ABSTRACT
Response measures to mitigate the coronavirus disease 2019 pandemic impacted access to routine vaccination services. We evaluate the impact of the pandemic on routine infant vaccination uptake by comparing vaccination coverage, vaccine delays and doses administered in 2019 and 2020, in Quebec, Canada. Using a population-based vaccination registry, we compared vaccination coverage at 3, 5, 13 and 19 months of age between 2019 and 2020 cohorts each month from January to November. For vaccine delays, we measured the cumulative proportion vaccinated in each targeted cohort monthly. We also compared the measles-containing vaccines administered before 24 months of age between the same period in 2019 and 2020. A decline in vaccination coverage and children vaccinated on time was observed in all cohorts during the first months of the pandemic. The greatest impact was observed for the 18-month vaccination visit with a difference in vaccination coverage between both cohorts of 30.9% in May. Measles-containing doses administered during the first months of the pandemic were lower in 2020 compared with 2019: −21.1% in March (95%CI-21.6;-20.4), and −39.2% in April (95%CI-40.0;-38.2). After May, the coverage increased for all cohorts to reach pre-pandemic levels after a few months for most target ages. Routine childhood vaccinations were affected during the first months of the pandemic, but catch-up occurred thereafter and vaccination coverage in affected cohorts were very close to levels of 2019 after a few months of follow-up. Real-time monitoring of childhood vaccination is essential but also for other vaccination programs, severely affected by the pandemic.
Introduction
The ongoing coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), started in late 2019 and spread rapidly to many countries resulting in a World Health Organization (WHO) declaration of a global pandemic on March 11, 2020.Citation1,Citation2 In the province of Quebec, in eastern Canada, the first case of COVID-19 was diagnosed February 27, 2020. On March 13, 2020, the government of Quebec declared a state of public health emergency. Response measures to mitigate the pandemic included a general shutdown of economic and social activities, as well as a stay-at-home order (Supplementary file 1).Citation3
The pandemic impacted access to routine vaccination services through cancellation of immunization appointments by providers, physical distancing measures and parental hesitancy to go to health facilities.Citation4 Given this context, public health authorities had to set new guidelines for routine vaccination programs. In Canada, the National Advisory Committee on Immunization (NACI) advised prioritizing the primary immunization series for infants and toddlers while postponing boosters for the 4–6 years age group, so long as they were received before school entry.Citation5 Within the province of Quebec, the Quebec Immunization Committee (QIC) initially recommended prioritizing vaccinations given in infancy (at 2, 4, and 12 months of age, statement released March 18, 2020). Due to the exceptional lack of health human resources caused by resource mobilization for pandemic response, and the applied physical distancing measures, the QIC also advised healthcare providers that, if necessary, they could defer all vaccination visits until the pressure on the healthcare system eased. On May 6, 2020, as Quebec’s COVID-19 situation became more stable, the QIC changed its recommendation to advise that immunization visits should be resumed for all vaccines given at 2, 4, 6 and 18 months of age.
Given the possible impact of these extraordinary circumstances on routine vaccination activities, it is essential to evaluate changes in vaccination coverage (VC) in order to estimate the risk of vaccine preventable disease outbreak in the population and determine next steps for vaccine programs.Citation6 Few published studies have examined this issue; however, results from both England and the United States (U.S) have all indicated a remarkable reduction in vaccination activities during the initial months of the COVID-19 pandemic.Citation7–10 Recently, Murthy et al.Citation10 noticed that the vaccine doses administered to children and adolescents during June-September 2020 were close to the pre-pandemic levels in most U.S. jurisdictions evaluated. Using a longer period of follow-up, the objective of this study was to evaluate the impact of the COVID-19 pandemic on routine infant vaccination uptake by measuring the VC, the vaccine delays and the number of administered doses in 2020 in comparison with the previous year 2019.
Method
Setting and data source
On March 10, 2021, data were extracted from the vaccine registry database in Quebec (population 8.5 million; about 83 000 births annually).Citation11 This population-based registry contains sociodemographic characteristics, vaccines received and date of vaccine administration. Sociodemographic data are updated by the Quebec universal public health insurance database, which includes about 97% of Quebecers. The registry was introduced gradually in Quebec starting June 2014 with public health settings, where about 80% of children were vaccinated. Furthermore, work to populate the registry with data from private settings has been ongoing since December 2018. All facilities that provide vaccines mandated to enter all administered vaccines in the registry, but vaccines given in a private setting have a lesser likelihood to be in the registry and this could affect the global VC.Citation12
Vaccination schedule
Quebec’s vaccination schedule has changed in recent years, with the most recent changes applying to children born since June 1, 2019 (). Thus, cohorts targeted by our evaluation had different vaccination schedules. To make cohorts comparable, we limited the analysis to routine vaccines that were offered to both cohorts. Only doses respecting recommended minimum/maximum age and minimum intervals between doses were included in this evaluation.
Table 1. Quebec vaccination schedule up to 24 months of age as applicable for children in this study
Outcomes
Vaccination coverage at target ages
To evaluate changes in VC during the pandemic, we compared VC among 2019 and 2020 cohorts one month after their due date of vaccination (i.e., on the day before they turned 3, 5, 13, and 19 months of age). We examined each month from January to November in 2019 (before the pandemic) and 2020 (during the pandemic). We looked for vaccines given up to November 30, 2020 as they would almost certainly be recorded in the registry by March 10, 2021 when we extracted data. For each month, VC was calculated as the proportion of children vaccinated among those eligible to be vaccinated. VC was calculated for DTaP-containing vaccine by 3, 5 and 19 months of age, rotavirus vaccine by 3 and 5 months of age, pneumococcal conjugate vaccine (PCV) by 13 months of age (third dose), and measles-containing vaccines (measles-mumps-rubella [MMR] or MMR-varicella [MMRV]) by 13 and 19 months of age.
Vaccination delay over time
To determine vaccine delay, we measured the proportion of vaccinated children in each age cohort (2 and 4 months old for DTaP-containing vaccines; 12 and 18 months old for measles-containing vaccines) for the months of March, April, May and June in both 2019 and 2020. We then measured the cumulative proportion vaccinated in each cohort monthly for the following 4 months (2-month-old cohort) or 6 months (all other cohorts) to compare the time to complete coverage in 2020 compared to 2019.
Vaccination counts
Using methods recommended by McDonald et al.Citation7, we calculated the number of weekly doses of measles-containing vaccines administered before 24 months of age from the first full week in January to the week beginning on November 22, 2020. We then calculated the weekly and monthly percent change in doses during 2020 compared with data from the same period in 2019.
Statistical analyses
Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary NC). We used the Chi-square test for VC comparisons between 2020 and 2019, with statistical significance set at α = 0.05. The 95% confidence intervals were estimated using an exact method for binomial proportions.
Ethical statement
Ethical approval for this study was obtained from the Health Research Ethics Board at the University of Alberta (PRO# 00102401). This evaluation was done as part of a public health evaluation and therefore an ethical approval from Quebec was not sought.
Results
Cohorts
The study included 200 901 children for the evaluation in 2019 and 198 477 children in 2020.
Changes in vaccination coverage at target ages
At the start of the evaluation period, a higher proportion of children were vaccinated by each target age in 2020 compared with 2019 (), with a marked difference for the rotavirus vaccine compared with DTaP vaccine at 3 and 5 months. VC subsequently decreased in all age cohorts during the first months of the pandemic. VC by 3 months for the DTaP-containing vaccine decreased from a high of 90.1% in March 2020 to 85.1% in April 2020, which was 4.2% lower than VC in the equivalent April 2019 cohort (, panel A). The VC estimates for 2020 remained below estimated 2019 coverage for all subsequent observation periods, with a difference varying between 2.7% to 5.0%. In November, the difference was 3.1% between both years. The same trends were observed for the rotavirus vaccine at 3 months; however, with smaller differences between 2019 and 2020 coverage. Similar results were observed among children aged 5 months, with VC for DTaP decreasing 6.7% between March and April 2020 (, panel B). However, for this age cohort, there was no difference in rotavirus VC between the two years from April to August. As VC for DTaP-containing vaccine and PCV vaccine were quite similar for both 3 and 5 months of age, we presented results for DTaP vaccine only.
Figure 1. Vaccination coverage of children who turned the milestone ages each month from January to November in 2019 and 2020. VC assessed by 3 months old (Panel A) and 5 months old (Panel B) for the first and second doses of DTaP and rotavirus vaccines scheduled at 2 and 4 months old. *P-value < .05 for the comparison in VC between 2019 and 2020 using a chi-square test.
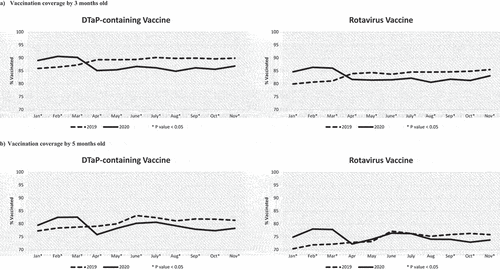
Figure 2. Vaccination coverage of children who turned the milestone ages each month from January to November in 2019 and 2020. VC assessed by 13 months old (Panel A) for the first dose of the measles-containing vaccine and for the third dose of the pneumococcal vaccine scheduled at 12 months old, and by 19 months old (Panel B) for the DTaP vaccine and the measles-containing vaccine (second doses) scheduled at 18 months old. *P-value < .05 for the comparison in VC between 2019 and 2020 using a chi-square test.
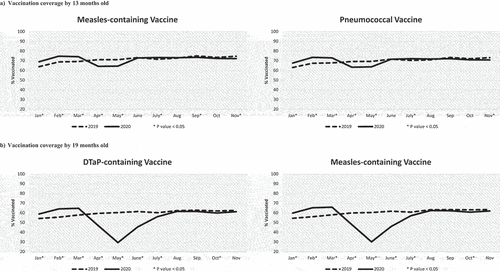
For the first dose of the measles-containing vaccine and the third dose of PCV vaccine, both due at 12 months, the decline in VC by 13 months from March to April 2020 was approximately 10%, with VC 6–7% lower than the same cohort in April 2019 (, panel A). The VC remained low in May 2020 and then increased from June to August to similar or higher levels than those reached in 2019. Thereafter, the 2020 VC remained slightly below 2019 with a 2–3% difference at the end of the follow-up.
The largest impact was observed for the 19-month age cohort (, panel B). VC for the 4th dose of DTaP-containing vaccine was only 29.5% in May 2020, a drop of 35.3% from March 2020, and 30.9% lower than the May 2019 cohort. VC steadily increased from June to August, reaching similar VC to 2019. We observed similar trends for the 2nd dose of the measles-containing vaccine, also scheduled at 18 months (, panel B).
Vaccination delays over time
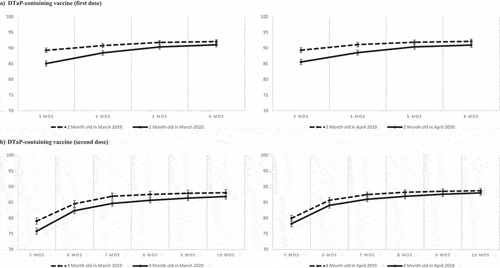
For children who turned 2 months old in March or April 2020, the cumulative proportion vaccinated by 3 months of age with the first dose of DTaP-containing vaccine was about 4% lower in 2020 compared with 2019. By 5 or 6 months of age, coverage was similar in both years (, panel A). Among children who turned 2 months old in May and June (Supplementary file 2), the cumulative proportion of those vaccinated each month was lower in 2020 compared to 2019; however, the difference was not statistically significantby 5 and 6 months old (in May only), with more than 90% adequately vaccinated by 6 months. Similar trends were observed for the 4-month vaccination visit, with no significant difference between 2019 and 2020 for children reaching 4 months of age in April and small differences in May and June (, Panel B and Supplementary file 2).
Figure 3. Cumulative proportion of children vaccinated for the first and second dose of DTaP-containing vaccine among children turning 2 months old (Panel A) and 4 months old (Panel B) in March 2019 and 2020 (left) and in April 2019 and 2020 (right). Bars represent 95% Confidence intervals calculated with the exact method.
For children reaching 12 months of age in March and April, the cumulative proportion of children vaccinated by 13 months of age with the first dose of measles-containing vaccine was about 7% lower in 2020 than in 2019 (, panel A). By 15 and 16 months, the difference was 2% or less, becoming non-significant by 17 months. For children who turned 12 months old in May and June (Supplementary file 3), the difference between 2019 and 2020 was not statistically significant.
Figure 4. Cumulative proportion of children vaccinated for the first and second dose of measles-containing vaccine among children turning 12 months old (Panel A) and 18 months old (Panel B) in March 2019 and 2020 (left) and in April 2019 and 2020 (right). Bars represent 95% Confidence intervals calculated with the exact method.
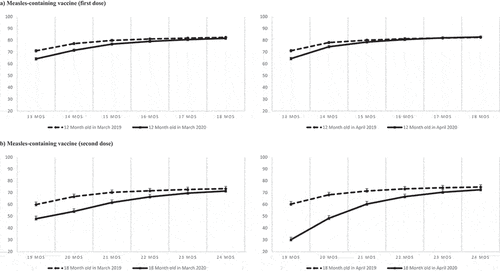
The greatest delay was observed at 18 months of age for the second dose of measles-containing vaccine. For those who turned 18 months old in April, the cumulative proportion vaccinated by 19 months was 30.1% lower in 2020 compared to 2019. By 24 months of age, the difference decreased to 2% but remained statistically significant (, panel B). For those who turned 18 months old in May and June, the difference between 2019 and 2020 cumulative proportions was no longer statistically significant after 22 and 20 months, respectively (Supplementary file 3).
Vaccination counts
During the first two months of 2020 (pre-pandemic), the number of measles-containing vaccine doses administered before 24 months of age was higher than the same period in 2019 (). This was followed by a considerable relative reduction of vaccination doses administered: −21.1% in March (95% CI −21.6; −20.4), and −39.2% in April (95% CI −40.0; −38.2). The reduction was greatest between the third week of March and the third week of May 2020 (corresponding to weeks 11 to 20) (Supplementary file 4).
Table 2. Doses of measles-containing vaccines (MMR or MMRV) administered before 24 months of age in 2020 compared with 2019, by month from January to November
From the fourth week of May 2020 through August, the vaccination counts tended to be higher in 2020 than in 2019. We observed another reduction in vaccination counts in September, October and some weeks in November (Supplementary file 4).
Discussion
This study suggests that the pandemic and associated restrictive measures in Quebec impacted vaccination activities for children under two years of age. The decline in both VC and proportion of children vaccinated on time was most marked from March to May 2020, corresponding to a period with intense measures put in place to control the pandemic. This decline was noticed for all vaccination visits, but it was greatest for the oldest cohort. After May 2020, the VC increased for those who turned milestone ages (i.e., 3, 5, 13, and 19 months of age), with small or non-significant differences between 2019 and 2020 at the end of the follow-up. VC by 3 and 5 months remained lower in 2020 compared with 2019. Similarly, the delay in coverage was more profound among children with a vaccination due in March and April 2020. However, coverage increased to pre-pandemic levels after a few months for most target ages. The pandemic impact was also documented in the number of doses of measles-containing vaccines administered before 24 months of age, with fewer doses administered in March, April and the first three weeks of May 2020, compared with the same periods in 2019.
The largest impact observed for older children during the first months of the pandemic might be explained by the QIC recommendations to prioritize infant vaccination visits (i.e., at 2, 4 and 12 months). The increase in the vaccination activities in the subsequent months might be linked to the successful implementation of the QIC’s May 2020 recommendation to resume all vaccination activities for children less than 24 months of age and the reinforcing message that vaccination is essential. Our results also suggest that there may have been strategies to catch up with children unvaccinated during the first months of the pandemic. Despite a large drop in VC by 19 months old in April and May 2020, the cumulative proportion of vaccinated children increased steadily for each following month, reaching only a 2% difference by 24 months compared with the same cohort in 2019. In addition, vaccination counts varied according to the intensity of pandemic control measures in place, the recommendations for vaccination activities and the epidemiologic context.Citation3
Our results are consistent with other published reports from outside Canada. Similar to results obtained by Santoli et al.Citation9 and Murthy et al.Citation10 in the U.S. and by McDonald et al.Citation7 in England, the reduction in the number of administered doses of recommended childhood vaccination started in March, corresponding to the period of national emergency declaration. Santoli et al.Citation9 observed a 50% reduction in the measles-containing vaccine doses administered for the week after the lockdown, compared to the preceding week. The reduction in the comparable period in Quebec was 27%. Santoli et al. also noticed an increase in vaccine administration starting in late March, potentially attributed to the early success of strategies implemented to promote vaccination during the pandemic. In the three weeks after the introduction of full measures, MacDonald et al.Citation7 noticed that MMR vaccination (first dose) was 19.8% lower than in 2019 and observed that vaccination counts increased thereafter despite restrictive measures remaining in place. In Quebec, the relative difference for all doses of measles-containing vaccine before 24 months during this period was about 34%, and vaccination counts increased from the fourth week of May 2020. As observed in our study, Murthy et al.Citation10 found that after a marked decline from March-May 2020, vaccine administration increased thereafter (i.e., during June-September). However, authors mentioned that this increase was not sufficient to catch-up children who missed vaccination during the first months of the pandemic. Similar to Bramer et al.Citation8 we observed a reduction in VC for all milestone age cohorts less than two years in May 2020. In our study, we observed a more pronounced impact for the 19-month age cohort. Results from McDonald’s study also suggest a bigger impact on older children.
Several plausible explanations for the reduction of routine vaccination coverage have been discussed in the literature. Besides the stay-at-home order, healthcare worker’s mobilization in response to the pandemic might have caused a shortage in the resources available for routine vaccination and other non-emergency activities. In addition, the use of teleconsultations during the pandemic could have reduced vaccination activities. Parental concerns about visiting health facilities with their children during this period could have also resulted in vaccine delays and a drop in VC. Likewise, vaccination activities might have been perceived as a nonessential activity by parents.Citation13 In an online parental survey conducted in Australia in June 2020, concern about catching COVID-19 during the vaccination visit was the main reason mentioned by parents for vaccine delays.Citation14
Limitations
Our findings are subject to some limitations. Firstly, it is possible that delays to data entry in the registry during the pandemic might have overestimated the drop in VC observed in 2020. These delays may have disproportionately affected VC in younger children from the 2020 cohort, because they had less opportunities for the data to be entered in the registry, in part due to changes in the vaccination schedule and the difference in time since vaccination. However, the impact on our results should be small as we can confirm that among doses administered from March to November 2020 to children before 3 years of age and available in the registry, nearly 99% were entered within three months of their date of administration. Data were extracted in mid-March 2021 and our evaluation period did not go beyond November 2020. Parental hesitancy about visiting health facilities during the pandemic could also explain why VC by 3 and 5 months of age remained lower in 2020 compared with 2019. Secondly, VC was higher in 2020 vs 2019 at the start of the assessment period, which would have hidden some of the impact of the pandemic on VC. However, we did see a continuous drop in VC from March through April/May 2020. Completeness of data in the registry is known to improve over time and this could also have underestimated the impact on VC for the comparison between years. In Quebec, there has been an increase in VC and a decrease in vaccine delays over the years.Citation15,Citation16 Thirdly, we presented only aggregated data for the province of Quebec. Decreases in VC due to the pandemic may have varied between regions, and a regional approach could help to prioritize efforts. Finally, we observed that the proportion of children adequately vaccinated using data from the provincial registry is lower than that estimated in our recent VC study, which combined multiple sources for vaccination data.Citation17 One plausible explanation is that this source remains incomplete. There is also a small probability that children might have been vaccinated outside Quebec, and thus not captured in our analysis, but pandemic travel restrictions between Canadian provinces and internationally make that unlikely.
Conclusion
Maintaining childhood vaccination activities during the pandemic is essential. Missed vaccination or delayed vaccination can leave young children vulnerable to vaccine-preventable diseases, and this could be an important issue post-pandemic, especially when international travel begins to increase. In Quebec, vaccination visits among children less than 2 years of age were affected during the first months of the pandemic, but catch-up occurred thereafter and VC in affected cohorts were very close to levels observed in 2019 after a few months of follow-up. Catch-up activities for children left behind due to the pandemic have been prepared and initiated in Quebec and other Canadian provinces. Vaccination services have also been revised to ensure greater access to vaccination while limiting the risk of COVID-19 transmission. We presented here the data for the impact until November 2020, but real-time monitoring of vaccination coverage and the ability to catch up is essential, both for early childhood vaccination and school-based programs.
Authors contribution
All authors have contributed meaningfully to the paper including conception and design or acquisition of data or analysis and interpretation of data. All authors have revised the article critically for important intellectual content as well as final approval of the version to be published.
Abbreviations
Supplemental Material
Download MS Power Point (64.9 KB)Supplemental Material
Download MS Power Point (72.3 KB)Supplemental Material
Download MS Power Point (69.4 KB)Supplemental Material
Download MS Word (51 KB)Acknowledgments
Remi Gagné, Institut national de santé publique du Quebec, Canada.
Nicole Boulianne, Institut national de santé publique du Quebec, Canada.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2021.2007707
Additional information
Funding
References
- World Health Organisation. Novel coronavirus – China [ Internet]. 2020 Jan [accessed 2020 Nov 17]. https://www.who.int/csr/don/12-january-2020-novel-coronavirus-china/en/.
- World Health Organisation. WHO Director-General’s opening remarks at the media briefing on COVID-19-11 March 2020 [ Internet]. [accessed 2020 Nov 17]. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19—11-march-2020.
- Institut national de santé publique du Québec. Ligne du temps COVID-19 au Québec [ Internet]. [accessed 2020 Nov 17]. https://www.inspq.qc.ca/covid-19/donnees/ligne-du-temps.
- World Health Organisation. Guiding principles for immunization activities during the COVID-19 pandemic [ Internet]. 2020 [accessed 2020 Nov 17]. https://apps.who.int/iris/bitstream/handle/10665/331590/WHO-2019-nCoV-immunization_services-2020.1-eng.pdf?ua=1 .
- National Advisory Committee on Immunization (NACI). Interim guidance on continuity of immunization programs during the COVID-19 pandemic [ Internet]. 2020 [accessed 2020 Dec 1]. https://www.canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/interim-guidance-immunization-programs-during-covid-19-pandemic.html.
- Causey K, Fullman N, Sorensen RJD, Galles NC, Zheng P, Aravkin A, Danovaro-Holliday MC, Martinez-Piedra R, Sodha SV, and Velandia-González MP, et al. Estimating global and regional disruptions to routine childhood vaccine coverage during the COVID-19 pandemic in 2020: a modelling study. Lancet Lond Engl 398 10299 . 2021 Aug 07 :522–8.
- McDonald HI, Tessier E, White JM, Woodruff M, Knowles C, Bates C, Parry J, Walker JL, Scott JA, Smeeth L, and Yarwood J , et al. Early impact of the coronavirus disease (COVID-19) pandemic and physical distancing measures on routine childhood vaccinations in England, January to April 2020. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull. 2020 May;25:19.
- Bramer CA, Kimmins LM, Swanson R, Kuo J, Vranesich P, Jacques-Carroll LA, and Shen AK. Decline in child vaccination coverage during the COVID-19 pandemic - Michigan care improvement registry, May 2016–May 2020. MMWR Morb Mortal Wkly Rep. 2020 May 22;69(20):630–1. doi:10.15585/mmwr.mm6920e1.
- Santoli JM, Lindley MC, DeSilva MB, Kharbanda EO, Daley MF, Galloway L, Gee J, Glover M, Herring B, and Kang Y. Effects of the COVID-19 pandemic on routine pediatric vaccine ordering and administration - United States, 2020. MMWR Morb Mortal Wkly Rep. 2020 May 15;69(19):591–3. doi:10.15585/mmwr.mm6919e2.
- Patel Murthy B, Zell E, Kirtland K, Jones-Jack N, Harris L, Sprague C, Schultz J, Le Q, Bramer CA, and Kuramoto S, et al. Impact of the COVID-19 pandemic on administration of selected routine childhood and adolescent vaccinations - 10 U.S. jurisdictions, March–September 2020. MMWR Morb Mortal Wkly Rep. 2021 June 11;70(23):840–5. doi:10.15585/mmwr.mm7023a2.
- Institut de la statistique du Québec. Naissances, décès et mariages par mois et par trimestre, Québec, 2010–2021 [Internet]. 2021 Jul [accessed 2020 Dec 4]. https://statistique.quebec.ca/fr/produit/tableau/naissances-deces-et-mariages-par-mois-et-par-trimestre-quebec.
- Gouvernement du Québec. Québec Vaccination Registry [ Internet]. 2020 [accessed 2021 Jan 6]. https://www.efits-of-the-registry/
- Brooks HE, McLendon LA, and Daniel CL. The impact of COVID-19 on pediatric vaccination rates in Alabama. Prev Med Rep. 2021 June;22:101320. doi:10.1016/j.pmedr.2021.101320.
- The Royal Children's Hospital National Child Health Poll. Routine childhood vaccinations: effects of the COVID-19 pandemic. Poll number 18 Supplementary Report. The Royal Children’s Hospital Melbourne [ Internet]. 2020 [accessed 2020 Sep 11]. https://www.rchpoll.org.au/polls/covid-19-pandemic-effects-on-the-lives-of-australian-children-and-families/
- Kiely M, Boulianne N, Talbot D, Ouakki M, Guay M, Landry M, Sauvageau C, and De Serres G. Impact of vaccine delays at the 2, 4, 6 and 12 month visits on incomplete vaccination status by 24 months of age in Quebec, Canada. BMC Public Health. 2018 Dec 11;18(1):1364. doi:10.1186/s12889-018-6235-6.
- Kiely M, Boulianne N, Talbot D, Ouakki M, Guay M, Landry M, Zafack J, Sauvageau C, and De Serres G. Impact of the addition of new vaccines in the early childhood schedule on vaccine coverage by 24 months of age from 2006 to 2016 in Quebec, Canada. Vaccine. 2018 July 5;36(29):4383–91. doi:10.1016/j.vaccine.2018.03.085.
- Kiely M, Ouakki M, De Serres G, Dubé E, Guay M, and Audet D. Étude sur la couverture vaccinale des enfants québécois âgés de 1 an, 2 ans et 7 ans en 2019. Institut national de santé publique du Québec, 2021 [Internet]. 2021 [accessed 2021 June 6]. https://www.inspq.qc.ca/sites/default/files/publications/2776-couverture-vaccinale-enfants-quebecois.pdf.
