ABSTRACT
In early 2020, the World Health Organization (WHO) declared the coronavirus disease 2019 (COVID-19) outbreak a global pandemic. In response, two novel messenger RNA (mRNA)-based vaccines: mRNA-1273 (Moderna) and BNT162b2 (Pfizer-BioNTech) were rapidly developed. A thorough understanding of the differences in workflow requirements between the two vaccines may lead to improved efficiencies and reduced economic burden, both of which are crucial for streamlining vaccine deployment and minimizing wastage. Vaccine administration workflow costs are borne by providers and reimbursed separately from dose acquisition in the United States. Currently, mRNA-1273 and BNT162b2 are the most administered COVID-19 vaccines in the United States. In this study, US-licensed and practicing pharmacists were interviewed to collect data on differences in terms of labor costs associated with the workflows for mRNA-1273 and BNT162b2. Results suggest the cost differential for mRNA-1273 compared to BNT162b2 is −$0.82 (or −$1.01 when assuming volume equivalency). If extrapolated to even just a proportion of the remaining unvaccinated US population, this can amount to significant workflow efficiencies and lower vaccine administration costs. Further, as key differences in the vaccine workflow steps between the two vaccines would be similar in other settings/regions, these findings are likely transferable to health-care systems worldwide.
CLASSIFICATION:
On March 11, 2020, the World Health Organization (WHO) declared the coronavirus disease 2019 (COVID-19) outbreak a global pandemic.Citation1 After genomic sequencing of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of COVID-19, messenger RNA (mRNA)-based vaccines were developed by Moderna (mRNA-1273) and Pfizer-BioNTech (BNT162b2).Citation2,Citation3 In December 2020, both vaccines were authorized by the US Food and Drug Administration for Emergency Use Authorization (EUA).Citation4 A third COVID-19 vaccine, an adenovirus vector vaccine manufactured by Janssen Biotech, was also granted EUA on February 27, 2021.Citation5
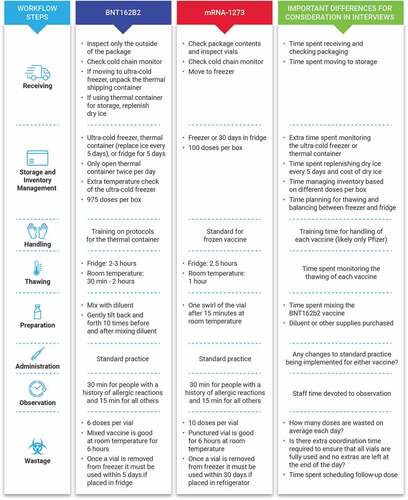
mRNA vaccines offer several potential advantages over conventional whole virus or recombinant protein-based vaccines, including ease and speed of manufacturing, scalability, reliability, and flexibility of antigen design.Citation6 However, these vaccines have important workflow requirements, including storage at very low and consistent temperatures (−20°C for mRNA-1273 and −70°C for BNT162b2), thawing processes, and reconstitution/mixing with diluent (BNT162b2 only); similarities and differences in workflow between the mRNA-1273 and BNT162b2 are summarized in .Citation7,Citation8 A greater understanding of the differences in workflow requirements between mRNA-1273 and BNT162b2, including the relative time burden to staff and financial burden to healthcare payers, may lead to improved efficiencies and reduced economic burden.
This study, based on interviews with US-licensed and practicing pharmacists, quantifies the differences in terms of labor costs associated with the workflows for the first two COVID-19 vaccines (mRNA-1273 and BNT162b2) to be approved for emergency use in the United States.Citation9 Currently, the purchase price of all COVID-19 vaccines is fully covered by the US Government; however, reimbursement applications are required by vaccine providers for the fees associated with vaccine administration.Citation10,Citation11 It is thus imperative to focus on not only the acquisition costs but also the labor and time costs associated with mRNA-1273 and BNT162b2. It is hoped that the information gained from this study informs vaccine providers of potential costs associated with vaccine administration, which are reimbursed separately from vaccine acquisition in the United States.
The data collection for this self-report time-based study was conducted based on interviews with US-licensed and practicing pharmacists; participating pharmacists had 2–30 y of dispensing experience working primarily in a hospital setting, were directly involved in all or most of the components of the vaccine workflow, and had direct experience with both mRNA-1273 and BNT162b2. Participants were excluded if they were working in Vermont or Massachusetts due to local reporting requirements.
In-depth hour-long telephone interviews were conducted on participants selected from a nationwide panel of hospital pharmacists. Screening and interviews were double-blinded. The study was deemed to be research not involving human subjects by the Institutional Review Board (Research Triangle Institute [RTI]). Respondents were asked to provide an estimate of the time spent beyond standard vaccination procedures on workflow steps for both mRNA-1273 and BNT162b2 using open-ended questions. Eight workflow steps from the clinical perspective were examined, all of which bore costs that were the responsibility of the vaccine provider ().
The total time spent on each step of the workflow is not directly comparable due to the time spent being dependent on whether the action is performed once per shipment, day, vial preparation, or dose. Workflow steps that occur once per day or once per shipment and do not vary based on the number of doses given are referred to as fixed-costs (eg, checking storage temperatures). Therefore, to be able to compare the two products, costs were standardized for each product to the amount of time required per dose (). The mean time per dose was then summed across all workflow steps, enabling the calculation of the difference in the total workflow times between the vaccines. To estimate cost/dose differences, time/dose differences were summed across all steps and multiplied by the median US wage of hospital pharmacy and clinic staff based on Bureau of Labor Statistics data.Citation12,Citation13
Table 1. Denominator and volume metrics used to convert the time of each workflow step to time/dose
As the number of doses administered each day impacts the per-dose cost of fixed-cost workflow steps and because the number of doses of each product may change over time, sensitivity analyses were performed that examined the impact of alternative numbers of doses administered/day on differences in cost/dose. Ten alternative volume scenarios between 100 and 1000 doses/day for each vaccine were evaluated.
Ten pharmacists practicing in academic hospitals (n = 2), large hospital systems (n = 4), and community hospitals (n = 4) completed the interviews between February 11, 2021, and February 16, 2021. Respondents were based in North Dakota, North Carolina, Missouri, Florida, California, Texas, Georgia, and Indiana, and had a mean of 19 years’ experience (range: 8 − 29 y). Respondents reported that workflow steps were primarily completed by pharmacists; therefore, the median pharmacist wage based on the Bureau of Labor Statistics data was applied ($89.77/hour adjusted for relevant employment benefits).Citation12,Citation13
Unadjusted analyses indicated that BNT162b2 took more time than mRNA-1273 for each workflow step excluding managing thawing (). Analyses indicated that there was substantial time spent training all staff in the organization for both vaccines; there was more training required for BNT162b2 than mRNA-1273. For administration and observation, respondents reported no difference from standard vaccination for either product.
Table 2. Mean time and cost spent on each vaccine workflow step
The sensitivity analyses indicated that at the sample average, the number of daily doses of BNT162b2 and mRNA-1273 was approximately 400 and 300, respectively (), with BNT162b2 costing $0.87/dose more than mRNA-1273. Assuming volume equivalency (400 doses/day), BNT162b2 cost $1.01 more per dose than mRNA-1273. The difference in cost between the two products narrows as the ratio of BNT162b2 volume to mRNA-1273 volume increases to the point where the difference flips to favor BNT162b2 at 300 doses/day when mRNA-1273 is administered at 100 doses/day. The additional cost of BNT162b2 substantially increases as the ratio of BNT162b2 to mRNA-1273 doses decreases, almost tripling when BNT162b2 volume is 100 doses/day, and mRNA-1273 volume is 1000 doses/day.
Table 3. Sensitivity analysis of alternative scenarios of vaccine doses administered per day
This study aimed to determine the labor and time costs associated with mRNA-1273 and BNT162b2, which at the time of this investigation were the only COVID-19 vaccines that had received EUA in the United States. Vaccine workflow time and cost analysis based on interviews with US-licensed and practicing pharmacists indicates that as of February 16, 2021, the cost of hospital pharmacist’s time spent on BNT162b2 was $0.82 more per dose than mRNA-1273. The primary driver of this difference was the time required for reconstituting/mixing BNT162b2 with diluent. Sensitivity analysis testing alternative vaccine volume scenarios found that the results were sensitive to changes in volume; when assuming volume equivalency (400 doses/day), BNT162b2 costs $1.01 more per dose than mRNA-1273.
After completion of this study, changes in workflow steps were approved for both BNT162b2 (eg, change in storage temperature from −80°C to −60°C) and mRNA-1273 (eg, increased extractable doses per vial).Citation14,Citation15 As the workflow for these vaccines is constantly evolving, additional changes in workflow since the time of the study may affect results; however, as the reconstitution/mixing with diluent step was the major cost driver identified for BNT162b2, alterations in this step would likely be required to alter the conclusions of this analysis.
There are important study limitations that must also be considered when interpreting these results. Due to the small sample size and the exclusive use of pharmacists from hospital-based settings, the translation of these findings to community/retail pharmacies and physician offices may be challenging. In addition, the US-specific wages applied to the cost analysis limits application of findings to healthcare systems of other countries. While the cost of vaccine acquisition for each country can vary significantly,Citation16,Citation17 key differences in the vaccine workflow steps between the two vaccines (reconstitution/mixing with diluent) were identified that are transferable across all settings/regions. Moreover, as this study was conducted solely in a hospital pharmacy setting, results likely underestimate the cost differences if smaller retail/rural pharmacies were to be included, due to lack of medical-grade freezers at many of these facilities.
Another key limitation of this study is the use of reported versus direct observations that could be obtained through time and motion measurements. However, applying such an approach was not conducive to the time-sensitive nature of this research during the height of the initial US COVID-19 vaccine rollout. That said, findings are consistent with several recent time and motion vaccine workflow studies revealing similar time savings with reduced vaccine reconstitution or preparation steps (~33 seconds).Citation18–21 In addition, other vaccine administration cost studies produced estimates ranging between $5 and $14/dose,Citation22–25 which help contextualize these results by showing the relative increase in cost of the additional workflow requirements of each vaccine over and above standard practice. Compared with results from these studies, the $1.64/dose allocated to time for BNT162b2 on extra workflow processes beyond standard requirements represents a 12% to 20% increase in time cost. The $0.82 spent in time for mRNA-1273 on the extra workflow processes beyond standard requirements represents a 6% to 10% increase in time cost. The estimated average overall difference in time cost between BNT162b2 and mRNA-1273 ($0.82) represents a 6% to 10% increase in time cost from standard practice. A better understanding of costs associated with vaccine workflow is important for future health economic evaluation of vaccination strategies.
Extrapolating these results to mass vaccination rollouts across the United States suggests the potential for substantial vaccination administration cost savings associated with mRNA-1273. As of the first week of April 2021, national surveillance data indicated that 112 million people, or 34% of the US population, received at least 1 dose of a COVID-19 vaccine, with up to 66 million (20%) fully vaccinated with either 2 shots of BNT162b2 or mRNA-1273 or 1 shot of Janssen COVID-19 vaccine. Given the challenges with the Janssen COVID-19 vaccine rollout,Citation26,Citation27 mRNA-1273 and BNT162b2 continue to drive COVID-19 vaccinations in the United States. The vaccination administration cost differential of −$0.82 associated with mRNA-1273 (or −$1.01 when assuming volume equivalency) when extrapolated to even just a proportion of the remaining unvaccinated US population can amount to significant workflow efficiencies and hundreds of millions of dollars of savings.
These estimates may also have important implications for reimbursement policy. In the United States, vaccine administration is reimbursed separately from the vaccine dose. Because policymakers seeking to maximize vaccination coverage want to encourage providers to administer large quantities of vaccines, it is essential to ensure vaccine administration reimbursement accounts for potential provider costs. Finally, US pharmacy decision-makers in the current environment have no choice of COVID-19 vaccine; hence, these findings are particularly relevant to inform their decisions given that vaccine product choice is anticipated in the future. Of note, vaccine prices in the United States for mRNA-1273 and BNT162b2 are expected to be similar once these products receive full market authorization and are commercially available, thus re-emphasizing the importance of understanding the labor and time costs associated with mRNA-1273 and BNT162b2 workflows.
As SARS-CoV-2 is still circulating at very high rates, vaccination remains our most powerful weapon against COVID-19.Citation28 Due to ease and speed of manufacturing, flexibility of antigen design, and the overall safety and efficacy profile, mRNA vaccines will likely remain a cornerstone of pandemic management. This study, based on interviews with US-licensed pharmacists, provides a greater understanding of workflow costs for mRNA-1273 and BNT162b2, which may lead to improved efficiencies and reduced economic burden for providers during the COVID-19 pandemic. Although this is a US-based study, the key difference in the vaccine workflow steps between the two vaccines (ie, reconstitution/mixing with diluent) would be similar to other settings/regions, making these findings likely transferable to healthcare systems across the globe.
Author contributions
BY conceptualized the study design, analyzed and interpreted the data, wrote and reviewed the manuscript, and approved the final version for submission. SB, EC, and POB wrote and reviewed the manuscript and approved the final version for submission.
Disclosure of potential conflicts of interest
Philip O. Buck is an employee of Moderna, Inc. and may hold stock/stock options. Benjamin Yarnoff, Steven Bodhaine, and Ed Cohen do not have any conflicts of interest to report.
Acknowledgments
The authors would like to thank Alicia Gilsenan, RTI International, for her input to the study.
Additional information
Funding
References
- Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91:157–60.
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–15. doi:10.1056/NEJMoa2034577.
- Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–16. doi:10.1056/NEJMoa2035389.
- United States Food and Drug Administration (U.S. FDA). Moderna COVID-19 vaccine; 2021.
- United States Food and Drug Administration (U.S. FDA). Janssen COVID-19 vaccine; 2020.
- Jackson NAC, Kester KE, Casimiro D, Gurunathan S, DeRosa F. The promise of mRNA vaccines: a biotech and industrial perspective. NPJ Vaccines. 2020;5:11. doi:10.1038/s41541-020-0159-8.
- (CDC) CfDCaP. Pfizer-BioNTech COVID-19 vaccine; 2021.
- Centers for Disease Control and Prevention (CDC). Moderna COVID-19 vaccine; 2021.
- Nature News. Moderna COVID vaccine becomes second to get US authorization; 2020.
- CDC. CDC COVID-19 vaccination program provider requirements and support; 2021 [accessed 2021 July 27]. https://www.cdc.gov/vaccines/covid-19/vaccination-provider-support.html.
- CDC. How to enroll as a COVID-19 vaccination provider; 2021 [accessed 2021 July 27. https://www.cdc.gov/vaccines/covid-19/provider-enrollment.html.
- U.S. Bureau of Labor Statistics. Occupational employment and wages, May 2020; 2020.
- U.S. Bureau of Labor Statistics. Employer costs for employee compensation historical listing; 2020.
- Administration USfd. Coronavirus (COVID-19) update: FDA makes two revisions to Moderna COVID-19 vaccine emergency use authorization to help increase the number of vaccine doses available; 2021.
- Administration USfd. Coronavirus (COVID-19) update: FDA allows more flexible storage, transportation conditions for Pfizer-BioNTech COVID-19 vaccine; 2021.
- UNICEF. COVID-19 vaccine market dashboard; 2021 [accessed 2021 July 26]. https://www.unicef.org/supply/covid-19-vaccine-market-dashboard.
- Dyer O. Covid-19: countries are learning what others paid for vaccines. BMJ. 2021;372:n281. doi:10.1136/bmj.n281.
- De Coster I, Fournie X, Faure C, Ziani E, Nicolas L, Soubeyrand B, Van Damme P. Assessment of preparation time with fully-liquid versus non-fully liquid paediatric hexavalent vaccines. A time and motion study. Vaccine. 2015;33:3976–82. doi:10.1016/j.vaccine.2015.06.030.
- Icardi G, Orsi A, Vitali Rosati G, Tognetto A, Checcucci Lisi G, Parisi S. Preferences of healthcare professionals regarding hexavalent pediatric vaccines in Italy: a survey of attitudes and expectations. J Prev Med Hyg. 2020;61:E424–E44.
- Lloyd AJ, Nafees B, Ziani E, Nicolas L, Fordham BA, Soubeyrand B, Bornhöft, C. What are the preferences of health care professionals in Germany regarding fully liquid, ready-to-use hexavalent pediatric vaccine versus hexavalent pediatric vaccine that needs reconstitution? Patient Prefer Adherence. 2015;9:1517–24.
- Pereira CC, Bishai D. Vaccine presentation in the USA: economics of prefilled syringes versus multidose vials for influenza vaccination. Expert Rev Vaccines. 2010;9:1343–49. doi:10.1586/erv.10.129.
- Glazner JE, Beaty B, Berman S. Cost of vaccine administration among pediatric practices. Pediatrics. 2009;124:S492–S8. doi:10.1542/peds.2009-1542H.
- Glazner JE, Beaty BL, Pearson KA, Berman S. The cost of giving childhood vaccinations: differences among provider types. Pediatrics. 2004;113:1582–87. doi:10.1542/peds.113.6.1582.
- Shen A, Khavjou O, King G, Bates L, Zhou F, Leidner AJ, Yarnoff B. Provider time and costs to vaccinate adult patients: impact of time counseling without vaccination. Vaccine. 2019;37:792–97. doi:10.1016/j.vaccine.2018.12.045.
- Yarnoff B, Kim D, Zhou F, Leidner AJ, Khavjou O, Bates L, Bridges CB. Estimating the costs and income of providing vaccination to adults and children. Med Care. 2019;57:410–16. doi:10.1097/MLR.0000000000001117.
- NPR. Johnson & Johnson says contractor botched part of vaccine production; 2021.
- Administration USFB. Joint CDC and FDA statement on Johnson & Johnson COVID-19 vaccine; 2021.
- Statista. COVID-19 deaths worldwide as of April 8, 2021, by country; 2021.