ABSTRACT
Tuberculosis (TB) has been a major public health problem worldwide, and the Bacillus Calmette–Guérin (BCG) vaccine is the only available vaccine against this disease. The BCG vaccine is no longer a single organism; it consists of diverse strains. The early-shared strains of the BCG vaccine are stronger immunostimulators than the late-shared strains. In this study, we have employed a simple in vitro human model to broadly evaluate the differences among four widely used BCG vaccines during the characterization of strain-specific host immune responses. In general, the BCG Moreau vaccine generated a higher inflammatory cytokine profile and lower TGF-β levels compared with the Russia, Pasteur, and Danish strains in the context of early sensitization with TB; however, no changes were observed in the IL-23 levels between infected and noninfected cultures. Unsurprisingly, the BCG vaccines provided different features, and the variances among those strains may influence the activation of infected host cells, which ultimately leads to distinct protective efficacy to tackle TB.
1. Introduction
Along with the recent coronavirus disease 2019 (COVID-19) pandemic, tuberculosis (TB) has also been a major public health problem worldwide. Since 2003, TB has remained as the leading cause of mortality due to an infectious pathogen, namely Mycobacterium tuberculosis, but this scenario is about to change shortly because by the time this text was written, COVID-19 deaths toll ranked close on (https://www.reddit.com/r/Coronavirus_COVID_19/comments/hgj86d/average_disease_deaths_per_day_worldwide_jun_2020/). However, COVID-19 is an epidemic, but TB has been endemic since the human modern history.
In 2019, an estimated 10 million people (88% adults, 56% male, and 8.2% people living with human immunodeficiency virus (HIV)) fell ill with TB. In addition, there has been an estimated 1.2 million TB deaths among HIV-negative people and 208,000 deaths among those who are HIV positive. The death toll from this infectious disease is still the highest among other diseases; thus, efforts to combat it must be accelerated.Citation1
TB is the classic model of cellular immunity, but both cell-mediated immunity (CMI) and humoral immune responses are implicated in protection against the disease.Citation2 Owing to the considerable complexity of TB and the highly heterogeneous nature of its progression, it has been difficult to demarcate different cytokines, chemokines, and types of effector T cells into protective versus destructive immune responses. Moreover, there is still a significant level of unawareness of the quantitative and qualitative relationships between T cell responses and their magnitude.
The live and attenuated Bacillus Calmette–Guérin (BCG) vaccine, originally derived from a serial passage of a virulent strain of M. bovis, has been the only available vaccine against TB since its introduction in 1921. It is most effective in preventing pediatric TB, miliary TB, and tuberculous meningitis.Citation3 However, it has a limited effect in preventing pulmonary TB, which occurs more frequently in adults.Citation4,Citation5 The protective efficacy of the present BCG vaccine against pulmonary TB ranges from 0%–85% in different clinical trials worldwide.Citation6 There have been several explanations for its variable efficacy, such as the hypothesis that prior infection with environmental mycobacteria in adults influences the subsequent efficacy of the BCG vaccine and the absence of antigens that are protective against M. tuberculosis.Citation6–9 The essence of such a hypothesis is that prior infection with some Mycobacterium species enhances the effect of BCG, whereas other species interfere.Citation10 In fact, guinea pigs vaccinated with BCG were found to have been protected against M. tuberculosis as early as 15 days postinfection. Cytokine analysis by qRT-PCR also revealed an increase in proinflammatory cytokine, indicating Th1-biased immune responses in BCG-vaccinated animals.Citation11
The BCG vaccine is no longer a single organism; it consists of genotypically and phenotypically different strains (reviewed byCitation12). Evolutionarily, the BCG vaccine was divided into “early-shared strains” (Group I) and more attenuated “late-shared strains” (Groups II to IV), with Group I strains being more effective than the others.Citation13,Citation14 Brazil has used BCG Moreau vaccine (Group I) to immunize neonates from 1930 to 2018, and some important data have been collected.Citation3 In 2012, this strain was listed as a WHO BCG Reference Reagent in an international collaborative study approved by the WHO Expert Committee on Biological Standardization.Citation15
There are substantial variations in the immunogenicity and specific immune responses of several BCG vaccine strains.Citation16,Citation17 Subsequent to the immunological characterization of most BCG vaccines, the early-shared strains (Moreau, Russia, Japan, Sweden, and Birkhaug) exhibited stronger induction activities of nitric oxide, IL-1β, IL-6, IL-8, IL-12, and TNF-α from A549 and THP-1 human cell lines in the presence of IFN-γ than did the late-shared strains (Danish, Pasteur, and others).Citation18 Thus, the early-shared strains appeared to be more potent immunostimulants than the late-shared strains. This could be attributed partially to several long fatty acids found in the cell wall of the BCG vaccine.Citation18 In addition, another study has demonstrated that in vivo immune responses to the BCG vaccine differ by strain, which may account for the variable outcomes of BCG immunization.Citation19 A given BCG strain may multiply and persist longer in organs after vaccination and this may be followed by different antigen priming and stimulation of T cell responses.Citation16 Remarkably, there is a lack of induction of CD8 + T cells following immunization with the BCG Japan. In addition, BCG Prague and BCG Japan were unable to protect mice against challenge whereas Glaxo, Pasteur, or Russia BCG strains eliminated mycobacteria very efficiently.Citation16 Also, BCG Danish immunized infants showed the highest IFN-γ, IL-13 and IL-10 levels. Conversely, clinical score differences, such as mortality were not significant.Citation17 These differences may play a role in BCG vaccination efficiency. In contrast, meta-analyses of human studies have also demonstrated that the effects of different BCG strains are comparable.Citation20,Citation21
To validate the hypothesis that the BCG Moreau vaccine is more effective than the other strains in terms of the induction of proinflammatory profile, we tested four widely used BCG vaccines in an in vitro human model in this study: BCG Moreau and BCG Russia (Group I), BCG Danish (Group III), and BCG Pasteur (Group IV) strains. Although BCG Russia may be much less effective than BCG Danish and BCG Japan (Group I),Citation22 since 2018, Brazil has been using BCG made from a seed strain of BCG Russia.Citation23 However, little is known about BCG Russia protective properties or its immune response in comparison with the other BCG strains, particularly the BCG Moreau vaccine. Although BCG Moreau induces a strong delayed-type hypersensitivity reaction in skin tests, relatively few in vitro studies have investigated the origin of this protective response (reviewed byCitation12).
2. Subjects, materials, and methods
The specimens tested in this study were collected from healthy adult donors (HD; n = 27) at a public blood bank (anonymous donations from individuals aged ≥18 years). Some samples were also used in a previous study to test BCG in vitro T cell immune responses.Citation24 Since 1967, Brazil has had a policy of providing universal BCG vaccination soon after birth. Although we acknowledge that maternal BCG scar rather than just a history of receipt of BCG is likely to modify an infant’s response to BCG,Citation25 and hence the importance of the BCG, as well as other mycobacteria exposure in a given population, we were unable to determine the BCG status of our cohort (or even to perform additional tests) due to the blood bank depository guidelines. The IOC-Fiocruz (protocol # 35775014.0.0000.5248) institutional review board approved the study procedures.
PBMCs were separated within no more than 24 h (average of 5 h) of obtaining blood specimens from all the study participants and cultured as described elsewhere.Citation24 In vitro infections of freshly isolated PBMCs with the BCG Moreau (at a single dose, individual batches of sealed glass vials containing liquid suspension with approximately 1 × 107 viable bacilli), Pasteur, Danish, or Russia strains (both at a single dose, individual batches of vials containing frozen suspensions with approximately 1 × 107 viable bacilli each) were performed at a multiplicity of infection (MOI) of 2:1 (bacilli/host cell). No difference was observed between the aforementioned frozen and liquid lots, or MOI.Citation24 The respective master batches were used once for each infection and then subsequently discarded. The BCG Pasteur, Danish, and Russia ampoules were thawed shortly before the infection of cells. Cultured PBMCs (1 × 106 cells each) in RPMI medium (Sigma Immunochemicals, USA) supplemented with 10% human AB serum were stored at 37°C in a humidified 5% CO2 atmosphere in individual 12 × 75 mm sterile polystyrene tubes (Falcon, Corning Inc., USA) for 48 h. Subsequently, the supernatants were stored at −70°C for further use in cytokine detection assays.
Except for the BCG Russia, analysis of the concentrations of human TGF-β, IL-1β, IL-6, IL-17, IL-23, and IFN-γ was included as part of our previous enzyme-linked immunosorbent assay (ELISA) and multiarray cytokine detection and induced by three BCG vaccine strains.Citation24 Cell-free supernatants were thawed once and subsequently assayed to determine the concentrations of human IL-1β (lower detection limit (DL): 0.4 pg/mL and upper DL: 2,877 pg/mL), IL-6 (lower DL: 3.1 pg/mL and upper DL: 2,539 ng/mL), IL-17 (lower DL: 1.2 pg/mL and upper DL: 6,631 pg/mL), and IFN-γ (lower DL: 0.7 pg/mL and upper DL: 11,377 pg/mL) using the Bio-Plex protein multiarray system kit, and Luminex-based technology was adopted (Bio-Rad, USA). Correspondingly, we performed ELISA on human IL-23 (lower DL: 125 pg/mL and upper DL: 8,000 pg/mL) and TGF-β (lower DL: 31.2 pg/mL and upper DL: 2,000 pg/mL) using commercial kits (R&D Systems, USA) according to the manufacturer’s instructions.
The cytokine production values are reported as median and interquartile range. The normality of the distribution of the variables was tested using the Shapiro–Wilk tests and Q–Q plots. Between-group comparisons were performed using the Kruskal–Wallis test followed by Dunn’s post hoc test. In addition, Spearman’s rank correlation coefficient was adopted to analyze the correlation between the cytokine level and BCG strain using GraphPad InStat 8.0 (GraphPad Software Inc., La Jolla, USA). P < .05 was considered statistically significant.
3. Results
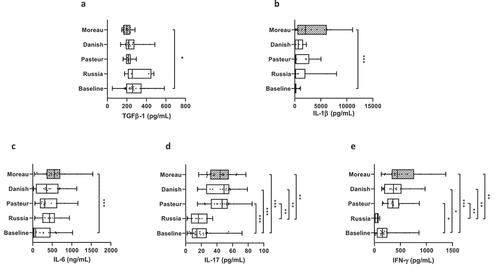
Better characterization of strain-specific host immune responses via comparison among the four widely used BCG strains (Moreau, Pasteur, Danish, and Russia) may facilitate in the development of rational approaches to improve the BCG vaccine. To achieve this, we conducted a merely cross-sectional, single-center cohort study. After appropriate sampling and in vitro stimulations, we simultaneously measured six cytokines from HD by multiparametric Luminex for IL-1β, IL-6, IL-17, and IFN-γ and ELISA for IL-23 and TGF-β. A lower level of TGF-β was detected only in BCG Moreau-infected culture compared with the matched medium rest background (baseline) immune responses (p < .03; ). Conversely, higher levels of IL-1β and IL-6 were detected exclusively in BCG Moreau-infected culture compared with baseline (p < .001; , respectively). Similarly, the IL-17 levels of BCG Moreau-, Pasteur-, and Danish-infected cultures were found to be higher compared with baseline (p < .001), as well as compared with the level of BCG Russia-infected culture (p < .006; ). Finally, the IFN-γ levels of BCG Moreau- and Danish-infected cultures were found to be higher compared with baseline (p < .03), as well as compared with the level of BCG Russia-infected culture (p < .001; ). No differences were observed in the IL-23 levels between infected and noninfected cultures (data not shown).
Figure 1. (A) TGF-β, (B) IL-1β, (C) IL-6, (D) IL-17 and (E) IFN-γ levels (pg/mL, except C ng/mL) in healthy donors (n = 27) representing the baseline, uninfected cells, and the BCG Moreau (hatched), Pasteur, Danish, and Russia strains in 48 h in vitro infection of human mononuclear cells. Box and whiskers plot denote the median and the interquartile range cytokine values in each condition. *p ≤ .05; ***p ≤ .0005 compared with baseline, and ##p ≤ .005 compared with BCG Russia, according to Dunn’s paired test.
According to a very recent study, the networks of cytokine correlations are quite complicated both in patient and in healthy control groups, and correlation analysis among the cytokines may explain the essential differences between them.Citation26 Thus, Spearman correlations of changes of four cytokines from all infected conditions demonstrated a positive correlation between IFN-γ and both the IL-1β (data below) and IL-17 levels (), only when relating to BCG Russia. Also, all infected conditions demonstrated a positive correlation between IFN-γ and the IL-17 levels as well as IL-6 and both IFN-γ and IL-17 levels, except for the IL-17 levels using the Russia strain (). Finally, the IL-1β levels exhibited a distinct pattern when compared with IL-6 (rho = 0.79, p < .01; rho = 0.91, p < .001; and rho = 0.51, p = ns), IFN-γ (rho = 0.84, p < .003; rho = 0.87, p < .002; and rho = 1.00, p < .001), and IL-17 levels (rho = 0.59, p = ns; rho = 0.75, p < .01; and rho = 0.98, p < .001) for BCG Danish, Pasteur, and Russia, respectively, but not for BCG Moreau. The four BCG strains were not found to be correlated with TGF-β and IL-23 levels (data not shown).
Table 1. Spearman’s correlation analysis of associations between cytokine levels using the four different BCG strains
4. Discussion
The success of M. tuberculosis, as one of the dreaded human pathogens, lies in its ability to utilize different defense mechanisms in response to the varied environmental challenges during the course of its intracellular infection, latency, and reactivation cycle.Citation27 This highlights the ability of M. tuberculosis for differential expression of genes required for its survival in the myriad of environmental conditions it faces during the course of infection.Citation28
To tackle TB, the BCG immunization policies are widely employed in countries burdened with high incidence of TB. Many countries have also continued the universal BCG vaccination despite the low incidence of TB. However, in other countries, BCG vaccination has been replaced by intensified case detection and supervised early treatment with contact tracing, whereas some chose to continue BCG vaccination but adopted a selective approach to be targeted only for the high-risk group.Citation29
As mentioned elsewhere, it is not surprising that diverse BCG strains could provide different adaptive features and that the variances between those strains may influence the activation of infected host cells.Citation30 Our present data has expanded and confirmed the results of a previous studyCitation24 and validated that the BCG Moreau vaccine is the most potent immunostimulant among the four strains in vitro tested herein as it demonstrated the highest level of proinflammatory cytokines and reduced the TGF-β levels. Earlier, it has been shown that the protective effect of bacterial immunostimulants, like OM-85BV (a preparation of lysates of eight bacterial pathogens), is ascribed to the improvement of nonspecific immunity, as well general activation of the CMI.Citation31 However, excessive inflammatory cytokine production can lead to tissue damage.Citation32 Conversely, local antiseptic, anti-inflammatory and mucolytic products are substantially less frequently used by OM-85BV treated patients.Citation31 In fact, although our knowledge of the BCG vaccine remains nascent, this field deserves more attention and deeper exploration. On the one hand, our study has found that there might be a recall and a specific immune response in adults with regard to the BCG Moreau vaccine strain, at least for three major monokines, i.e., IL-1β, IL-6, and TGF-β. Since the HC subjects had been previously immunized with BCG-Moreau as infants, it was not unexpected that this strain would also induce the largest in vitro cytokine responses after stimulation with the same vaccine. A study has pointed out a strain-specific immune response at in vitro level with regard to two BCG vaccine strains, although different from the four strains used in the present study.Citation30 On the other hand, in our study IL-17 and IFN-γ were broadly induced by the BCG vaccine from three distinct groups: I, III, and IV. Additionally, this study found no correlation when IL-1β levels were compared with IL-17 (for BCG Danish) and IL-6 (for BCG Russia). Surprisingly, the BCG Russia vaccine did not induce any cytokine tested herein, albeit this strain is classified as the closest to BCG Moreau.Citation13
Previously, BCG Russia has been shown to be less immunogenic than other BCG strains, albeit it is currently the most widely administered BCG strain worldwide.Citation22 BCG Russia induced lower frequencies of mycobacteria-specific polyfunctional CD4+ and cytotoxic CD8 + T cells, lower Th1 cytokine secretion and smaller skin test reactions when compared with other BCG strains in a small research clinical trial in Australia.Citation33 In another setting, the BCG Russia immune response of African infant vaccinees corroborated with the poor polyfunctional scores related to BCG Danish and tended to accumulate in a CD4 + T cell naïve-like state.Citation34 Thus, the data suggested that BCG Russia did not enhance innate immunity in the same way as other BCG vaccines and possibly deviates the immune system away from an anti-inflammatory IL-10 immune response.
Our study also found a positive association involving IFN-γ, IL-17 and IL-6 levels in almost all BCG-infected conditions, although the IL-1β levels exhibited a distinct pattern when compared with those cytokines. In fact, reports describing the effect of IFN-γ on IL-1β production are conflicting.Citation35 It has been recognized that IL-1β produced by macrophages and dendritic cells has a pro-inflammatory profile comparable to IFN-γ.Citation36 Two studies have found that IL-1 synergized with IL-6Citation37 and also IL-23Citation38 to maintain cytokine expression in effector Th17 cells. Typically, IL-1β, TGF-β and IL-23 are essential driving forces of cell differentiation from Th0 to Th17 profile, resulting in increased IL-17 production.Citation39 In another setting, IFN-γ potentiated IL-1β release from human cells.Citation35 Although still debated, an improved pro-inflammatory milieu has been related to the BCG vaccine, and ascribed as one of the main protection mechanisms provided by BCG against TB infection.Citation24,Citation39
The WHO published the last BCG reportCitation29 in 2018 and recommended that a single dose of the BCG vaccine be given to all infants at birth in countries where TB is highly endemic or where there is a high risk of exposure to TB, whereas countries with low incidence of TB may limit BCG vaccination to selected high-risk groups. Accordingly, the International Union Against Tuberculosis and Lung Diseases (IUATLD)Citation40 had set criteria to help countries in defining low endemicity and to consider the discontinuation of BCG vaccination or changing of its policy from a universal to a targeted approach. As the incidence of TB continues to decline in most developed countries,Citation1 selective BCG vaccination strategies in high-risk populations are increasingly being considered as an alternative to universal vaccination,Citation41–44 particularly among countries that satisfy the IUATLD criteria for discontinuation.Citation40
There is general evidence that vaccines have important heterologous, off-target effects on children in low-income countries.Citation45 The hypothesis states that, until a different vaccine is administered, live attenuated vaccines induce a beneficial nonspecific immunity. In randomized clinical trials, the BCG Danish vaccine nearly halves all-cause mortality in neonates by dropping deaths from infections other than TB, and this multifaceted effect is stronger if the mother has had the BCG vaccine and much stronger after the child’s second or subsequent dose of the BCG vaccine.Citation45–47 These heterologous protections are mediated, at least in part, by epigenetic effects on innate immune function.Citation45 A recent study related to the agonistic effect, leading to a heterologous and rapid protection of the BCG vaccine, pointed out that BCG administration reduces human neonatal sepsis, suggesting that this defense mechanism requires the increase of G-CSF provided by the BCG vaccine.Citation48 More recently, though, there has been a debate about the nonspecific beneficial effects of the BCG vaccine on viral infections: In the strategy of vaccine repositioning, could BCG afford protection against COVID-19?.Citation49 Consequently, clinical trials addressing this question are underway (https://mvec.mcri.edu.au/clinical-trial-of-bcg-vaccine-against-covid-19-brace/). Thus, at present, the WHO does not recommend BCG vaccination for the prevention of COVID-19.
The development of an improved TB vaccine, which can act as an efficient prophylactic vaccine to boost the immune system of BCG-vaccinated individuals, is urgently needed. Currently, the improvement of BCG is still considered as one of the best choices for the rational design of a new antituberculosis vaccine to recall the primed CMI. Reportedly, a BCG-based delivery system is attributed with greater potency of live bacterial vectors in stimulating adaptive immune responses than peptides, proteins, or DNA vaccines; the ability to mimic a natural infection and to induce innate immune responses via toll-like receptors; and providing an inflammatory milieu for antigen capture by dendritic cells.Citation50,Citation51 Thus, the BCG vaccine itself is a vehicle with inherent adjuvant properties, expressing different genes in a live-attenuated vaccine format.Citation52–54
The most important limitation of the current study was the small sample size. There were other limitations of this study. First, the difficulty in determining the significant differences in vitro among the different BCG vaccine strains after immunization in humans, since many of the strains used in vaccine manufacture contain more than one genotype.Citation55 More evidence on the efficacy, effectiveness, and adverse effects of the BCG vaccine strain is needed. Second, the strain specificity topic, since it is necessary to test our hypothesis using alternative cohort of matched individuals already vaccinated with any nonrelated BCG Moreau vaccine. Third, although unable to explore better in this study, we appreciate the use of pre-exposure markers to mycobacteria, such as the IGRA, in future studies, thus allowing relating the stimulus provided by the BCG vaccine to any eventual recall immune response to mycobacteria.Citation56
Taken together, the BCG Moreau vaccine generates an in vitro higher inflammatory cytokine profile and lower TGF-β levels compared with the Russia, Pasteur, and Danish strains in the context of early sensitization with mycobacteria (i.e., healthy adults vaccinated with BCG Moreau when they were young). It will be important to find a long-term association between adequate early vaccination with specific BCG strains, subsequent skin test reaction kinetics, and survival. Considering that this centennial vaccine has been safely used worldwide, we have obtained a significant amount of information about it. It would be remiss to not remark that astonishing progress has been made in TB vaccine research in response to this disease. Conclusively, this study demonstrates how harnessing the BCG vaccine field has led to new insights into its protective efficacy to tackle TB.
Acknowledgments
The authors are grateful to Ana L. Freitas, Joao PRC Barbosa, Leandro PS Santos, Maria ARS Soares, Staelen Santiago, Thatiane M. Kokumai (Clinical Immunology Laboratory, IOC/FIOCRUZ) for their help during technical procedures. The BCG Moreau vaccine was a gift of the Ataulpho de Paiva Foundation, whereas BCG Pasteur, Danish and Russia strains were provided by Dr. Milton O. Moraes (Leprosy Laboratory, IOC/FIOCRUZ). We also thank the Hemotherapy Service of the Hospital Clementino Fraga Filho (UFRJ) for providing buffy coats, the FIOCRUZ flow cytometry core lab and Dr. Eduardo CT Santos (Laboratory of Biochemistry of Trypanosomatids, IOC/FIOCRUZ) for kindly granting access to the spectrophotometer. This work was partly supported by the Coordination for the Improvement of Higher Education Personnel Vice-Presidência de Educação, Informação e Comunicação Coordenação-Geral de Educação Av. Brasil, 4365– Castelo Mourisco, sala 07 – Manguinhos – 21040-900 – Rio de Janeiro, Rj - Brasil e-mail: [email protected]/Telefones: 21 3885-1718/1077 (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - CAPES) - Finance Code 001. CP, LA and MR are the recipients of CNPq scholarships, AS, MA and SR are the recipients of CAPES scholarships, and PRZA is granted with CNPq research fellowship (PQ-2).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- WHO. Global tuberculosis report 2020. World Health Organization, 2020.
- Abebe F, Bjune G. The protective role of antibody responses during Mycobacterium tuberculosis infection. Clin Exp Immunol. 2009;157(2):235–7. doi:10.1111/j.1365-2249.2009.03967.x.
- Antas PR, Castello-Branco LR. New vaccines against tuberculosis: lessons learned from BCG immunisation in Brazil. Trans R Soc Trop Med Hyg. 2008;102(7):628–30. doi:10.1016/j.trstmh.2008.03.014.
- Zodpey SP. The BCG controversy: a reappraisal of the protective effect against tuberculosis and leprosy. Indian J Public Health. 2004;48:70–77.
- Pereira SM, Dantas OM, Ximenes R, Barreto ML. [BCG vaccine against tuberculosis: its protective effect and vaccination policies]. Rev Saude Publica. 2007;41(Suppl 1):59–66. doi:10.1590/S0034-89102007000800009.
- Colditz GA, Brewer TF, Berkey CS, Wilson ME, Burdick E, Fineberg HV, Mosteller F. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA. 1994;271:698–702. doi:10.1001/jama.1994.03510330076038
- Pe F. Variation in protection by BCG: implications of and for heterologous immunity. Lancet. 1995;346(8986):1339–45. doi:10.1016/S0140-6736(95)92348-9.
- Brandt L, Feino Cunha J, Weinreich Olsen A, Chilima B, Hirsch P, Appelberg R, Andersen P. Failure of the Mycobacterium bovis BCG vaccine: some species of environmental Mycobacteria block multiplication of BCG and induction of protective immunity to tuberculosis. Infect Immun. 2002;70(2):672–78. doi:10.1128/IAI.70.2.672-678.2002.
- Triccas JA. Recombinant BCG as a vaccine vehicle to protect against tuberculosis. Bioeng Bugs. 2010;1(2):110–15. doi:10.4161/bbug.1.2.10483.
- Stanford JL. Improving on BCG. APMIS. 1991;99(1–6):103–13. doi:10.1111/j.1699-0463.1991.tb05127.x.
- Grover A, Taylor J, Troudt J, Keyser A, Arnett K, Izzo L, Rholl D, Izzo A. Kinetics of the immune response profile in Guinea pigs after vaccination with Mycobacterium bovis BCG and infection with Mycobacterium tuberculosis. Infect Immun. 2009;77(11):4837–46. doi:10.1128/IAI.00704-09.
- Antas PR. Crucial requirement for standardization during the development of novel recombinant BCG vaccines: does the corresponding substrain background matter? Hum Vaccin Immunother. 2016;12(12):3099–102. doi:10.1080/21645515.2016.1212145.
- Brosch R, Gordon SV, Garnier T, Eiglmeier K, Frigui W, Valenti P, Dos Santos S, Duthoy S, Lacroix C, Garcia-Pelayo C. Genome plasticity of BCG and impact on vaccine efficacy. Proc Natl Acad Sci U S A. 2007;104(13):5596–601. doi:10.1073/pnas.0700869104.
- Horwitz MA, Harth G, Dillon BJ, Maslesa-Galić S. Commonly administered BCG strains including an evolutionarily early strain and evolutionarily late strains of disparate genealogy induce comparable protective immunity against tuberculosis. Vaccine. 2009;27(3):441–45. doi:10.1016/j.vaccine.2008.10.058.
- Dagg B, Hockley J, Rigsby P, Ho MM. The establishment of sub-strain specific WHO reference reagents for BCG vaccine. Vaccine. 2014;32(48):6390–95. doi:10.1016/j.vaccine.2014.09.065.
- Lagranderie MR, Balazuc AM, Deriaud E, Leclerc CD, Gheorghiu M. Comparison of immune responses of mice immunized with five different Mycobacterium bovis BCG vaccine strains. Infect Immun. 1996;64(1):1–9. doi:10.1128/iai.64.1.1-9.1996.
- Anderson EJ, Webb EL, Mawa PA, Kizza M, Lyadda N, Nampijja M, Elliott AM. The influence of BCG vaccine strain on mycobacteria-specific and non-specific immune responses in a prospective cohort of infants in Uganda. Vaccine. 2012;30(12):2083–89. doi:10.1016/j.vaccine.2012.01.053.
- Hayashi D, Takii T, Fujiwara N, Fujita Y, Yano I, Yamamoto S, Kondo M, Yasuda E, Inagaki E, Kanai K. Comparable studies of immunostimulating activities in vitro among Mycobacterium bovis bacillus Calmette-Guérin (BCG) substrains. FEMS Immunol Med Microbiol. 2009;56(2):116–28. doi:10.1111/j.1574-695X.2009.00559.x.
- Wang JF, Dai FY, Gong XL, Bao L. Commonly administered bacille Calmette-Guerin strains induce comparable immune response. Int J Clin Exp Med. 2015;8:15834–39.
- Abubakar I, Pimpin L, Ariti C, Beynon R, Mangtani P, Sterne JA, Fine P, Smith PG, Lipman M, Elliman D, et al. Systematic review and meta-analysis of the current evidence on the duration of protection by bacillus Calmette-Guérin vaccination against tuberculosis. Health Technol Assess. 2013;17(37):v–vi. doi:10.3310/hta17370.
- Mangtani P, Abubakar I, Ariti C, Beynon R, Pimpin L, Fine PE, Rodrigues LC, Smith PG, Lipman M, Whiting PF, et al. Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin Infect Dis. 2014;58(4):470–80. doi:10.1093/cid/cit790.
- Shann F. Editorial commentary: different strains of Bacillus Calmette-Guerin vaccine have very different effects on tuberculosis and on unrelated infections. Clin Infect Dis. 2015;61:960–62. doi:10.1093/cid/civ454.
- Antas PRZ, Flores-Valdez M, Shann F. An opportunity to compare the effects of BCG-Moreau and BCG-Russia in Brazil. Int J Tuberc Lung Dis. 2018;22(9):1108–09. doi:10.5588/ijtld.18.0271.
- Ponte C, Hacker M, Moraes M, Castello-Branco L, Silva F, Antas P. The patterns of in vitro cell-death and inflammatory cytokines induced by distinct BCG vaccine strains are differentially induced in human mononuclear cells. Hum Vaccin Immunother. 2018;14(1):28–35. doi:10.1080/21645515.2017.1382788.
- Mawa PA, Webb EL, Filali-Mouhim A, Nkurunungi G, Sekaly RP, Lule SA, Prentice S, Nash S, Dockrell HM, Elliott AM, et al. Maternal BCG scar is associated with increased infant proinflammatory immune responses. Vaccine. 2017;35(2):273–82. doi:10.1016/j.vaccine.2016.11.079.
- Ma X, Zhang X, Liu J, Liu Y, Zhao C, Cai H, Lei W, Ma J, Fan H, Zhou J, et al. The correlations between Th1 and Th2 cytokines in human alveolar echinococcosis. BMC Infect Dis. 2020;20(1):414. doi:10.1186/s12879-020-05135-y.
- Pawaria S, Lama A, Raje M, Dikshit KL. Responses of Mycobacterium tuberculosis hemoglobin promoters to in vitro and in vivo growth conditions. Appl Environ Microbiol. 2008;74(11):3512–22. doi:10.1128/AEM.02663-07.
- Cosma CL, Sherman DR, Ramakrishnan L. The secret lives of the pathogenic mycobacteria. Annu Rev Microbiol. 2003;57(1):641–76. doi:10.1146/annurev.micro.57.030502.091033.
- World Health Organization. BCG vaccine: WHO position paper, February 2018 - Recommendations. Vaccine 2018.
- Sanarico N, Colone A, Grassi M, Speranza V, Giovannini D, Ciaramella A, Colizzi V, Mariani F. Different transcriptional profiles of human monocyte-derived dendritic cells infected with distinct strains of Mycobacterium tuberculosis and Mycobacterium bovis bacillus Calmette-Guérin.Clin Dev Immunol. 2011;2011:741051. doi:10.1155/2011/741051
- Rozy A, Chorostowska-Wynimko J. Bacterial immunostimulants–mechanism of action and clinical application in respiratory diseases. Pneumonol Alergol Pol. 2008;76:353–59.
- Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, Li Y, Wang X, Zhao L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2018;9(6):7204–18. doi:10.18632/oncotarget.23208.
- Ritz N, Dutta B, Donath S, Casalaz D, Connell TG, Tebruegge M, Robins-Browne R, Hanekom WA, Britton WJ, Curtis N. The influence of bacille Calmette-Guerin vaccine strain on the immune response against tuberculosis: a randomized trial. Am J Respir Crit Care Med. 2012;185(2):213–22. doi:10.1164/rccm.201104-0714OC.
- Kiravu A, Osawe S, Happel AU, Nundalall T, Wendoh J, Beer S, Dontsa N, Alinde OB, Mohammed S, Datong P, et al. Bacille Calmette-Guérin vaccine strain modulates the ontogeny of both Mycobacterial-specific and heterologous T cell immunity to vaccination in infants. Front Immunol. 2019;10:2307. doi:10.3389/fimmu.2019.02307.
- Masters SL, Mielke LA, Cornish AL, Sutton CE, O’Donnell J, Cengia LH, et al. Regulation of interleukin-1beta by interferon-gamma is species specific, limited by suppressor of cytokine signalling 1 and influences interleukin-17 production. EMBO Rep. 2010;11(8):640–46. doi:10.1038/embor.2010.93.
- Dinarello CA. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol Rev. 2018;281:8–27.
- Pollara G, Turner CT, Rosenheim J, Chandran A, Bell LCK, Khan A, et al. Exaggerated IL-17A activity in human in vivo recall responses discriminates active tuberculosis from latent infection and cured disease. Sci Transl Med. 2021;13.
- Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, Ma L, Watowich SS, Jetten AM, Tian Q, et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30(4):576–87. doi:10.1016/j.immuni.2009.02.007.
- Fatima S, Kumari A, Das G, Dwivedi VP. Tuberculosis vaccine: a journey from BCG to present. Life Sci. 2020;252:117594. doi:10.1016/j.lfs.2020.117594.
- Criteria for discontinuation of vaccination programmes using Bacille Calmette-Guerin (BCG) in countries with a low prevalence of tuberculosis. A statement of the International Union Against Tuberculosis and Lung Disease. Tuber Lung Dis. 1994;75(3):179–80. doi:10.1016/0962-8479(94)90003-5.
- Dierig A, Tebruegge M, Krivec U, Heininger U, Ritz N; (ptbnet) PTNETg. Current status of Bacille Calmette Guérin (BCG) immunisation in Europe – a ptbnet survey and review of current guidelines. Vaccine. 2015;33(38):4994–99. doi:10.1016/j.vaccine.2015.06.097.
- Tu HA, Vu HD, Rozenbaum MH, Woerdenbag HJ, Postma MJ. A review of the literature on the economics of vaccination against TB. Expert Rev Vaccines. 2012;11(3):303–17. doi:10.1586/erv.11.197.
- Hersh AL, Tala-Heikkilä M, Tala E, Tosteson AN, Fordham von Reyn C. A cost-effectiveness analysis of universal versus selective immunization with Mycobacterium bovis bacille Calmette-Guérin in Finland. Int J Tuberc Lung Dis. 2003;7:22–29.
- Feiring B, Laake I, Molden T, Håberg SE, Nøkleby H, Seterelv SS, Magnus P, Trogstad L. Do selective immunisation against tuberculosis and hepatitis B reach the targeted populations? A nationwide register-based study evaluating the recommendations in the Norwegian Childhood Immunisation Programme. Vaccine. 2016;34(17):2015–20. doi:10.1016/j.vaccine.2016.02.060.
- Aaby P, Roth A, Ravn H, Napirna BM, Rodrigues A, Lisse IM, Stensballe L, Diness BR, Lausch KR, Lund N. Randomized trial of BCG vaccination at birth to low-birth-weight children: beneficial nonspecific effects in the neonatal period? J Infect Dis. 2011;204(2):245–52. doi:10.1093/infdis/jir240.
- Stensballe LG, Ravn H, Birk NM, Kjærgaard J, Nissen TN, Pihl GT, et al. BCG vaccination at birth and rate of hospitalization for infection until 15 months of age in Danish children: a randomized clinical multicenter trial. J Pediatric Infect Dis Soc. 2018.
- Biering-Sørensen S, Aaby P, Lund N, Monteiro I, Jensen KJ, Eriksen HB, et al. Early BCG-Denmark and Neonatal mortality among infants weighing <2500 g: a randomized controlled trial. Clin Infect Dis. 2017;65:1183–90.
- Brook B, Harbeson DJ, Shannon CP, Cai B, He D, Ben-Othman R, et al. BCG vaccination-induced emergency granulopoiesis provides rapid protection from neonatal sepsis. Sci Transl Med. 2020;12.
- O’Neill LAJ, Netea MG. BCG-induced trained immunity: can it offer protection against COVID-19? Nat Rev Immunol. 2020;20(6):335–37. doi:10.1038/s41577-020-0337-y.
- Gomez M, Doukhan L, Nair G, Smith I. sigA is an essential gene in Mycobacterium smegmatis. Mol Microbiol. 1998;29(2):617–28. doi:10.1046/j.1365-2958.1998.00960.x.
- Kaufmann SH, Gengenbacher M. Recombinant live vaccine candidates against tuberculosis. Curr Opin Biotechnol. 2012;23(6):900–07. doi:10.1016/j.copbio.2012.03.007.
- Collins DM. New tuberculosis vaccines based on attenuated strains of the Mycobacterium tuberculosis complex. Immunol Cell Biol. 2000;78(4):342–48. doi:10.1046/j.1440-1711.2000.00937.x.
- Hanson MS, Lapcevich CV, Haun SL. Progress on development of the live BCG recombinant vaccine vehicle for combined vaccine delivery. Ann N Y Acad Sci. 1995;754:214–21. doi:10.1111/j.1749-6632.1995.tb44453.x.
- Ohara N, Yamada T. Recombinant BCG vaccines. Vaccine. 2001;19(30):4089–98. doi:10.1016/S0264-410X(01)00155-4.
- Wada T, Maruyama F, Iwamoto T, Maeda S, Yamamoto T, Nakagawa I, Yamamoto S, Ohara N. Deep sequencing analysis of the heterogeneity of seed and commercial lots of the bacillus Calmette-Guérin (BCG) tuberculosis vaccine substrain Tokyo-172. Sci Rep. 2015;5(1):17827. doi:10.1038/srep17827.
- Hanekom WA. The immune response to BCG vaccination of newborns. Ann N Y Acad Sci. 2005;1062(1):69–78. doi:10.1196/annals.1358.010.
