ABSTRACT
The prognosis of patients with advanced pancreatic cancer is poor despite the recent introduction of immune checkpoint inhibitors. Therefore, the development of new therapeutic approaches is urgently required. In the present phase I/II study, we have evaluated the safety, the efficacy and the prognostic factors of Wilms’ tumor 1 (WT1) and/or mucin 1 (MUC1) peptide-loaded dendritic cell (DC) vaccination in combination with a chemotherapy employing gemcitabine plus nab-paclitaxel or a combination chemotherapy regimen consisting of oxaliplatin, irinotecan, fluorouracil and leucovorin (FOLFIRINOX) in patients with advanced or relapsed pancreatic ductal adenocarcinoma (PDAC). Forty-eight eligible patients were enrolled and received the vaccinations approximately every 2–4 weeks at least seven times. No severe adverse events related to the vaccinations were observed. Median progression free survival and overall survival were 8.1 months and 15.1 months, respectively. DC vaccinations augmented tumor specific immunity which might be related to clinical outcome. The multivariate analyses demonstrated that WT1 or MUC1-specific interferonɤ enzyme-linked immunospot number prior to DC vaccination was an independent prognostic factor related to overall survival. These results indicate that DC-based immunotherapy combined with a conventional chemotherapy is safe and has clinical benefits for patients in advanced stage of PDAC. The precise evaluation of the baseline antitumor specific immunity is critical to predict clinical outcome.
Introduction
Pancreatic cancer is the fourth leading cause of cancer-related death with an estimated 60,430 new cases and 48,220 deaths in the United States in 2020Citation1. The numbers of new cases and deaths worldwide are approximately 458,900 and 432,200 according to GLOBOCAN 2018 estimates.Citation2 The prognosis of patients with advanced pancreatic ductal adenocarcinoma (PDAC) is extremely poor with 5-year survival rate less than 10%.Citation3 PFS and OS in patients with metastatic and inoperable disease are reported to be approximately from 3.7 to 11.7 months and from 6.1 to 15.9 months, respectively from the initiation of the standard chemotherapy.Citation4 Surgical resection is the only curative treatment of PDAC. However, the majority of patients are already in unresectable advanced stage at the time of diagnosis and need multimodal therapy.Citation3 Although multiagent chemotherapy has been shown to prolong survival by several months, clinical benefit remains unsatisfactory.Citation3 While immune checkpoint inhibitors have demonstrated remarkable efficacy in several solid tumors, clinical trials targeting immune checkpoint molecules have failed in PDAC.Citation5,Citation6 Therefore, the development of new therapeutic approaches is urgently needed.
Dendritic cells (DCs) are potent antigen presenting cells capable of presenting tumor-associated antigens (TAAs) to T lymphocytes.Citation7 PDAC cells express various TAAs which elicit T cell responses against them.Citation8 These include Wilms’ tumor 1 (WT1), mucin 1 (MUC1), human telomerase reverse transcriptase, p53, mutated K-RAS, survivin, carcinoembryonic antigen, HER-2/neu, and α-enolase. Both WT1 and MUC1 are considered to be the most suitable tumor-associated antigens for immunotherapy based on the fact that these antigens are highly immunogenic and over-expressed or over-expressed in an incompletely glycosylated form in various cancers including pancreatic cancer, while the expression in normal tissues is limited.Citation9,Citation10 The expression of WT1 in normal tissue is demonstrated in mesothelium, glomerular podocytes and mesangial cells of the kidney, hematopoietic progenitor cells, Sertoli cells of the testis, stromal cells, surface epithelium, and granulosa cells of the ovary and myometrium and endometrial stromal cells of the uterus.Citation11,Citation12 No expression of WT1 in normal pancreatic tissue is reported.Citation9 MUC1 is reported to be overexpressed in an incompletely glycosylated form in various human cancers.Citation13 However, the expression of MUC1 is not observed in specimens from normal pancreas, chronic pancreatitis, or ductal hyperplasia of the pancreas.Citation14 Clinical trials using these peptides or peptide-loaded DC vaccination in patients with advanced PDAC have demonstrated immune responses against tumor antigens with limited clinical efficacy.Citation15–20
Gemcitabine (GEM) plus nab-paclitaxel (nab-PTX) or a combination chemotherapy regimen consisting of oxaliplatin, irinotecan, fluorouracil, and leucovorin (FOLFIRINOX) is recommended as the first line chemotherapy for patients with advanced or relapsed PDAC.Citation3 GEM plus nab-PTX and FOLFIRINOX improved clinical outcome compared with GEM or S-1 (tegafur gimeracil oteracil).Citation21,Citation22 However, median overall survival is less than a year.
Immune-regulatory functions have been reported in several chemotherapeutic agents; GEM induces an upregulation of TAAs expression on tumor cells and the proliferation of monocytes and DCs.Citation23,Citation24 5-FU increases the frequency of tumor-infiltrating T cells and favors myeloid-derived suppressor cells (MDSCs) differentiation.Citation25,Citation26 Paclitaxel favors tumor infiltration by natural killer (NK) cells and cytotoxic T cells (CTLs).Citation27 Oxaliplatin increases the CTL/regulatory T cells (Tregs) ratio and depletes MDSCs.Citation28 Based on these facts, we hypothesized that the combined DC vaccination with conventional chemotherapies might improve clinical outcome. In fact, an add-on effect of DC or peptide vaccination to a chemotherapy including GEM and/or S-1 in inoperable pancreatic cancer was suggested by us and others.Citation15–19 However, clinical benefits of the combination of DC vaccination and GEM plus nab-PTX or FOLFIRINOX remain to be elucidated.
In the present phase I/II study, we have evaluated the safety, the clinical and immunological responses and analyzed the prognostic factors related to survival in patients with metastatic and unresectable or relapsed PDAC who received WT1 and/or MUC1 peptide-loaded DC vaccination in combination with a toll-like receptor (TLR) 4 agonist, OK-432, and a chemotherapy employing GEM plus nab-PTX or FOLFIRINOX regimen.
Methods
Patients and eligibility criteria
Sixty-two patients with metastatic and unresectable or relapsed PDAC were referred from local community hospitals for DC vaccination. Fourteen patients were excluded due to incompatibility to the inclusion criteria and 48 patients were enrolled in the present study from May 2017 to December 2019. The inclusion criteria were as follows: (1) pathologically diagnosed PDAC with metastatic and unresectable or recurrent disease; (2) WT1 or MUC1 expression was confirmed by an immunohistochemistry; (3) PS 0–2; (4) an expected prognosis of over 4 months; (5) white blood cell (WBC) count more than 2500 cells/mm3; (6) hemoglobin more than 9.0 g/dL; (7) platelet count more than 90,000 cells/mm3; and (8) no serious dysfunction of vital organs.
This study was approved by the Institutional Review Board (IRB) at Sapporo Hokuyu Hospital and performed in accordance with the Declaration of Helsinki. All patients signed informed consent forms before enrolling in this study. This study is registered in University Hospital Medical Information Network (UMIN) in Japan. The registration number is UMIN 000027279. The registration in clinicaltrials.gov was not requested by the IRB at Sapporo Hokuyu Hospital, so we registered in the UNIM in Japan.
Preparation of DC
DCs were prepared from PBMCs obtained by leukapheresis as described previously.Citation29–31 DCs were loaded with HLA-A2-, HLA-A24-, or HLA-A26-restricted 9-mer WT1 peptides and/or MUC-1 peptide and cryopreserved until the day of administration. The phenotype characteristic to mature DC (CD14−/low/HLA-DR+/HLA-ABC+/CD80+/CD83+/CD86+/CD40+) was confirmed by flow cytometry (FACS) analysis. Negativity of mycoplasma (polymerase chain reaction [PCR] method) and endotoxins (Endospecy™; Seikagaku Co.) in cell suspension were confirmed before cryopreservation. Three HLA-restricted 9-mer WT1 peptides were used depending on the HLA-A allele the patients had. Amino acid sequences of WT1 peptides are as follows: HLA-A2 peptide, RMFPNAPYL; HLA-A24 peptide, CYTWNQMNL; HLA-A26, VTFDGTPSY. WT1 peptides were obtained from NeoMPS Inc. and AnyGen Co. Ltd. MUC1 peptide (TRPAPGSTAPPAHGVTSAPDTRPAPGSTAP) was used for patients whose tumor was positive for MUC1 regardless of HLA type. MUC1 peptide was obtained from AnyGen Co. Ltd.
Patient treatment
The vaccination regimen has been described previously.Citation29–31 Briefly, WT1 and/or MUC1 peptide-loaded mature DCs (approximately 1 × 107) were injected intradermally at four positions at each side of axilla approximately every 2–4 weeks if WBC count was above 2000 cells/µl. Patients without progressive disease upon the completion of the standard 7th vaccination received additional vaccinations until the occurrence of disease progression or the administration of the last tube. The median number of vaccinations was 9 times (range: 5–29 times). OK-432 (0.5–2.0 KE), a penicillin-killed and lyophilized preparation of Streptococcus pyogenes (Chugai Pharmaceutical Co. Ltd.), was administered subcutaneously at each side of axilla in the proximity of vaccine sites to activate DC functions.
Patients were treated with either GEM plus nab-PTX or FOLFIRINOX regimen concurrently depending on the referring physician’s decision. In the GEM plus nab-PTX regimen, nab-PTX 125 mg/m2 was administered on day 1 and GEM 1000 mg/m2 was administered on day 1, 8, and 15 and repeated every 4 weeks. In FOLFIRINOX regimen, oxaliplatin 85 mg/m2, l-leucovorin 200 mg/m2, irinotecan 180 mg/m2 were administered on day 1. 5-FU 400 mg/m2 was administered i.v. bolus followed by 2400 mg/m2 continuous i.v. infusion over 46 hours. 5-FU i.v was discontinued when the dose reduction was required due to a serious toxicity. This regimen was repeated every 2 weeks. Dose reduction by up to 40% was permitted depending on the degree of adverse events. To prevent cytotoxicity of chemotherapeutic agents, DC were administered on day 3 and day 17 in the GEM plus nab-PTX regimen and day 5 in in the FOLFIRINOX regimen.
Evaluation of toxicity and clinical responses
Patients were examined for signs of adverse events during the treatment. The US National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 was used to classify the grades. Physical examinations and laboratory tests were conducted at each vaccination. A computed tomography (CT) was performed within a month prior to and post DC vaccination and repeated approximately every 1–3 months until the disease progression. The clinical response was evaluated on the basis of the Response Evaluation Criteria in Solid Tumors (RECIST) (version 1.1). Follow-up information was obtained from the referring physicians.
ELIspot assay
Cryopreserved PBMCs (1–2 × 106), obtained from the patients before and after the vaccination, were cultured with WT1 or MUC1 peptide (10 μg/ml each) and interleukin-2 (50 U/ml, Peprotech Inc.) for 2 weeks. After harvesting, washing, and evaluating the viability of cells, serially threefold diluted cells (3x105–1x103) were incubated with WT1, MUC1 or HIV peptide-pulsed antigen presenting cells (APCs; K562 human erythroleukemia cells transduced with HLA-A*02:01 or HLA-A*2402 gene) in duplicate in a separate well of a nitrocellulose plate pre-coated with anti-human IFNγ moAb. Irrelevant HIV peptide-pulsed APCs were used as a negative control to evaluate nonspecific response. The viability of harvested cells, examined by a trypan blue dye exclusion method, was in the range of 80–95%. The presence of IFNɤ-secreting WT1 or MUC1-specific T cells were examined by an IFNɤ ELIspot assay kit (Invitrogen/ThermoFisher Scientific, Inc.) following the procedure recommended by the manufacturer. The number of IFNɤ spots were measured by an image analyzer and software (Cellular Technology Ltd.). The number of WT1 or MUC1 peptide-specific spots was calculated by subtracting the mean spot number of duplicated cells stimulated with HIV peptide-pulsed APCs from that of duplicated cells stimulated with WT1 or MUC1 peptide-pulsed APCs.
FACS analysis
The expression of surface molecules on DCs or PBMCs was examined by FACS using various fluorescent dye-conjugated monoclonal antibodies (moAbs) as described previously;Citation29–31 fluorescein isothiocyanate (FITC)-conjugated moAbs against cluster of differentiation (CD) 4, CD8 or T cell receptor (TCR) ɤδ (Becton Dickinson Japan Co.), Phycoerythrin (PE)-conjugated moAbs against CD11b, CD56,CD62L or CD127 (Becton Dickinson Japan Co.). Peridinin chlorophyll protein (PerCP)-conjugated moAbs against CD3, CD4 or HLA-DR and PE-conjugated moAbs against CD33 or HLA-ABC (BioLegend Inc.). FITC-conjugated moAbs against CD25, CD45RO, CD80 or CD83, and PE-conjugated moAbs against CD86 (Beckman Coulter Co.). FITC-conjugated anti-CD14 moAb (Miltenyi Biotec Co.). After 15 minutes incubation, cells were washed with FACS buffer, applied to FACSCalibur and analyzed using CellQuest software (Becton Dickinson Japan Co.).
Immunohistochemistry and HLA typing
The expression of WT1 and MUC1 on tumor cells in biopsy samples was examined by immunohistochemistry using rabbit polyclonal anti-WT1 Ab (C-19; Santa Cruz Biotechnology, Inc.) and anti-MUC1 moAb (VU4H5; Santa Cruz Biotechnology, Inc.) as described by Oji et al.Citation9 HLA typing was carried out by using a HLA DNA typing kit obtained from Wakunaga Pharmaceutical Co. following the procedure recommended by the manufacturer.
Statistical analysis
Statistical significance of the differences was calculated by the Wilcoxon signed-ranks test or the Mann-Whitney U-test. P < .05 was considered significant. OS and PFS were analyzed by the Kaplan-Meier method and the survival was measured in months from the date of the first vaccine to the date of death or final follow-up and the date of disease progression, respectively. Survival curve comparisons were conducted with the log-rank test. Univariate or multivariate analysis of the factors related to survival was conducted with the log-rank test and the Cox’s proportional hazards regression model, respectively. Statistical analyses were performed using Statcel software (OMS Publishing Co.) and EZR (version 1.36; Saitama Medical Center, Jichi Medical University).
Results
Patient characteristics and treatment
Characteristics of patients enrolled in this study are shown in . Median age of patients was 64 years (range; 41–83). The male to female ratio was 1 to 1.29. Eastern Cooperative Oncology Group performance status (PS) at the first DC vaccination was below 1 except 2 patients in PS 2. Pancreatic tumor location in head or body/ tail was 22 and 26 patients, respectively. Metastatic sites at DC vaccination were local in 8, liver in 16, lung 9, peritoneum in 5 and multiple sites in 10 patients. Median serum CA19.9 and CEA levels were 499.8 U/ml and 7.0 ng/ml, respectively. Previous treatments were chemotherapy employing S-1 and/or GEM, GEM plus nab-PTX or FOLFIRINOX in 21 patients, surgery in 11 patients and radiation in 3 patients. 13 patients were chemo-naïve. Concurrent treatments were GEM plus nab-PTX in 33 patients and FOLFIRINOX in 15 patients. The median treatment cycle of GEM plus nab-PTX and FOLFIRINOX was 7.0 (range; 3.0–15.0) and 9.0 (range; 5.0–21.0), respectively. Postvaccination chemotherapies were S-1 and/or GEM in 19 patients, GEM plus nab-PTX in 25 patients and FOLFIRINOX in 12 patients. The median number of DC vaccinations were 9.0 times (range; 5.0–29.0). Six patients received vaccinations five or six times due to the insufficient amount of DCs generated.
Table 1. Patient characteristics
Clinical outcome
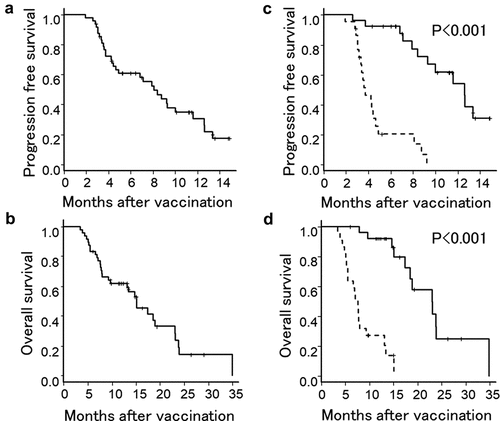
Although no patient had complete response, 7 patients had partial response (PR), 20 patients had stable disease (SD), and the remaining 21 patients had progressive disease (PD) following the 7th DC injection. Therefore, an objective response rate and a disease control rate were 14.6% and 56.3%, respectively. Median progression free survival (PFS) and overall survival (OS) was 8.1 months (95% confidence interval, 4.4–10.0 months) and 15.1 months (95% confidence interval, 9.3–18.9 months) from the initiation of DC vaccination, respectively with the median observation period of 13.1 months. One year PFS and OS was 30.6% and 61.9%, respectively ().
Figure 1. Kaplan-Meier estimates of PFS and OS of patients treated with a combined DC vaccination with a chemotherapy. (a and b) PFS and OS of all patients. (c and d) PFS and OS of patients who showed positive immune responses in either WT1 or MUC1 ELIspot assay (solid line) and patients who showed negative immune responses in both assays (dotted line).
Adverse events
Adverse events (AEs) observed in more than 20% of patients during the treatment period are shown in . Grade 3 or 4 leukocytopenia, neutropenia, anemia, and thrombocytopenia occurred in 44%, 46%, 13%, and 8% of patients, respectively. Febrile neutropenia occurred in 4 (8%) patients. Seventeen (35%) patients were treated with granulocyte colony stimulating factor to resolve neutropenia. The most frequent nonhematological AEs were fatigue (48%), nausea (46%), alopecia (44%), anorexia (42%), and peripheral sensory neuropathy (42%) followed by pain (38%) and constipation (33%). The most common AEs directory related to DC vaccination were transient low grade erythema and/or induration at the injected sites (100%) and mild fever related to OK-432 (40%).
Table 2. Adverse events†
Immunological monitoring
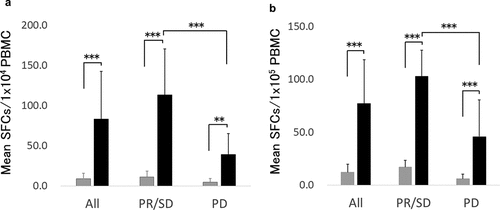
WT1 or MUC1 specific immune responses were evaluated by an Interferonɤ (IFNɤ) enzyme-linked immunospot (ELIspot) assay following in vitro culture with WT1 or MUC1 peptide. As shown in , WT1 specific IFNɤ spot number per 1 × 104 peripheral blood mononuclear cells (PBMCs) increased from 9.0 to 83.4 on average following vaccination (p < .001). The increment in spot number was significantly higher in the patients who had PR or SD (responding patients) in comparison with PD patients (p < .001). Similarly, we observed a significant increase in the MUC1 specific IFNɤ spot number following vaccination (p < .001) and significantly greater magnitude of increase in responding patients (p < .001, )). These results demonstrated that WT1 and MUC1 specific immunity were augmented significantly by WT1 and/or MUC1 peptide-loaded DC vaccination.
The relationship between clinical outcome and immune responses was demonstrated by a survival analysis using a log-rank test. Median PFS and OS were significantly longer in patients who showed positivity in either WT1 or MUC1 ELIspot assay than in patients who were negative in both assays (p < .001) (). These results suggested that augmentation of WT1 or MUC1 specific immunity might contribute to a better clinical outcome.
Prognostic factors related to PFS and OS
The univariate analyses with log-rank tests showed that none of the clinico-laboratory factors such as age, gender, PS, primary tumor localizations, metastatic sites, and previous treatments were significantly correlated with PFS and OS. Similarly, neither concurrent chemotherapy regimens nor difference of peptides were prognostic factors for PFS and OS (). Instead, neutrophil to lymphocyte ratio (NLR) more than 5 (PFS, p = .0206; OS p = .0164), prognostic nutritional index (PNI) less than 40 (p = .0174; OS, p = .0109) and modified Glasgow prognostic score (mGPS) 1 or 2 (PFS, p = .0169: OS p = .0251) were significantly related to shorter PFS and OS among the clinico-laboratory factors. On the other hand, carcinoembryonic antigen (CEA) above median (p = .0444) and platelet to lymphocyte ratio (PLR) more than 150 was significantly related to shorter PFS (p = .028) (). Because there was a variation in the staining level in WT1 and MUC1 in immunohistochemistry, we compared PFS and OS between the patient groups depending on the staining level. We found no significant difference in PFS and OS between the patient groups with moderate or weak staining of WT1 and MUC1 ().
Table 3. Univariate analysis of the association of clinical factors with PFS and OS
Table 4. Univariate analysis of the association of laboratory factors with PFS and OS
Among the immune-related factors, we found that Treg number in peripheral blood (PB) below median (PFS, p = .00306; OS, p = .0159), marked skin reaction (≧30 mm) (PFS, p = .0107; OS, p = .00174), WT1-specific IFNɤ ELIspot number above median (PFS, p= .000914; OS p < .0001), and MUC1-specific IFNɤ ELIspot number above median (PFS, p < .0001; OS, p < .0001) were significantly related to longer PFS and OS ().
Table 5. Univariate analysis of the association of immune-related factors with PFS and OS
The multivariate analyses with a Cox’s proportional hazards regression model among the significant factors in the univariate analyses demonstrated that WT1-specific IFNɤ ELIspot number was an independent prognostic factor related to OS (p= .038) and that MUC1-specific IFNɤ ELIspot number was an independent prognostic factor related to both PFS (p = .012) and OS (p = .014) ().
Table 6. Multivariate analysis
Taken together, these results indicated that baseline tumor-specific and nonspecific immunity and the augmentation of tumor-specific immune response following DC vaccination play a pivotal role and influence the clinical outcome.
Discussion
Previous studies of immunotherapy in PDAC demonstrated both immunological responses and clinical efficacy. However, the clinical benefit is limited.Citation8 In the present study, we aimed to examine the hypothesis that a combination of DC vaccination and the first-line chemotherapy employing GEM plus nab-PTX or FOLFIRINOX might improve clinical outcome in patients with advanced or relapsed PDAC. We also investigated the contribution of both the baseline immunity and the immune responses following DC vaccination to the clinical outcome.
Findings in this study are the followings; (1) We demonstrated a substantial prolongation of median PFS and OS. Median PFS and OS were 8.1 months and 15.1 months, respectively, from the initiation of vaccination, which exceeded the previously reported median PFS and OS. (2) We observed a significant enhancement of the immune responses specific to WT1 or MUC1 following DC vaccination in the majority of patients which was correlated with the prolongation of median PFS and OS. (3) WT1 specific IFNɤ ELIspot number prior to vaccination was an independent prognostic factor related to OS and MUC1 specific IFNɤ ELIspot number prior to vaccination was an independent prognostic factor related to both PFS and OS.
A phase III trial (MPAC trial) or a phase II/III trial (PRODIGE/ACCORD trial) in patients with metastatic pancreatic cancer demonstrated that median PFS and OS in GEM plus nab-PTX were 5.5 months and 8.5 months, respectively and those in FOLFIRINOX were 6.4 months and 11.1 months, respectively.Citation21,Citation22 A systemic review of 34 studies including 6915 patients with metastatic or advanced pancreatic cancer who were treated with either GEM plus nab-PTX or FOLFIRINOX in the first-line setting showed a range of median OS and PFS as follows; median OS of GEM plus nab-PTX and FOLFIRINOX ranged from 6.1 to 14.4 months and from 8.6 to 15.9 months, respectively, and median PFS of GEM plus nab-PTX and FOLFIRINOX ranged from 4.0 to 8.5 months and from 3.7 to 11.7 months, respectively.Citation4 Based on these data and considering the fact that more than 70% of the patients in this study relapsed or were refractory to the first-line treatment and that there seemed to be no marked differences in the characteristics of patients in this study compared with the previous studies particularly regarding median age and PS, the clinical outcome of this study might be favorable, suggesting an add-on effect of DC vaccination.
Although we observed grade 3 or 4 hematological toxicity, anorexia, fatigue, and alanine aminotransferase/aspartate aminotransferase elevation, the majority of adverse events were less than grade 2 (). There were no adverse events newly observed in this study compared with the previous studies employing GEM plus nab-PTX or FOLFIRINOX.Citation21,Citation22 Therefore, we considered that a combined DC vaccination with GEM plus nab-PTX or FOLFIRINOX was safe and tolerable. A major concern using GEM plus nab-PTX or FOLFIRINOX regimen was toxicity to immune cells, because a hematological toxicity was more profound compared with GEM and/or S-1.Citation21,Citation22 However, the results showed that the enhancement of WT1 or MUC1-specific immune responses was demonstrated to be independent of leukocytopenia. These results are consistent with our previous reports and eliminated a potential detrimental effect of chemotherapeutic agents on anti-tumor immunity.Citation29–31
OK-432 has been used as an immunotherapeutic agent in various cancers.Citation36–38 Because OK-432 facilitates maturation of DCs and stimulates the secretion of type-1 helper T cell (Th1)-type cytokines such as IFNɤ, tumor necrotizing factorα, interleukin-12 and interleukin-18 through toll-like receptor 4 signaling,Citation39 we employed OK-432 in the preparation of DCs in standard operating procedure (SOP) and used as an adjuvant for DC vaccine to improve clinical outcome in a similar manner to previous studies.Citation16,Citation17,Citation29–31,Citation40 Considering AEs by OK-432, we administrated lower dose of OK-432 (0.5–2KE) compared with that used in the treatment for patients with various cancers (5–10KE).Citation37,Citation38 The AEs directly related to OK-432 were transient mild fever and low grade erythema and/or induration at the injection sites, which is consistent with the frequently observed AEs in the previous DC vaccination trials employing OK-432Citation16,Citation17,Citation29–31,Citation40 and the reported AEs in the treatment of cancer patients with OK-432.Citation36–38
Many studies have demonstrated various prognostic factors related to the survival in patients with PDAC. These include PS, tumor localization, carbohydrate antigen 19–9, carcinoembryonic antigen, NLR, PLR, biochemical parameters such as albumin, lactate dehydrogenase, alkaline phosphatase, blood urea nitrogen, aspartate aminotransferase, bilirubin, C-reactive protein, and various molecular markers.Citation41 We found that NLR, PNI, and mGPS were the significant prognostic factors related to FPS and OS among clinico-laboratory factors examined, which is consistent with the previous studiesCitation17,Citation32,Citation33 (). Inflammation associated with cancer is a well-known risk factor for the progression of various cancers and a critical factor for survival.Citation42 NLR, PLR, and mGPS have been demonstrated as indicators of systemic inflammatory response and have been identified as independent prognostic factors for both inoperable and surgically resected pancreatic cancer patients.Citation32,Citation33,Citation34
Host immunity against tumors is prerequisite in immunotherapy. Therefore, host immune-related factors may offer a useful tool for predicting prognosis. There was a trend toward better prognosis in high absolute number of subset of lymphocytes such as CD4+ T cells, CD8+ T cells, type 1 helper T cells (Th1 cells), ɤδT cells, natural killer T cells (NKT cells), and low number of MDSCs. However, these factors did not reach statistical significance (). A univariate analysis revealed that only an absolute number of Treg prior to therapy was a prognostic factor for PFS and OS among various subsets of immune cells, indicating the importance of Treg in cancer immunotherapy.Citation43 Consistent with our findings, the prognostic significance of Treg number in PB or at tumor sites has been reported.Citation44,Citation45 Liu et al. showed that a high initial Treg level or CD4/CD8 ratio before treatment was associated with a poor prognosis of the patients with unresectable pancreatic cancer who were treated with gemcitabine-based chemotherapy.Citation46
We have demonstrated that a skin reaction after DC vaccination, which is considered to be a delayed type hypersensitivity reaction against WT1 and/or MUC1 peptide, is an independent treatment-related prognostic factor for patients with inoperable pancreatic cancer receiving WT1 and/or MUC1 peptide-pulsed DC vaccination together with a chemotherapy consisting of gemcitabine and/or S-1.Citation17 However, precise immunological baseline factors related to survival remain to be solved. In the present study, baseline skin reactions were not identified as an independent prognostic factor for both OS and PFS in a multivariate analysis, though it was extracted as a prognostic factor in a univariate analysis (). Therefore, it is of particular interest that WT1 and MUC1 specific T cell numbers, detected by an IFNɤ ELIspot assay prior to DC vaccination, emerged as independent prognostic factors for OS. The finding indicates that the tumor specific immunity prior to DC vaccination play an essential role and affects clinical outcome. Because a limited subgroup of patients will really benefit from this combined DC vaccination with chemotherapy, it would be critical to identify the patients who could have a better prognosis by the precise evaluation of tumor specific immunity prior to therapy.
The primary limitation of this study is the relatively small number of patients and the fact that it was a nonrandomized study. Although the existence of an add-on effect of DC vaccination to a concurrent chemotherapy was strongly suggested, it is too early to conclude the existence of an add-on effect. A phase III study is warranted to draw a definitive conclusion.
In conclusion, we showed the safety and the clinical benefits of vaccination with WT1 and/or MUC1 peptide-loaded DC and OK-432 with a concurrent chemotherapy with GEM plus nab-PTX or FOLFIRINOX in patients with advanced PDAC. WT1 or MUC1-specific IFNɤ ELIspot number prior to DC vaccination was demonstrated to be an independent prognostic factor related to OS by the multivariate analyses, emphasizing the importance of a baseline anti-tumor immunity. These results suggest that WT1 and/or MUC1 peptide-loaded DC vaccination in combination with a chemotherapy might be a promising novel strategy for the treatment of patients with relapsed or refractory PDAC who have better prognostic factors.
Acknowledgments
We would like to express our thanks to patients who participated in this study and the referring physicians who performed a concomitant therapy and provided follow-up information. We would like to thank Mr. Timothy Grose for reviewing and correcting the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Siegel R, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. doi:10.3322/caac.21654.
- Bray F, Ferlay J, Soerjomataram I, Siegal R, Torre L, Jemal A. Global cancer statistics 2018: BLOBOCAN estimates of incidence and mortality worldwide for 36 cancers. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492.
- Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet. 2020;395(10242):2008–20. doi:10.1016/S0140-6736(20)30974-0.
- Chiorean EG, Cheung WY, Giordano G, KimG, Al-Batran SE. Real-world comparative effectiveness of nab-paclitaxel plus gemcitabine versus FOLFIRINOX in advanced pancreatic cancer: a systematic review. Ther Adv Med Oncol. 2019;11:1–17. doi:10.1177/1758835919850367.
- Wainberg ZA, Hochster HS, Kim EJ, George B, Kaylan A, ChioreanEG, Waterhouse DM, Guiterrez M, Parikh A, Jain R, et al. Open-label, phase I study of nivolumab combined with nab-paclitaxel plus gemcitabine in advanced pancreatic cancer. Clin Cancer Res. 2020;26(18):4814–22. doi:10.1158/1078-0432.CCR-20-0099.
- Wu AA, Bever KM, Ho WJ, Fertig EJ, Niu N, Zheng L, Parkinson RM, Durham JN, Onners B, Ferguson AK, et al. A Phase II study of allogeneic GM-CSF-transfected pancreatic tumor vaccine (GVAX) with ipilimumab as maintenance treatment for metastatic pancreatic cancer. Clin Cancer Res. 2020;26(19):5129–39. doi:10.1158/1078-0432.CCR-20-1025.
- Bancherereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–52. doi:10.1038/32588.
- Amedei A, Niccolai E, Prisco D. Pancreatic cancer: role of the immune system in cancer progression and vaccine-based immunotherapy. Hum Vaccin Immunother. 2014;10(11):3354–68. doi:10.4161/hv.34392.
- Oji Y, Nakamori S, Fujikawa M, Nakatsuka S, Yokota A, Tatsumi N, Abeno S, Ikeba A, TakashimaS, Tsujie M, et al. Overexpression of the Wilms’ tumor gene WT1 in pancreatic ductal adenocarcinoma. Cancer Sci. 2004;95(7):583–87. doi:10.1111/j.1349-7006.2004.tb02490.x.
- Hinoda Y, Ikematsu Y, Horinochi M, SatoS, YamamotoK, Nakano T, Fukui M, Suehiro Y, Hamanaka Y, Nishikawa Y, et al. Increased expression of MUC1 in advanced pancreatic cancer. JGastroenterol. 2003;38(12):1162–66. doi:10.1007/s00535-003-1224-6.
- Scharnhost V, van der Eb AJ, Jochemsen AG. WT1 proteins: functions in growth and differentiation. Gene. 2001;273(2):141–61. doi:10.1016/s0378-1119(01)00593-5.
- Nakatsuka S, OjiY, Horiuchi T, Kanda T, Kitagawa M, Takeuchi T, Kawano K, Kuwae Y, Yamauchi A, Okumura M, et al. Immunohistochemical detection of WT1 protein in a variety of cancer cells. Mod Pathorol. 2006;19(6):804–14. doi:10.1038/modpathol.3800588.
- Gendler SJ, Lancaster CA, Taylor-Papadimitriou J, Duhig T, Peast N, Burchell J, Pemberton L, Lalani EN, Wilson D. Molecular cloning and expression of human tumor-associated polymorphic epithelial mucin. J Biol Chem. 1990;265(25):15286–93. doi:10.1016/S0021-9258(18)77254-2.
- Masaki Y, OkaM, Ogura Y, Ueno T, Nishihara K, Tangoku A, TakahashiM, Yamamoto M, Irimura T. Sialylated MUC1 mucin expression in normal pancreas, benign pancreatic lesions and pancreatic ductal adenocarcinoma. Hepatogastroenterology. 1999;46:2240–45.
- Nishida S, Koid S, Takeda Y, HommaS, KomitaH, Takahara A, Morita S, Ito T, Morimoto S, HaraK, et al. Wilms tumor gene (WT1) peptide-based cancer vaccine combined with gemcitabine for patients with advanced pancreatic cancer. J Immunother. 2014;37(2):105–14. doi:10.1097/CJI.0000000000000020.
- Kimura Y, Tsukada J, Tomoda T, Takahashi H, Imai K, Shimamura K, Sunamura M, Yonemitsu Y, ShimodairaS, KoidoS, et al. Clinical and immunologic evaluation of dendritic cell-based immunotherapy in combination with gemcitabine and/or S-1 in patients with advanced pancreatic carcinoma. Pancreas. 2012;41(2):195–205. doi:10.1097/MPA.0b013e31822398c6.
- Kobayashi M, Shimodaira S, Nagai K, Ogasawara M, Takahashi H, Abe H, Tanii M, Okamoto M, Tsujitani S, Yusa S, et al. Prognostic factors related to add-on dendritic cell vaccines on patients with inoperable pancreatic cancer receiving chemotherapy: a multicenter analysis. Cancer Immunol Immunother. 2014;63(8):797–806. doi:10.1007/s00262-014-1554-7.
- Koido S, Homma S, Okamoto M, TakakuraK, Mori M, Yoshizaki S, Tsukinaga S, Odahara S, Koyama S, Imazu H, et al. Treatment with chemotherapy and dendritic cells pulsed with multiple Wilms’ Tumor 1 (WT1)-specific MHC class I/II-restricted epitopes for pancreatic cancer. Clin Cancer Res. 2014;20(16):4228–39. doi:10.1158/1078-0432.CCR-14-0314.
- Mayanagi S, Kitago M, Sakurai T, Matsuda T, Fujita T, Higuchi H, Taguchi H, Itano O, Aiura K, Hamamoto Y, et al. Phase I pilot study of Wilms tumor gene 1 peptide-pulsed dendritic cell vaccination combined with gemcitabine in pancreatic cancer. Cancer Sci. 2015;106(4):397–406. doi:10.1111/cas.12621.
- Kondo H, Hazama S, Kawaoka T, Yoshino S, Yoshida S, Tokuno K, Takashima M, Ueno T, Hinoda Y, Oka M. Adaptive immunotherapy for pancreatic cancer using MUC1 peptide-pulsed dendritic cells and activated T lymphocytes. Anticancer Res. 2008;28(1B):379–88. PMID: 18383873.
- Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–703. doi:10.1056/NEJMoa1304369.
- Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarm Y, Adenis A, Raoul J-L, Gourgou-Bourgade S, de la Fouchardiere C, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–25. doi:10.1056/NEJMoa1011923.
- Takahara A, Koido S, Ito M, Nagasaki E, Sagawa Y, Iwamoto T, Komita H, Ochi T, Fujiwara H, Yasukawa M, et al. Gemcitabine enhances Wilms’ tumor gene WT1 expression and sensitizes human pancreatic cancer cells with WT1-specific T-cell-mediated antitumor immune response. Cancer Immunol Immunother. 2011;60(9):1289–97. doi:10.1007/s00262-011-1033-3.
- Soeda A, Morita-Hoshi Y, Makiyama H, Morizane C, Ueno H, Ikeda M, Okusaka T, Yamagata S, Takahashi N, Hyodo I, et al. Regular dose of gemcitabine induces an increase in CD14+ monocytes and CD11c+ dendritic cells in patients with advanced pancreatic cancer. Jpn J Clin Oncol. 2009;39(12):797–806. doi:10.1093/jjco/hyp112.
- Lim SH, Chua W, Cheng C, Descallar J, Ng W, Solomon M, Bokey L, Wong K, Lee MT, de Souza P, et al. Effect of neoadjuvant chemoradiation on tumor-infiltrating/associated lymphocytes in locally advanced rectal cancers. Anticancer Res. 2014;34(11):6505–13. PMID: 25368252.
- Kanterman J, Sade-Feldman M, Biton M, Ish-Shalom E, Lasry A, Goldshtein A, Hubert A, Baniyash M. Adverse immunoregulatory effects of 5FU and CPT11 chemotherapy on myeloid-derived suppressor cells and colorectal cancer outcomes. Cancer Res. 2014;74(21):6022–35. PMID: 25209187. doi:10.1158/0008-5472.CAN-14-0657.
- Demaria S, Volm MD, Shapiro RL, Yee HT, Oratz R, Formenti SC, Muggia F, Symmans WF. Development of tumor-infiltrating lymphocytes in breast cancer after neoadjuvant paclitaxel chemotherapy. Clin Cancer Res. 2001;7(10):3025–30. PMID: 11595690.
- Gonzalez-Aparicio M, Alzuguren P, Mauleon I, Medina-Echeverz J, Hervas-Stubbs S, Mancheno U, Berraondo P, Crettaz J, Gonzalez-Aseguinolaza G, Prieto J, et al. Oxaliplatin in combination with liver-specific expression of interleukin 12 reduces the immunosuppressive microenvironment of tumours and eradicates metastatic colorectal cancer in mice. Gut. 2011;60(3):341–49. doi:10.1136/gut.2010.211722.
- Ogasawara M, Miyashita M, Ota S. Vaccination of urological cancer patients with WT1 peptide-pulsed dendritic cells in combination with molecular targeted therapy or conventional chemotherapy induces immunological and clinical responses. Ther Apher Dial. 2018;22(3):266–77. doi:10.1111/1744-9987.12694.
- Ogasawara M, Miyashita M, YamagishiY, Ota S. Phase I/II pilot study of Wilms’ tumor 1 peptide-pulsed dendritic cell vaccination combined with conventional chemotherapy in patients with head and neck cancer. Ther Apher Dial. 2019;23(3):279–88. doi:10.1111/1744-9987.12831.
- Ogasawara M, Miyashita M, Yamagishi Y, Ota S. Immunotherapy employing dendritic cell vaccination for patients with advanced or relapsed esophageal cancer. Ther Apher Dial. 2020;24(5):482–91. doi:10.1111/1744-9987.13542.
- Stotz M, Gerger A, Eisner F, Szkandera J, Loibner H, Ress AL, Kornprat P, AlXoughbi W, Seggewies FS, Lacner C, et al. Increase neutrophil-lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer. Br J Cancer. 2013;109(2):416–21. doi:10.1038/bjc.2013.332.
- Imaoka H, Mizuno N, Hara K, Hijioka S, Tajika M, TanakaT, Ishihara M, Yogi T, Tsutsumi H, Fujiyoshi T, et al. Evaluation of modified Glasgow prognostic score for pancreatic cancer: a retrospective cohort study. Pancreas. 2016;45(2):211–17. doi:10.1097/MPA.0000000000000446.
- Dolan RD, McSorley ST, Horgan PG, Laird B, McMillan DC. The role of the systemic inflammatory response in predicting outcomes in patients with advanced inoperable cancer: systematic review and meta-analysis. Crit Rev Oncol Hematol. 2017;116:134–46. doi:10.1016/j.critrevonc.2017.06.002.
- Kanai T, Ito Z, Oji Y, Suka M, Nishida S, Takakura K, Kajihara M, Saruta M, Fujioka S, Misawa T, et al. Prognostic significance of Wilms’ tumor 1 expression in patients with pancreatic ductal adenocarcinoma. Oncol Lett. 2018;16(2):2682–92. doi:10.3892/ol.2018.8961.
- Ryoma Y, MoriyaY, Okamoto M, Kanaya I, Saito M, Sato M. Biological effect of OK-432 (picibanil) and possible application to dendritic cell therapy. Anticancer Res. 2004;24(5C):3295–301. PMID: 15515424.
- Gochi A, Orita K, Fuchimoto S, Tanaka N, Ogawa N. The prognostic advantage of preoperative intratumoral injection of OK-432 for gastric cancer patients. Br J Cancer. 2001;84(4):443–51. doi:10.1054/bjoc.2000.1599.
- Kasahara K, Shibata K, Shintani H, Iwasa K-I, Sone T, Kimura H, Nobata H, Hirose T, Yoshimi Y, Katayama N, et al. Randomized phase II trial of OK-432 in patients with malignant pleural effusion due to non-small cell lung cancer. Anticancer Res. 2006;26(2B):1495–99. PMID: 16619563.
- Okamoto M, Furuichi S, Nishioka Y, Oshikawa T, Tano T, Ahmed SU, Takada K, Arita S, Ryoma Y, Moriya Y, et al. Expression of toll-like receptor 4 on dendritic cells is significant for anticancer effect of dendritic cell-based immunotherapy in combination with an active component of OK-432, a streptococcal preparation. Cancer Res. 2004;64(15):5461–70. doi:10.1158/0008-5472.CAN-03-4005.
- Nagai K, Adachi T, Harada H, Eguchi S, Sugiyama H, Miyazaki Y. Dendritic cell-based immunotherapy pulsed with Wilms tumor 1 peptide and mucin 1 as an adjuvant therapy for pancreatic ductal adenocarcinoma after curative resection: a phase I/IIa clinical trial. Anticancer Res. 2020;40(10):5765–76. doi:10.21873/anticanres.14593.
- Bilici A. Prognostic factors related with survival in patients with pancreatic adenocarcinoma. World J Gastroenterol. 2014;20(31):10802–12. doi:10.3748/wjg.v20.i31.10802.
- Zhou Y, Cheng S, Fathy AH, Qian H, Zhao Y. Prognostic value of platelet-to-lymphocyte ratio in pancreatic cancer: a comprehensive meta-analysis of 17 cohort studies. Onco Targets Ther. 2018;11:1899–908. doi:10.2147/OTT.S154162.
- Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Research. 2017;27(1):109–18. doi:10.1038/cr.2016.151.
- Cheng H, LuoG, Lu Y, JinK, Guo M, XuJ, Kong J, LiuL, YuX, Liu C. The combination of systemic inflammation-based marker NLR and circulating regulatory T cells predicts the prognosis of resectable pancreatic cancer patients. Pancreatology. 2016;16(6):1080–84. doi:10.1016/j.pan.2016.09.007.
- Tang Y, Xu X, Guo S, Zhang C, Tang Y, Tian Y, NiB, Lu B, Wang H. An increased abundance of tumor-infiltrating regulatory T cells is correlated with the progression and prognosis of pancreatic ductal adenocarcinoma. PLoS One. 2014;9(3):e91551. doi:10.1371/journal.pone.0091551.
- Liu C, Cheng H, Luo G, Lu Y, Jin K, Guo M, Ni Q, Yu X. Circulating regulatory T cell subsets predict overall survival of patients with unresectable pancreatic cancer. Int J Oncol. 2017;51(2):686–94. doi:10.3892/ijo.2017.4032.