Torres.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- MacNeil JR, Blain AE, Wang X, Cohn AC. Current epidemiology and trends in meningococcal disease—United States, 1996–2015. Clin Infect Dis. 2017;66(8):1276–81. doi:10.1093/cid/cix993.
- Mbaeyi SA, Joseph SJ, Blain A, Wang X, Hariri S, MacNeil JR. Meningococcal disease among college-aged young adults: 2014-2016. Pediatr. 2019 Jan;143(1). doi:10.1542/peds.2018-2130.
- Bauman K, Cranney S. School enrollment in the United States: 2018. Current Population Reports: US Census Bureau; September 2020 [accessed 2021 April 28]. www.census.gov/library/publications/2020/demo/P20-584.html .
- National Center for Education Statistics. Table 302.60. Percentage of 18- to 24-year-olds enrolled in college, by level of institution and sex and race/ethnicity of student: 1970 through 2019 [accessed 2021 April 22]. https://nces.ed.gov/programs/digest/d20/tables/dt20_302.60.asp?current=yes .
- Imrey PB, Jackson LA, Ludwinski PH, England AC III, Fella GA, Fox BC, Isdale LB, Reeves MW, Wenger JD. Meningococcal carriage, alcohol consumption, and campus bar patronage in a serogroup C meningococcal disease outbreak. J Clin Microbiol. 1995;33(12). doi:10.1128/jcm.33.12.3133-3137.1995.
- Imrey PB, Jackson LA, Ludwinski PH, England AC III, Fox BC, Isdale LB, Reeves MW, Wenger JD. Outbreak of serogroup C meningococcal disease associated with campus bar patronage. Am J Epidemiol. 1996;143(6):624–30. doi:10.1093/oxfordjournals.aje.a008792.
- Bruce MG, Rosenstein NE, Capparella JM, Shutt KA, Perkins BA, Collins M. Risk factors for meningococcal disease in college students. JAMA. 2001;286(6):688–93. doi:10.1001/jama.286.6.688.
- Mandal S, Wu HM, MacNeil JR, Machesky K, Garcia J, Plikaytis BD, Quinn K, King L, Schmink SE, Wang X, et al. Prolonged university outbreak of meningococcal disease associated with a serogroup B strain rarely seen in the United States. Clin Infect Dis. 2013;57(3):344–48. doi:10.1093/cid/cit243.
- McNamara LA, Thomas JD, MacNeil J, Chang HY, Day M, Fisher E, Martin S, Poissant T, Schmink SE, Steward-Clark E, et al. Meningococcal carriage following a vaccination campaign with MenB-4C and MenB-fHbp in response to a university serogroup B meningococcal disease outbreak—Oregon, 2015–2016. J Infect Dis. 2017;216(9):1130–40. doi:10.1093/infdis/jix446.
- Breakwell L, Whaley M, Khan UI, Bandy U, Alexander-Scott N, Dupont L, Vanner C, Chang HY, Vuong JT, Martin S, et al. Meningococcal carriage among a university student population – United States, 2015. Vaccine. 2018;36(1):29–35. doi:10.1016/j.vaccine.2017.11.040.
- Bilukha OO, Rosenstein N. Prevention and control of meningococcal disease. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2005;54:1–21.
- Mbaeyi SA, Blain A, Whaley MJ, Wang X, Cohn AC, MacNeil JR. Epidemiology of meningococcal disease outbreaks in the United States, 2009-2013. Clin Infect Dis. 2019;68(4):580–85. doi:10.1093/cid/ciy548.
- Oliver S. Meningococcal vaccine policies among colleges and universities -United States, 2017. National Immunization Conference; May 16, 2018. Accessed April 28, 2021.
- Centers for Disease Control and Prevention. Enhanced meningococcal disease surveillance report, 2016. Updated 2017; [accessed 2021 July 27]. https://www.cdc.gov/meningococcal/downloads/NCIRD-EMS-Report.pdf .
- Centers for Disease Control and Prevention. Enhanced meningococcal disease surveillance report; 2017. Updated 2018. [accessed 2021 July 27]. www.cdc.gov/meningococcal/downloads/NCIRD-EMS-Report-2017.pdf .
- Centers for Disease Control and Prevention. Enhanced meningococcal disease surveillance report; 2018. Updated 2019 [accessed 2021 July 27]. www.cdc.gov/meningococcal/downloads/NCIRD-EMS-Report-2018.pdf .
- Centers for Disease Control and Prevention. Enhanced meningococcal disease surveillance report; 2015. Updated 2017 [accessed 2021 July 27]. www.cdc.gov/meningococcal/downloads/NCIRD-EMS-Report-2015.pdf .
- Kumar A, Murray DL, Havlichek DH. Immunizations for the college student: a campus perspective of an outbreak and national and international considerations. Pediatr Clin North Am. 2005;52(1):229–41. doi:10.1016/j.pcl.2004.10.009.
- Martinón-Torres F. Deciphering the burden of meningococcal disease: conventional and under-recognized elements. J Adolesc Health. 2016;59(2):S12–S20. doi:10.1016/j.jadohealth.2016.03.041.
- Pace D, Pollard AJ. Meningococcal disease: clinical presentation and sequelae. Vaccine. 2012;30:B3–B9. doi:10.1016/j.vaccine.2011.12.062.
- Mbaeyi SA, Bozio CH, Duffy J, Rubin LG, Hariri S, Stephens DS, MacNeil JR. Meningococcal vaccination: recommendations of the advisory committee on immunization practices, United States, 2020. MMWR Recomm Rep. 2020;69(9):1–41. doi:10.15585/mmwr.rr6909a1.
- Schaffner W, Baker CJ, Bozof L, Engel J, Offit PA, Turner JC. Addressing the challenges of serogroup B meningococcal disease outbreaks on campuses, infectious diseases in clinical practice. Bethesda (MD): National Foundation for infectious diseases; 2014 .
- Cohn A, MacNeil J, Harrison L, Hatcher C, Theodore J, Schmidt M, Pondo T, Arnold KE, Baumbach J, Bennett N, et al. Changes in Neisseria meningitidis disease epidemiology in the United States, 1998–2007: implications for prevention of meningococcal disease. Clin Infect Dis. 2010;50(2):184–91. doi:10.1086/649209.
- Soeters HM, McNamara LA, Blain AE, Whaley M, MacNeil JR, Hariri S, Mbaeyi SA. University-based outbreaks of meningococcal disease caused by serogroup B, United States, 2013–2018. Emerg Infect Dis. 2019;25(3):434–40. doi:10.3201/eid2503.181574.
- Harrison LH, Pelton SI, Wilder-Smith A, Holst J, Safadi MA, Vazquez JA, Taha MK, LaForce FM, von Gottberg A, Borrow R, et al. The global meningococcal initiative: recommendations for reducing the global burden of meningococcal disease. Vaccine. 2011;29(18):3363–71. doi:10.1016/j.vaccine.2011.02.058.
- Parikh SR, Campbell H, Bettinger JA, Harrison LH, Marshall HS, Martinon-Torres F, Safadi MA, Shao Z, Zhu B, von Gottberg A, et al. The everchanging epidemiology of meningococcal disease worldwide and the potential for prevention through vaccination. J Infect. 2020;81(4):483–98. doi:10.1016/j.jinf.2020.05.079.
- MacNeil JR, Rubin L, Folaranmi T, Ortega-Sanchez IR, Patel M, Martin SW. Use of Serogroup B meningococcal vaccines in adolescents and young adults: recommendations of the advisory committee on immunization practices, 2015. MMWR Morb Mortal Wkly Rep. 2015;64(41):1171–76. doi:10.15585/mmwr.mm6441a3.
- Wu K, Mulholland EK, Edwards KM. Choosing a mass immunization program against meningococcal B. N Engl J Med. 2020;382(4):379–82. doi:10.1056/NEJMclde1916746.
- Rivero-Calle I, Raguindin PF, Gómez-Rial J, Rodriguez-Tenreiro C, Martinón-Torres F. Meningococcal group B vaccine for the prevention of invasive meningococcal disease caused by Neisseria meningitidis serogroup B. Infect Drug Resist. 2019;12:3169.
- Centers for Disease Control and Prevention. Meningococcal disease: surveillance data tables; 2019 [accessed 2021 April 28]. https://www.cdc.gov/meningococcal/surveillance/surveillance-data.html
- Meyer S. Epidemiology of meningococcal disease among college students - United States, 2014-2016. Advisory Committee on Immunization Practices Meeting. Atlanta (GA): Centers for Disease Control and Prevention; February 22, 2018 [accessed 2021 Apr 28]. https://stacks.cdc.gov/view/cdc/59918 .
- Ramsay ME, Andrews NJ, Trotter CL, Kaczmarski EB, Miller E. Herd protection from meningococcal serogroup C conjugate vaccination in England: database analysis. BMJ. 2003;326(7385):365–66. doi:10.1136/bmj.326.7385.365.
- Trotter CL, Gay NJ, Edmunds WJ. Dynamic models of meningococcal carriage, disease, and the impact of serogroup C conjugate vaccination. Am J Epidemiol. 2005;162(1):89–100. doi:10.1093/aje/kwi160.
- Maiden MC, Ibarz-Pavón AB, Urwin R, Gray SJ, Andrews NJ, Clarke SC, Walker AM, Evans MR, Kroll JS, Neal KR, et al. Impact of meningococcal serogroup C conjugate vaccines on carriage and herd protection. J Infect Dis. 2008;197(5):737–43. doi:10.1086/527401.
- Harrison LH, Stephens DS. Good news and bad news — 4CMenB vaccine for group B Neisseria meningitidis. N Engl J Med. 2020;382(4):376–78. doi:10.1056/NEJMe1916440.
- Jackson LA, Baxter R, Reisinger K, Karsten A, Shah J, Bedell L, Dull PM; V59P13 Study Group. Phase III comparison of an investigational quadrivalent meningococcal conjugate vaccine with the licensed meningococcal ACWY conjugate vaccine in adolescents. Clin Infect Dis. 2009;49(1):e1–10. doi:10.1086/599117.
- Reisinger KS, Baxter R, Block SL, Shah J, Bedell L, Dull PM. Quadrivalent meningococcal vaccination of adults: phase III comparison of an investigational conjugate vaccine, MenACWY-CRM, with the licensed vaccine, Menactra. Clin Vaccine Immunol. 2009;16(12):1810–15. doi:10.1128/CVI.00207-09.
- Chang LJ, Hedrick J, Christensen S, Pan J, Jordanov E, Dhingra MS. A Phase II, randomized, immunogenicity and safety study of a quadrivalent meningococcal conjugate vaccine, MenACYW-TT, in healthy adolescents in the United States. Vaccine. 2020;38(19):3560–69. doi:10.1016/j.vaccine.2020.03.017.
- Dhingra MS, Peterson J, Hedrick J, Pan J, Neveu D, Jordanov E. Immunogenicity, safety and inter-lot consistency of a meningococcal conjugate vaccine (MenACYW-TT) in adolescents and adults: a Phase III randomized study. Vaccine. 2020;38(33):5194–201. doi:10.1016/j.vaccine.2020.06.013.
- Weston WM, Friedland LR, Wu X, Howe B. Immunogenicity and reactogenicity of co-administered tetanus–diphtheria–acellular pertussis (Tdap) and tetravalent meningococcal conjugate (MCV4) vaccines compared to their separate administration. Vaccine. 2010;29(5):1017–22. doi:10.1016/j.vaccine.2010.11.057.
- Keyserling H, Papa T, Koranyi K, Ryall R, Bassily E, Bybel MJ, Sullivan K, Gilmet G, Reinhardt A. Safety, immunogenicity, and immune memory of a novel meningococcal (groups A, C, Y, and W-135) polysaccharide diphtheria toxoid conjugate vaccine (MCV-4) in healthy adolescents. Arch Pediatr Adolesc Med. 2005;159(10):907–13. doi:10.1001/archpedi.159.10.907.
- Baxter R, Reisinger K, Block SL, Percell S, Odrljin T, Dull PM, Smolenov I. Antibody persistence after primary and booster doses of a quadrivalent meningococcal conjugate vaccine in adolescents. Pediatr Infect Dis J. 2014;33(11):1169–76. doi:10.1097/INF.0000000000000438.
- Gill CJ, Baxter R, Anemona A, Ciavarro G, Dull P. Persistence of immune responses after a single dose of Novartis meningococcal serogroup A, C, W-135 and Y CRM-197 conjugate vaccine (Menveo®) or Menactra® among healthy adolescents. Hum Vaccin. 2010;6(11):881–87. doi:10.4161/hv.6.11.12849.
- Robertson CA, Hedrick J, Bassily E, Greenberg DP. Persistence of bactericidal antibodies 4 years after a booster dose of quadrivalent meningococcal diphtheria toxoid conjugate vaccine (MenACWY-D). Vaccine. 2019;37(8):1016–20. doi:10.1016/j.vaccine.2019.01.008.
- Robertson CA, Greenberg DP, Hedrick J, Pichichero M, Decker Md, Saunders M. Safety and immunogenicity of a booster dose of meningococcal (groups A, C, W, and Y) polysaccharide diphtheria toxoid conjugate vaccine. Vaccine. 2016;34(44):5273–78. doi:10.1016/j.vaccine.2016.09.003.
- Tipton M, Daly W, Senders S, Block SL, Lattanzi M, Mzolo T, Barbi S, Pellegrini M, Keshavan P. MenACWY-CRM conjugate vaccine booster dose given 4-6 years after priming: results from a phase IIIb, multicenter, open label study in adolescents and adults. Vaccine. 2019;37(42):6171–79. doi:10.1016/j.vaccine.2019.08.065.
- Áñez G, Hedrick J, Simon MW, Christensen S, Jeanfreau R, Yau E, Pan J, Jordanov E, Dhingra MS. Immunogenicity and safety of a booster dose of a quadrivalent meningococcal tetanus toxoid-conjugate vaccine (MenACYW-TT) in adolescents and adults: a Phase III randomized study. Hum Vaccin Immunother. 2020;16(6):1292–98. doi:10.1080/21645515.2020.1733867.
- Soeters HM, Whaley M, Alexander-Scott N, Kanadanian KV, MacNeil JR, Martin SW, McNamara LA, Sicard K, Vanner C, Vuong J, et al. Meningococcal carriage evaluation in response to a serogroup B meningococcal disease outbreak and mass vaccination campaign at a college—Rhode Island, 2015–2016. Clin Infect Dis. 2017;64(8):1115–22. doi:10.1093/cid/cix091.
- Østergaard L, Vesikari T, Absalon J, Beeslaar J, Ward BJ, Senders S, Eiden JJ, Jansen KU, Anderson AS, York LJ, et al. A bivalent meningococcal B vaccine in adolescents and young adults. N Engl J Med. 2017;377(24):2349–62. doi:10.1056/NEJMoa1614474.
- Perrett KP, McVernon J, Richmond PC, Marshall H, Nissen M, August A, Percell S, Toneatto D, Nolan T. Immune responses to a recombinant, four-component, meningococcal serogroup B vaccine (4CMenB) in adolescents: a phase III, randomized, multicentre, lot-to-lot consistency study. Vaccine. 2015;33(39):5217–24. doi:10.1016/j.vaccine.2015.06.103.
- Nolan T, Santolaya ME, de Looze F, Marshall H, Richmond P, Henein S, Rheault P, Heaton K, Perrett KP, Garfield H, et al. Antibody persistence and booster response in adolescents and young adults 4 and 7.5 years after immunization with 4CMenB vaccine. Vaccine. 2019;37(9):1209–18. doi:10.1016/j.vaccine.2018.12.059.
- Østergaard L, Vesikari T, Senders SD, Flodmark CE, Kosina P, Jiang HQ, Maguire JD, Absalon J, Jansen KU, Harris SL, et al. Persistence of hSBA titers elicited by the meningococcal serogroup B vaccine menB-FHbp for up to 4 years after a 2- or 3-dose primary series and immunogenicity, safety, and tolerability of a booster dose through 26 months. Vaccine. 2021;39(32):4545–54. doi:10.1016/j.vaccine.2021.06.005.
- Deceuninck G, Lefebvre B, Tsang R, Betala-Belinga JF, De Serres G, De Wals P. Impact of a mass vaccination campaign against Serogroup B meningococcal disease in the Saguenay-Lac-Saint-Jean region of Quebec four years after its launch. Vaccine. 2019;37(31):4243–45. doi:10.1016/j.vaccine.2019.06.021.
- Read RC, Dull P, Bai X, Nolan K, Findlow J, Bazaz R, Kleinschmidt A, McCarthy M, Wang H, Toneatto D, et al. A phase III observer-blind randomized, controlled study to evaluate the immune response and the correlation with nasopharyngeal carriage after immunization of university students with a quadrivalent meningococcal ACWY glycoconjugate or serogroup B meningococcal vaccine. Vaccine. 2016;35(3):427–34. doi:10.1016/j.vaccine.2016.11.071.
- Marshall HS, McMillan M, Koehler AP, Lawrence A, Sullivan TR, MacLennan JM, Maiden MCJ, Ladhani SN, Ramsay ME, Trotter C, et al. Meningococcal B vaccine and meningococcal carriage in adolescents in Australia. N Engl J Med. 2020;382(4):318–27. doi:10.1056/NEJMoa1900236.
- Ladhani SN, Andrews N, Parikh SR, Campbell H, White J, Edelstein M, Bai X, Lucidarme J, Borrow R, Ramsay ME. Vaccination of infants with meningococcal group B vaccine (4CMenB) in England. N Engl J Med. 2020;382(4):309–17. doi:10.1056/NEJMoa1901229.
- Wheeler CM, Harvey BM, Pichichero ME, Simon MW, Combs SP, Blatter MM, Marshall GS, Catteau G, Dobbelaere K, Descamps D, et al. Immunogenicity and safety of human papillomavirus-16/18 AS04-adjuvanted vaccine coadministered with tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine and/or meningococcal conjugate vaccine to healthy girls 11 to 18 years of age: results from a randomized open trial. Pediatr Infect Dis J. 2011;30(12):e225–34. doi:10.1097/INF.0b013e31822d28df.
- Arguedas A, Soley C, Loaiza C, Rincon G, Guevara S, Perez A, Porras W, Alvarado O, Aguilar L, Abdelnour A, et al. Safety and immunogenicity of one dose of MenACWY-CRM, an investigational quadrivalent meningococcal glycoconjugate vaccine, when administered to adolescents concomitantly or sequentially with Tdap and HPV vaccines. Vaccine. 2010;28(18):3171–79. doi:10.1016/j.vaccine.2010.02.045.
- Velentgas P, Amato AA, Bohn RL, Chan KA, Cochrane T, Funch DP, Dashevsky I, Duddy AL, Gladowski P, Greenberg SA, et al. Risk of Guillain-Barré syndrome after meningococcal conjugate vaccination. Pharmacoepidemiol Drug Saf. 2012;21(12):1350–58. doi:10.1002/pds.3321.
- Myers TR, McNeil MM, Ng CS, Li R, Marquez PL, Moro PL, Omer SB, Cano MV. Adverse events following quadrivalent meningococcal diphtheria toxoid conjugate vaccine (Menactra®) reported to the Vaccine Adverse Event Reporting System (VAERS), 2005-2016. Vaccine. 2020;38(40):6291–98. doi:10.1016/j.vaccine.2020.07.039.
- Tseng HF, Sy LS, Ackerson BK, Hechter RC, Tartof SY, Haag M, Slezak JM, Luo Y, Fischetti CA, Takhar HS, et al. Safety of quadrivalent meningococcal conjugate vaccine in 11- to 21-year-olds. Pediatrics. Jan 2017;139(1). doi:10.1542/peds.2016-2084.
- Ostergaard L, Lucksinger GH, Absalon J, Beeslaar J, Eiden J, Jansen KU, York LJ, Quinn A, Graversen ME, Perez JL. A phase 3, randomized, active-controlled study to assess the safety and tolerability of meningococcal serogroup B vaccine bivalent rLP2086 in healthy adolescents and young adults. Vaccine. 2016;34(12):1465–71. doi:10.1016/j.vaccine.2016.01.044.
- Muse D, Christensen S, Bhuyan P, Absalon J, Eiden JJ, Jones TR, York LJ, Jansen KU, O'Neill RE, Harris SL, et al. A phase 2, randomized, active-controlled, observer-blinded study to assess the immunogenicity, tolerability and safety of bivalent rLP2086, a meningococcal serogroup B vaccine, coadministered with tetanus, diphtheria and acellular pertussis vaccine and serogroup A, C, Y and W-135 meningococcal conjugate vaccine in healthy US adolescents. Pediatr Infect Dis J. 2016;35(6):673–82. doi:10.1097/inf.0000000000001124.
- Senders S, Bhuyan P, Jiang Q, Absalon J, Eiden JJ, Jones TR, York LJ, Jansen KU, O'Neill RE, Harris SL, et al. Immunogenicity, tolerability and safety in adolescents of bivalent rLP2086, a meningococcal serogroup B vaccine, coadministered with quadrivalent human papilloma virus vaccine. Pediatr Infect Dis J. 2016 May;35(5):548–54. doi:10.1097/inf.0000000000001072.
- Duffy J, Marquez P, Dores GM, Ng C, Su J, Cano M, Perez-Vilar S. Safety surveillance of bivalent meningococcal group B vaccine, vaccine adverse event reporting system, 2014-2018. Open Forum Infect Dis. 2020;7(12):ofaa516. doi:10.1093/ofid/ofaa516.
- Turner JC, Keller A. College health surveillance network: epidemiology and health care utilization of college students at US 4-year universities. J Am Coll Health. 2015;63(8):530–38. doi:10.1080/07448481.2015.1055567.
- American College Health Association. Immunization recommendations for college students. [accessed 2021 March 19]. www.acha.org/documents/resources/guidelines/ACHA_Immunization_Recommendations_Oct2018.pdf .
- Patton ME, Stephens D, Moore K, MacNeil JR. Updated recommendations for use of MenB-FHbp serogroup B meningococcal vaccine - advisory committee on immunization practices, 2016. MMWR Morb Mortal Wkly Rep. 2017;66(19):509–13. doi:10.15585/mmwr.mm6619a6.
- Marshall GS, Tan L. Understanding the category B recommendation for serogroup B meningococcal vaccine. Pediatr. 2017 May;139(5). doi:10.1542/peds.2016-3484.
- Centers for Disease Control and Prevention. ACIP shared clinical decision-making recommendations. [accessed 2020 Dec 15]. www.cdc.gov/vaccines/acip/acip-scdm-faqs.html .
- Leeds IL, Namasivayam V, Bamogo A, Sankhla P, Thayer WM. Cost effectiveness of meningococcal serogroup B vaccination in college-aged young adults. Am J Prev Med. 2019;56(2):196–204. doi:10.1016/j.amepre.2018.09.020.
- Chung GS, Hutton DW. Epidemiological impact and cost-effectiveness of universal meningitis b vaccination among college students prior to college entry. PLoS One. 2020;15(10):e0239926. doi:10.1371/journal.pone.0239926.
- Centers for Disease Control and Prevention. Guidance for the evaluation and public health management of suspected outbreaks of meningococcal disease. Version 2.0. September 28, 2019. [accessed 2021 April 28]. https://www.cdc.gov/meningococcal/downloads/meningococcal-outbreak-guidance.pdf.
- Elam-Evans LD, Yankey D, Singleton JA, Sterrett N, Markowitz LE, Williams CL, Fredua B, McNamara L, Stokley S. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years - United States, 2019. MMWR Morb Mortal Wkly Rep. 2020;69(33):1109–16. doi:10.15585/mmwr.mm6933a1.
- Shaw J, Mader EM, Bennett BE, Vernyi-Kellogg OK, Yang YT, Morley CP. Immunization mandates, vaccination coverage, and exemption rates in the United States. Open Forum Infect Dis. 2018 Jun 2;5(6):ofy130. doi:10.1093/ofid/ofy130.
- Franco M, Mazzucca S, Padek M, Brownson RC. Going beyond the individual: how state-level characteristics relate to HPV vaccine rates in the United States. BMC Public Health. 2019;19(1):246–246. doi:10.1186/s12889-019-6566-y.
- Bradford WD, Mandich A. Some state vaccination laws contribute to greater exemption rates and disease outbreaks in the United States. Health Aff (Millwood). 2015;34(8):1383–90. doi:10.1377/hlthaff.2014.1428.
- Niccolai LM, Yakely AE, Hansen CE. Up-to-date coverage with meningococcal vaccine among adolescents age 17 years: patterns and correlates in the United States, 2017. Vaccine. 2019;37(40):5934–38. doi:10.1016/j.vaccine.2019.08.015.
- Immunization Action Coalition. State mandates on immunization and vaccine-preventable diseases. February 19, 2020. [accessed 2021 Mar 19]. www.immunize.org/laws/#inf .
- National Meningitis Association. NMA advocacy. February 4, 2017. [accessed 2021 Mar 19]. https://nmaus.org/nma-advocacy/.
- Haimowitz R, Torres R, Caleb S, Thompson D, Smith A, Ciotoli C, Dannenbaum M, Fu LY. Serogroup B meningococcal vaccination practice patterns on college campuses. Vaccine. 2020. doi:10.1016/j.vaccine.2020.09.035.
- National Vaccine Advisory Committee (NVAC). Standards for adult immunization practice. May 2, 2016. [accessed 2021 Apr 9]. www.cdc.gov/vaccines/hcp/adults/for-practice/standards/index.html .
- Daly KL, Halon PA, Aronowitz T, Ross G. A university health initiative to increase human papillomavirus vaccination rates. J Nurse Pract. 2016;12(6):e281–e286. doi:10.1016/j.nurpra.2016.02.013.
- Monn J. An evidence-based project to improve influenza immunization uptake. J Nurse Pract. 2016;12:e159–e162.
- Fu LY, Smith A Ciotoli C, Dannenbaum M, Jacobs M. An immunization quality improvement learning collaborative in the college health setting [In press]. J Am Coll Health. doi:10.1080/07448481.2021.1979560.
- Caleb S, Thompson D, Haimowitz R, Ciotoli C, Dannenbaum M, Fu LY. How colleges intervene to increase student body vaccination coverage. J Am Coll Health. May 2020:1–8. doi:10.1080/07448481.2020.1752698.
- Abelson J, Dungca N, Kornfield M, Tran A. At college health centers, students battle misdiagnoses and inaccessible care. The Washington Post. July 13, 2020.
- American College Health Association. 2010–2011 College health salary and staffing survey report; 2011. Silver Spring (MD): American College Health Association; 2011.
- Oliver SE, Patton ME, Hoban M, Leino V, Mbaeyi SA, Hariri S, MacNeil JR. Evaluation of meningococcal vaccination policies among colleges and universities - United States, 2017. J Am Coll Health. 2019;1–6. doi:10.1080/07448481.2019.1687484.
- Centers for Disease Control and Prevention. COVID-19: considerations for institutions of higher education. December 31, 2020. [accessed 2021 Mar 24]. www.cdc.gov/coronavirus/2019-ncov/community/colleges-universities/considerations.html .
- American College Health Association. COVID-19 resources. March 24, 2021. [accessed 2021 Mar 24]. www.acha.org/COVID-19.
- American College Health Association. The COVID-19 pandemic’s effect on campus health and well-being services. Report #3, August 4-7, 2020. [accessed 2021 Sep 1]. www.acha.org/documents/Resources/COVID_19/COVID-19_Effect_On_Campus_Health_Services_REPORT_3_August_2020.pdf .
- Centers for Disease Control and Prevention. CDC vaccine price list. March 4, 2021. [accessed 2021 Mar 31]. www.cdc.gov/vaccines/programs/vfc/awardees/vaccine-management/price-list/index.html .
- Huang L, Goren A, Lee LK, Li VW, Dempsey A, Srivastava A. Disparities in healthcare providers’ interpretations and implementations of ACIP’s meningococcal vaccine recommendations. Hum Vaccin Immunother. 2019:1–12. doi:10.1080/21645515.2019.1682845.
- Sponsler BA, Egelman G. The impact of the affordable care act on campus healthcare services. July 2013. [accessed 2021 Apr 28]. www.naspa.org/files/dmfile/RPI_Policy_Brief_Affordable_Care_Act_July.13.pdf .
- American College Health Association. Standards for student health insurance/benefits coverage. July 2020. [accessed 2021 Apr 28]. www.acha.org/documents/resources/guidelines/ACHA_Standards_SHIBPs_July2020.pdf .
- American College Health Association. Update: concerns for colleges promoting students short-term limited duration insurance or other plans that are not compliant with the affordable care act [accessed 2021 Mar 24]. www.acha.org/documents/ACHA_Update_STLDI.pdf .
- Affordable Colleges Online. Student guide to health insurance plans. March 24, 2021 [accessed 2021 Apr 28]. www.affordablecollegesonline.org/college-resource-center/student-health-insurance/ .
- 111th Congress. Patient protection and affordable care act. Public Law 111-148, March 23, 2010.
- Fawole OA, Srivastava T, Fasano C, Feemster KA. Evaluating variability in immunization requirements and policy among U.S. colleges and universities. J Adolesc Health. 2018;63(3):286–92. doi:10.1016/j.jadohealth.2018.06.013.
- Noesekabel A, Fenick AM. Immunization requirements of the top 200 universities: implications for vaccine-hesitant families. Vaccine. 2017;35(29):3661–65. doi:10.1016/j.vaccine.2017.05.038.
- Jewett A, Bell T, Cohen NJ, Buckley K, Leino EV, Even S, Beavers S, Brown C, Marano N. US college and university student health screening requirements for tuberculosis and vaccine-preventable diseases, 2012. J Am Coll Health. 2016;64(5):409–15. doi:10.1080/07448481.2015.1117465.
- Sandler K, Srivastava T, Fawole OA, Fasano C, Feemster KA. Understanding vaccine knowledge, attitudes, and decision-making through college student interviews. J Am Coll Health. 2019:1–8. doi:10.1080/07448481.2019.1583660.
- Ravert RD, Fu LY, Zimet GD. Reasons for low pandemic H1N1 2009 vaccine acceptance within a college sample. Adv Prev Med. 2012:242518. doi:10.1155/2012/242518.
