ABSTRACT
Background
High-density microarray patch (HD-MAP) vaccines may increase vaccine acceptance and use. We aimed to ascertain whether professional immunizers (PIs) and other healthcare workers (HCWs) in Australia, a High-Income Country (HIC), found the HD-MAP applicator usable and acceptable for vaccine delivery.
Methods
This feasibility study recruited PIs and HCWs to administer/receive simulated HD-MAP administration, including via self-administration. We assessed usability against essential and desirable criteria. Participants completed a survey, rating their agreement to statements about HD-MAP administration. A subset also participated in an interview or focus group. Survey data were analyzed using descriptive statistics, and interviews were transcribed and subject to thematic analysis.
Results
We recruited 61 participants: 23 PIs and 38 HCWs. Findings indicated high usability and acceptability of HD-MAP use across both groups by a healthcare professional or trained user and for self-administration with safety measures in place. Most administrations met essential criteria, but PIs, on average, applied the HD-MAP for slightly less time than the required 10-seconds, which the HCWs achieved. PIs perceived safety concerns about home administration but found layperson self-administration acceptable in an emergency, pandemic, and rural or remote settings.
Conclusions
Participants found HD-MAP administration usable and acceptable. Usability and acceptability are likely to be improved through end-user education and training.
Plain language summary
Professional immunizers and healthcare workers found high-density microarray patch devices highly usable and acceptable to administer vaccines.
HD-MAPs may have advantages over intramuscular injections in clinical settings and in pandemics.
Vaccination with HD-MAP may improve acceptance for those with needle-related anxiety.
Introduction
Vaccination using patches covered by an array of micro-projections may increase vaccine acceptance and use. Vaxxas’ high-density microarray patch (HD-MAP) is 1 cm2 of a biocompatible polymer covered by thousands (1500 to 3000) of micro-projections each 300 µm in length, coated with a dried vaccine formulation. The HD-MAP efficiently delivers the vaccine to the outer layers of the skin; the epidermis and the upper dermis.Citation1 Compared to vaccination with an intramuscular injection (IM), potential benefits of HD-MAP vaccines include: reducing pain and fear of pain of the hypodermic needle; no needle-stick injuries or sharps waste; no cold-chain requirement; no need for vaccine reconstitution; reduced vaccine dose and adjuvant-sparing; cost-savings, convenience, and the potential for self-administration.Citation2 If HD-MAP vaccines replace IM as a standard vaccine delivery method, they need to be highly acceptable and usable to vaccination providers, end-users and equal or superior to existing platforms in immunogenicity, efficacy, administration, and logistics.Citation3
Preferences for a specific delivery mode, and beliefs regarding safety and efficacy, may influence willingness to receive or use the HD-MAP to administer a vaccine.Citation4 Few studies have reported on acceptability and usability of vaccine microarray patch (MAP) administration with healthcare workers (HCWs): some have reported on actual administration or simulation,Citation5 while others have examined hypothetical administration.Citation6,Citation7 Some studies have examined preferences amongst adult laypeople, which suggest that MAP vaccination is favored over IM.Citation4,Citation8–12
Previously, we have reported on the acceptability of the Vaxxas HD-MAP in healthy adults aged 18 to 45 years as part of clinical trials.Citation8,Citation9 Building on an earlier study – a simulation of HD-MAP vaccine administration with children in Low and Middle-Income Countries (LMICs),Citation5 the primary objective of this study was to assess acceptability and usability of the Vaxxas prototype HD-MAP applicator through a simulation vaccination experience in a High-Income Country (HIC) real-world setting. We aimed to ascertain whether professional immunizers (PIs) and HCWs found the clinical HD-MAP applicator usable and acceptable for vaccine delivery. We hypothesized that PIs and HCWs would find the HD-MAP applicator highly usable and acceptable in healthcare settings, including for self-administration; and, that both groups would find self-administration usable and acceptable under emergency circumstances such as pandemic vaccine rollout.
Methods
We conducted this study in 2019 and early 2020 before the COVID-19 pandemic.
Design
This feasibility study assessed the usability and acceptability of a prototype HD-MAP applicator as a delivery method for vaccination in clinical settings. We followed the CONSORT extension to randomized pilot and feasibility trials, reviewed related methodology papers, and adapted existing guidelines to report our non-randomized study.Citation13–16,Citation17
To guide our approach, we employed an ecological framework to understand individual, social and organizational levels of influence on PIs and HCWs in a healthcare workplace.Citation18 The Integrative Behavioral Model (IBM), which includes constructs from the Theory of Reasoned Action and the Theory of Planned Behavior, informed survey development and interview guides.Citation19
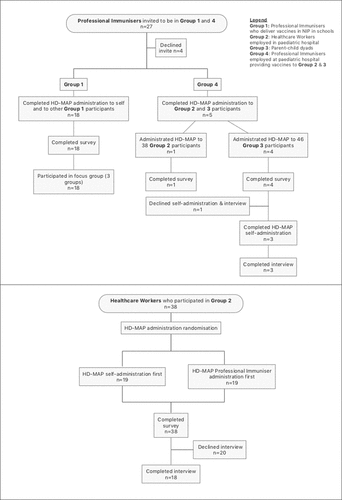
The HD-MAP applicator was administered to all participants in a simulation experience without HD-MAP or vaccine. We studied the usability and acceptability of the HD-MAP applicator in four participant groups, three of which are presented here.
Setting and participants
In Sydney, Australia, at two sites: a Public Health Unit responsible for delivering scheduled vaccines to adolescents in schools and a pediatric hospital (staff and child) immunization clinic.
Study groups included:
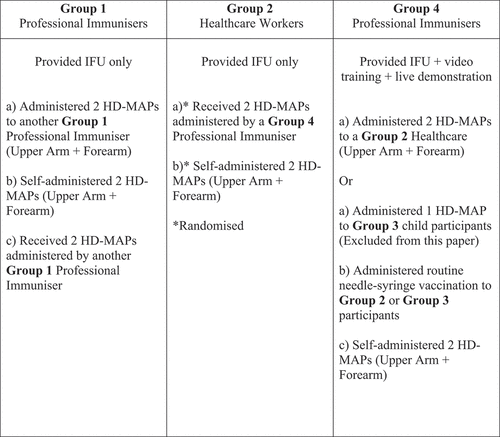
Group one: PIs who deliver vaccines in schools to adolescents as part of the National Immunization Program (NIP) (in this study PIs refers to trained immunization nurses);
Group two: HCWs employed or volunteering in a pediatric hospital (in this study, a HCW refers to a person working in a healthcare setting, including but not limited to health professionals);
Group three: Parent-child dyads (excluded from this paper; manuscript in preparation);
Group four: PIs employed at a pediatric hospital immunization clinic and who provided required vaccines to HCWs in group two, and to children under five in group three.
Eligible participants provided informed consent to participate in the study.
Recruitment
Study researchers approached interested PIs (groups one and four) and explained the study in-depth. Group four PIs introduced the study to all HCWs (group two) who presented for routine vaccination.
Follow-up interview participation was introduced to all individuals with the intention that a subset would self-select to participate in a semi-structured interview (from groups two and four) or focus group (from group one). (See ).
Intervention
The Vaxxas prototype spring-activated applicator did not contain a HD-MAP but otherwise represented the appearance and functionality of the device. An applicator is needed to apply the HD-MAP to the skin consistently and is supplied ready-for-use. The applicator is actuated by the user pushing a button with around 20 N of force, inverting the spring, and applying the HD-MAP. Once triggered, the applicator cannot be reused, and the entire device (applicator and integrated HD-MAP) can be discarded.
All study participants experienced administering and/or receiving simulated HD-MAP vaccinations. Administration sites included the subject’s upper arm (UA [deltoid]) and forearm ([volar] FA). Required wear-time was 10 seconds per administration. Group one PIs and two HCWs were given instructions for use (IFU) to read but no training. Group four PIs were assigned IFU and trained with a video and live demonstration. Group one PIs administered two HD-MAPs to another group one PI (UA and FA) and then self-administered two HD-MAPs. Group two HCWs received two HD-MAPs (UA and FA) administered by a group four PI; they then self-administered two HD-MAPs before receiving their routine vaccination. The HD-MAP administrations for group two HCWs were randomized to self-administration first or PI administration first. (See ).
Outcomes
In this study, following the international standard,Citation20 usability refers to the ease of use to achieve effectiveness, efficiency, and satisfaction through the device’s administration, maintenance, and removal. Acceptability refers to a ‘willingness and intention to vaccinate or receive a vaccination, and not actual vaccination.’Citation21 Acceptability includes how well an intervention is received by the target audience and the extent to which it meets individual and organizational needs.Citation22
The outcomes of this study presented here were predefined as follows:
Usability of the HD-MAP applicator for HCWs as administered by a) PI and b) self-administration, in association with pictorial/short written IFU.
Acceptability of the HD-MAP applicator for HCWs as administered by a) PI and b) self-administration, in association with pictorial/short written IFU.
Usability of the HD-MAP applicator for PIs as administered by a) another PI and b) self-administration, in association with pictorial/short written IFU and training video.
Acceptability of the HD-MAP applicator for PIs as administered by a) another PI and b) self-administration, in association with pictorial/short written IFU and training video.
Data collection
Usability and acceptability were assessed using the following data collection tools:
Observation Checklist
All HD-MAP administrations were video-recorded and scored using an observation checklist developed to assess HD-MAP applicator usability (see Appendix A). The observation checklist had eleven criteria, of which five were considered essential for the effectiveness of delivery, and six were desirable. Essential criteria were defined as the series of actions without which HD-MAP administration would fail, and desirable criteria were defined as actions to enable administration success.
(b) Survey
After the HD-MAP administrations, all participants completed a survey assessing perceptions of usability and acceptability (see Appendix B). The IBM detailed above, and a questionnaire used in a similar study, informed survey development.Citation4,Citation19 The survey was pilot tested with several PIs and HCWs for face validity. Participants rated their agreement with statements about their experience when compared to vaccination by IM. Group one and group two completed the survey directly after the HDMAP administrations. Group four were surveyed after completing all HD-MAP administrations to study participants in groups two and three.
(c) Interview
We conducted focus groups with group one PIs on the day of the HD-MAP administrations. We conducted interviews with group two HCWs between 0–14 days post-HD-MAP administrations and group four PIs after completing all HD-MAP administrations to groups two and three. The interviews probed participant experiences about HD-MAP usability, acceptability, beliefs, and attitudes regarding vaccine delivery preferences (see Appendix C).
Sample size
As this was a feasibility study, we aimed to recruit at least 20–60 participants in each group.
Randomization
After consent, an investigator (CD) randomly allocated participants by IDs to either “PI administration first” or “self-administration first.” This process ensured that we captured a range of experiences. The investigator preparing the randomized envelopes noted this assignment in the participant ID log for later data analysis. Research staff recruiting subjects were blinded to group allocation.
Data analysis
Quantitative analysis
We completed a descriptive statistical analysis of survey data, including mean agreement and standard deviations, and present usability and acceptability findings. We also categorized observation checklist data into ‘essential’ and ‘desirable’ criteria and present descriptive statistics. Comparisons involving the same participant (for example, comparing administrations sites) were undertaken to identify potential statistical associations.
Qualitative analysis
We stopped recruitment once data saturation (or data adequacy) was achieved. Interviews were recorded, transcribed and coded in NVivo12 where data was subject to thematic analysis.Citation18,Citation23 We used inductive and deductive approaches to generate codes employed across the data set. CD, MT, and CK developed codes: coding was undertaken sentence-by-sentence, identifying, and discussing themes. We reached conceptual saturation when no new codes were generated. CD performed an overall analysis to ensure that diverse themes emerging from the data set were represented.
Ethics and informed consent
The study was approved by the Human Research Ethics Committee of the participating sites. It was conducted following the principles of the International Conference on Harmonization’s Good Clinical Practice guidelines, as adopted in AustraliaCitation24 which builds upon the ethics codes contained in the Declaration of HelsinkiCitation25 (SCHN HREC approval 2019/ETH11789).
Results
There were 61 participants: 18 PIs (all female) in group one, 38 HCWs (30 female, eight male) in group two, and five PIs (all female) in group four (See ). Participants were recruited from September 23rd, 2019, until recruitment ceased due to COVID-19 restrictions on March 3rd, 2020, however data saturation had been reached for all groups. Thirty-nine study participants (68%) also took part in interviews: 18 Professional Immunizers from group one (all female), 18 HCW (15 female, three male) from group two and 3 PIs from group four. (See ).
Table 1. Participant demographics
Quantitative findings
Essential criteria
For self-administration of the HD-MAP applicator, PIs achieved essential criteria 100% of the time compared to HCWs who achieved essential criteria 83% of the time. When administering the HD-MAP applicator to another participant, PIs achieved the essential criteria over 90% of the time. There were no significant patterns in criteria that were not achieved. The most frequently failed criterion was correctly pressing the device onto the correct immunization site (Criterion 5). PIs missed these criteria 1% of the time, and HCWs missed it 7% of the time.
Wear time
PIs met the 10-second wear time on 36% of the administrations, with the HD-MAP applicator held on the skin for a mean of 8.4 seconds (SD 1.9 seconds) across self-administrations and 9.2 seconds (SD 2.5 seconds) when administering to others. HCWs met the 10-second wear time on 58% of their administrations, with the HD-MAP applicator held on the skin for a mean 10.2 seconds (SD 3.5 seconds) across all self-administrations. (See ).
Table 2. Percentage of HD-MAP administrations achieving all 5 essential criteria and mean HD-MAP wear time
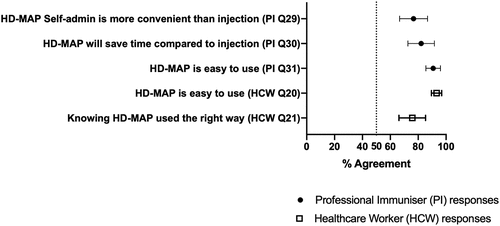
Usability of HD-MAP applicator administration
PIs generally found the HD-MAP applicator extremely easy to use, with the corresponding survey item (Professional Immunizer Survey Question 31 [PI Q31]) rated 91% (SD 13%) in agreement. HCWs also found the HD-MAP applicator easy to use, regardless of whether they performed self-administration first or received PI administration first (Healthcare Worker Survey Question 20 [HCW Q20]), 93% agreement, [SD 11%]). HCWs reported that they could easily tell if they applied the HD-MAP correctly when self-administering, rating the corresponding survey item (HCW Q21) at 76% (SD 30%) agreement. (; see Appendix B for survey items).
Acceptability of HD-MAP applicator administration
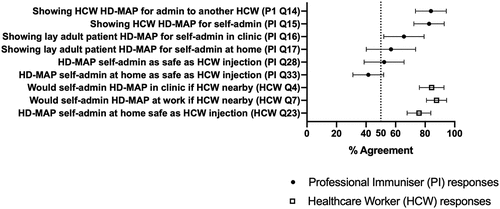
PIs rated acceptability items about healthcare professional use very highly. PIs mostly agreed that they could show healthcare professionals how to self-administer or administer to other healthcare professionals (agreement 83%, SD 25% for PI Q15 and 84%, SD 26% for PI Q14, respectively). Regarding showing an adult layperson how to self-administer in a clinical practice setting, PIs reported moderate agreement (66%; SD 33% [PI Q16]) agreement. Regarding showing a layperson how to self-administer in the home setting, PIs reported moderate agreement (57%; SD 41% [PI Q17]). Regarding self-administration at home, PIs reported low to moderate agreement (42%; SD 25% [PI Q33]), compared to vaccination with an IM injection from a nurse or doctor.
Conversely, HCWs rated all acceptability survey items very highly (; Appendix B for survey items). HCWs rated layperson self-administration with a healthcare professional nearby very highly: agreement to self-administration at GP practice was 84% (SD 25%; HCW Q4), and at a healthcare workplace 88% (SD 21%.; HCW Q7). HCWs considered a healthcare setting or the supervision by healthcare professionals important. When comparing the safety of self-administration in the home compared to IM from a healthcare professional, HCWs reported a 76% (SD 21%; HCW Q23) agreement to both being similarly safe.
Qualitative findings
Usability of HD-MAP applicator administration
Both PIs and HCWs found the HD-MAP highly usable: participants reported that administration and removal were straightforward and “could be used easily with one hand” (group one). PIs also said that the IFU was “straightforward” (group one) and understandable for most adults. Similarly, HCWs found the IFU easy to understand and use (regardless of randomization group). However, some other participants reported difficulty: “it was too complicated” (group two). A few participants randomized to self-administration first noted that they might have had greater confidence if they had observed PI administration before self-administration.
PIs reported that administering a HD-MAP to a patient would be less time-consuming than an IM injection as “there’s no mixing … which can take more than a couple of seconds to do” (group four). HCWs highlighted the significant potential for the HD-MAP to improve the speed of a mass vaccination program with supervised self-administration due to reduced waiting times (See ).
Table 3. Illustrative quotes from Professional Immunizers (Groups 1 and 4) and Healthcare Workers (Group 2)
Acceptability of HD-MAP applicator administration
All participants generally found the HD-MAP applicator acceptable, especially if administered by a healthcare professional. Compared to IM, the HD-MAP appeared “less daunting” (group two), especially for those who may experience a stress-related response to vaccines administered by IM.
PIs supported healthcare professional and supervised layperson self-administration in mass vaccination settings so that “there’s someone with emergency training and adrenalin nearby” (group one). They also suggested that a suitable ratio of healthcare professionals per layperson self-administration would be required, followed by 15 minutes of observation post-vaccination. PIs reported less support for unsupervised HD-MAP self-administration at home by laypeople due to safety concerns.
While PIs expressed safety concerns about adult layperson self-administration at home, they generally agreed that in an emergency or pandemic, risks associated with self-administration would outweigh the risks of acquiring a serious infectious disease. They reported: “Post it out … whack it on, and you’re done” (group one) during a pandemic or emergency. They also considered layperson self-administration at home in rural or remote settings appropriate if laypeople were trained and could respond to adverse events. PIs suggested that community healthcare professionals could be trained to assist with layperson HD-MAP self-administration in a community setting. In an emergency, PIs proposed checks and balances to ensure informed consent: “Maybe before you get given it the prescriber or the doctor has to make you sign all these things, before they hand it over to you” (group one). PIs wanted to be informed about who would be accountable in the case of an adverse event that was mishandled if they had been responsible for dispensing the vaccine to the patient.
HCWs reported the need for more support for self-administration at home than PIs. They explained they would prefer to complete self-administration under supervision from a healthcare professional, “just to be on the safe side in case things go wrong” (group two). Non-healthcare professional HCWs reported feeling more confident in the healthcare professional’s ability to administer the HD-MAP due to their health knowledge and experience. HCWs also reported trusting the safety and reliability of the HD-MAP if administered by a healthcare professional, especially in the context of rolling out a new medical device. However, HCWs also indicated they would be happy to receive an HD-MAP from adult laypeople, given that the applicator was “fool-proof” (group two) enough to be handled by people without a health background. One participant indicated: “we train people all the time to administer medications and use medical devices who have no medical training” (group two).
Like PIs, HCWs also wanted HD-MAP users trained. They reported that this training should be mandatory but not necessarily as rigorous and intensive as training required for vaccines administered by IM (See ).
Discussion
Study participants found the HD-MAP applicator administration to be highly usable and acceptable. PIs and HCWs considered HD-MAP administration to be quick and more convenient for patients than IM, with an easy-to-follow IFU. HCWs reported that HD-MAP healthcare professional administration and self-administration were acceptable in all settings. PIs found the HD-MAP applicator highly acceptable in clinical settings. PIs had lower-level agreement to HD-MAP self-administration, unless in an emergency, pandemic, or remote setting.
Compliance with HDMAP essential criteria was high during PI administration to HCWs and during HCW self-administration. PIs did not always meet the ten-second wear-time requirement. By comparison, HCW self-administration, on average, achieved consistently accurate wear time. This difference may be due to routine immunization practice: administration of IM is embedded in the behavior of PIs, while it is not for HCWs. PIs are used to delivering a vaccination and then moving to the next task, rather than waiting the 10 seconds that may be required for HD-MAP administration.
However, PIs also reported that HD-MAP administration would save time overall, given that there is no cold-chain requirement or vaccine reconstitution. Generally, successful administration of IM vaccines is in part indicated visually as the vaccine leaves the syringe and enters the deltoid. While effort and intention are required to change routine practices for highly specialized professionals, an objective measure of HD-MAP wear-time and/or a visual or tactile indicator of administration success may be necessary to ensure correct HD-MAP administration, which may infer effective vaccine delivery. Participants in the earlier LMIC study also requested a visible cue on the HD-MAP to indicate a full vaccine dose.Citation5
Our HD-MAP simulation demonstrated that all groups achieved successful self-administration; there was no difference by group regardless of training and expertise. This simulation reflects clinical trial results showing no significant differences in delivery efficiency between Vaxxas HD-MAPs administered by PIs compared to self-administration by healthy adults regardless of administration site (UA and FA) (manuscript under review). Even though in our simulation study, some HCWs reported less confidence with HD-MAP administration in interviews, if randomized to self-administration first, this group fulfilled all essential usability criteria. To help improve confidence with self-administration, HCWs should have access to a simple video demonstration, e.g., easily accessible through a mobile device, or a live demonstration in a mass vaccination setting, in addition to an IFU leaflet accompanying the HD-MAP.
Over 90% of PIs and HCWs in our study found the HD-MAP applicator highly usable and acceptable, especially when administered in a clinical setting, including self-administration with healthcare professional supervision. These findings are similar to a Vaxxas HD-MAP applicator simulation with HCWs in LMICs (91.8% acceptability).Citation5 Our findings suggest that HD-MAP vaccines should initially be used in clinical settings to increase end-user familiarity (including self-administration) and establish safety and effectiveness before self-administration at home. Home self-administration could increase vaccine access, coverage, and equity. Adult layperson use at home may be supported by written and/or audio-visual IFU in the end-user’s language and potentially a telehealth consultation. Our findings also reflect results from other studies with healthcare professionals, including hypothetical administration.Citation7
PI acceptability was lower for self-administration at home versus supervised in a clinic setting, in questionnaire data. However, interview data showed that all groups supported self-administration with appropriate safety measures when the risks of acquiring an infectious disease outweighed adverse events following immunization (AEFI). The main concern for PIs is the small but real risk of anaphylaxis. The attributable risk of anaphylaxis after commonly administered vaccines is 1/100000 to 1/1 000 000.Citation26,Citation27 We do not yet know the rate of anaphylaxis with HD-MAP and will not know until it is used in a population-based vaccination program. If HD-MAPs are used in a future vaccination program, including with the option for self-administration, types, and risks of serious AEFI and reactogenicity will need to be monitored and well-described.
PIs expressed more caution about self-administration without supervision than HCWs. It is essential to measure HD-MAP acceptability for PIs because it is a departure from already established trusted practice. Their attitudes may affect the uptake of this new technology. All groups described needle-related anxiety associated with IM as a barrier to vaccine uptake, a finding also reflected in the literature.Citation28–30 Vaccination with HD-MAP may improve this issue given the absence of an IM.Citation8,Citation9,Citation12
Limitations
Simulation may have limited participants’ understanding of the actual HD-MAP vaccination experience. The impact on acceptability of potential skin site reactions following immunization were not able to be captured with this study design. Usability and acceptability studies including a HD-MAP with vaccine, will enable real-world end-user experience. The small sample size meant that statistical comparisons were not possible.
Conclusions
PIs and HCWs found the HD-MAP applicator highly usable and acceptable. In particular, the HD-MAP applicator was considered more straightforward to use than IM. An objective time indicator may be required to support wear-time compliance, even for short wear times of 10 seconds. Overall, targeted education and training may enhance the acceptability and usability of the HD-MAP device.Citation31 This education should include HD-MAP safety, effectiveness, and administration compliance. HD-MAPs have a range of potential advantages over IM both in the routine clinical setting and in a pandemic emergency. PIs indicated that self-administration would be acceptable in a supervised or pandemic response setting.
Abbreviations
| HD-MAP | = | High-density microarray patch |
| MAP | = | Microarray patch |
| HCWs | = | Healthcare workers |
| PIs | = | Professional Immunizers |
| LMICs | = | Low and Middle-Income Countries |
| IBM | = | Integrative Behavioral Model |
| NIP | = | National Immunization Program |
| IFU | = | Instructions for use |
| HIC | = | High-Income Country |
| UA | = | Upper Arm |
| FA | = | Forearm |
Supplemental Material
Download MS Word (64.3 KB)Acknowledgments
We would like to acknowledge the contributions to this study by Dr Leon Heron, Dr Jodie Robinson and Elizabeth Barnes. We also wish to acknowledge the invaluable input of the research participants in this study: the parents/guardians and the healthcare workers and immunisation team members that allowed the research to be undertaken. In particular, we would like to thank to the following immunisation nurses: Lois Leacey, Melanie Coorey, Joanne Maude, Deidre Brogan Ailsa Colclough and Caroline Scott. Davies is a researcher and Skinner is an investigator within the Wellbeing Health & Youth (WH&Y) NHMRC Centre of Research Excellence in Adolescent Health (APP 1134984). All authors attest they meet the ICMJE criteria for authorship.
Supplementary Material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2021.2018863.
Disclosure statement
CD’s institution has received monies for consultancy work provided to Vaxxas; her salary is paid partly through the research grant which funds this study. CR and AF are paid employees of Vaxxas Pty Ltd, the company that co-funded this study. RB consults to Vaxxas for this work and to Abbott, GSK, Jansen, MSD Merck, Novartis, Novavax, Pfizer, Roche, Sanofi Pasteur and Seqirus for activities outside the presented work. SRS has received funding from Seqirus and Merck for her contribution to educational activities for the general-public and professionals. MT, LD, CK, SB report no conflicts of interest.
Additional information
Funding
References
- Jeong SY, Park JH, Lee YS, Kim YS, Park JY, Kim SY. The current status of clinical research involving microneedles: a systematic review. Pharmaceutics. 2020;12:1–15. doi:10.3390/pharmaceutics12111113.
- Forster AH, Witham K, Depelsenaire ACI, Veitch M, Wells JW, Wheatley A, Pryor M, Lickliter JD, Francis B, Rockman S, et al. Safety, tolerability, and immunogenicity of influenza vaccination with a high-density microarray patch: results from a randomized, controlled phase 1 clinical trial. PLoS Med. 2020;17. doi:10.1371/journal.pmed.1003024.
- Hossain MK, Ahmed T, Bhusal P, Subedi RK, Salahshoori I, Soltani M, Hassanzadenganroudsari, M . Microneedle systems for vaccine delivery: the story so far. Expert Rev Vaccines. 19:1153–1166. 2021; doi:10.1080/14760584.2020.1874928.
- Norman JJ, Arya JM, McClain MA, Frew PM, Meltzer MI, Prausnitz MR. Microneedle patches: usability and acceptability for self-vaccination against influenza. Vaccine. 2014;32:1856–62.
- Guillermet E, Alfa DA, Phuong Mai LT, Subedi M, Demolis R, Giersing B, Jaillard P. End-user acceptability study of the nanopatchTM; a microarray patch (MAP) for child immunization in low and middle-income countries. Vaccine. 2019;37:4435–43. doi:10.1016/j.vaccine.2019.02.079.
- Jacoby E, Jarrahian C, Hull HF, Zehrung D. Opportunities and challenges in delivering influenza vaccine by microneedle patch. Vaccine. 2015;33(37):4699–704. doi:10.1016/j.vaccine.2015.03.062.
- Birchall JC, Clemo R, Anstey A, John DN. Microneedles in clinical practice–an exploratory study into the opinions of healthcare professionals and the public. Pharm Res. 2011;28:95–106. doi:10.1007/s11095-010-0101-2.
- Fernando GJP, Hickling J, Flores J, Cesar M, Griffin P, Anderson CD, Davies C, Witham K, Pryor M, Bodle J. Safety, tolerability, acceptability and immunogenicity of an influenza vaccine delivered to human skin by a novel high-density microprojection array patch (Nanopatch™). Vaccine. 2018;36:3779–88. doi:10.1016/j.vaccine.2018.05.053.
- Griffin P, Elliott S, Krauer K, Davies C, Rachel Skinner S, Anderson CD, Forster A. Safety, acceptability and tolerability of uncoated and excipient-coated high density silicon micro-projection array patches in human subjects. Vaccine. 2017;35:6676–84. doi:10.1016/j.vaccine.2017.10.021.
- Frew PM, Paine MB, Rouphael N, Schamel J, Chung Y, Mulligan MJ, Prausnitz, MR . Acceptability of an inactivated influenza vaccine delivered by microneedle patch: results from a phase I clinical trial of safety, reactogenicity, and immunogenicity. Vaccine. 2020;38:7175–81. doi:10.1016/j.vaccine.2020.07.064.
- Rouphael NG, Paine M, Mosley R, Henry S, McAllister DV, Kalluri H, Pewin W, Frew PM, Yu T, Thornburg NJ, et al. The safety, immunogenicity and acceptability of inactivated influenza vaccine delivered by microneedle patch: a randomized, partially blind, placebo-Controlled Phase 1 trial. Lancet. 2017;390:649–58. doi:10.1016/S0140-6736(17)30575-5.
- Arya S, Henry S, Kalluri H, McAllister DV, Pewin WP, Prausnitz MR. Tolerability, usability and acceptability of dissolving microneedle patch administration in human subjects. Biomaterials. 2017;128:1–7. doi:10.1016/j.biomaterials.2017.02.040.
- Eldridge S, Chan C, Campbell M, Bond C, Hopewell S, Thabane L, Lancaster GA. CONSORT statement: extension to randomised pilot and feasibility trials. Br Med J. 2016;355:i5239. doi:10.1136/bmj.i5239.
- Eldridge S, Lancaster GA, Campbell M, Thabane L, Hopewell S, Coleman C, Bond CM. Defining feasibility and pilot studies in preparation for randomised controlled trials: using consensus methods and validation to develop a conceptual framework. PloS One. 2016;11:e0150205. doi:10.1371/journal.pone.0150205.
- Thabane L, Hopewell S, Lancaster GA, Bond C, Coleman C, Campbell M, Eldridge, SM . Methods and processes for development of a CONSORT extension for reporting pilot randomized controlled trials. Pilot and Feasability Studies. 2016;2:25.
- Thabane L, Lancaster GA. A guide to the reporting of protocols of pilot and feasibility trials. Pilort Feasability Stud. 2019;5. doi:10.1186/s40814-019-0423-8.
- Lancaster GA, Thabane L. Guidelines for reporting non-randomised pilot and feasibility studies. Pilot and Feasability Studies. 2019;5:17.
- Lucie R, Gauvin L, Raine K. Ecological models revisited: their uses and evolution in health promotion over two decades. Annu Rev Public Health. 2011;32:307–26. doi:10.1146/annurev-publhealth-031210-101141.
- Montano DE, Kasprzyk D. Theory of reasoned action, theory of planned behaviour, and the integrated behavioural model. In: Glanz K, Rimer BK, Viswanath K, editors. Health behavior: theory research and practice. New York, NY: Wiley; 2014. p. 95–124.
- ISO-9241-11. Ergonomics of human-system interaction.
- Cunningham MS, Davison C, Aronson KJ. HPV vaccine acceptability in Africa: a systematic review. Prev Med. 2014;69:274–79. doi:10.1016/j.ypmed.2014.08.035.
- Ayala GX, Elder JP. Qualitative methods to ensure acceptability of behavioral and social interventions to the target population. J Public Health Dent. 2011;71(Suppl 1):S69–S79. doi:10.1111/j.1752-7325.2011.00241.x.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3:77–101. doi:10.1191/1478088706qp063oa.
- TGA. Note for Guidance on Good Clinical Practice (CPMP/ICH/135/95). Therapeutics Goods Association: Commonwealth Department of Health and Aged Care. 2000.
- WMA. Declaration of Helsinki. Ethical principles for medical research involving human subjects. Helsinki (Finland): World Medical Association; 1964.
- Dudley MZ, Halsey NA, Omer SB, Orenstein WA, O’Leary ST, Limaye RJ, Salmon, DA . The state of vaccine safety science: systematic reviews of the evidence. Lancet Infect Dis. 2020;20:e80–e9. doi:10.1016/S1473-3099(20)30130-4.
- Dreskin SC, Halsey NA, Kelso JM, Wood RA, Hummell DS, Edwards KM, Caubet J-C, Engler RJM, Gold MS, Ponvert C. International Consensus (ICON): allergic reactions to vaccines. World Allergy Organ J. 2016;9. doi:10.1186/s40413-016-0120-5.
- McMurty CM. Managing immunization stress-related response: a contributor to sustaining trust in vaccines. Can Commun Dis Rep. 2020;6:210–18. doi:10.14745/ccdr.v46i06a10.
- WHO. Reducing pain at the time of vaccination: WHO position paper – September 2015. Weekly epidemiological record. Geneva (Switzerland): World Health Organization; 2015. p. 505–16.
- Davies C, Marshall HS, Zimet G, McCaffery K, Brotherton JML, Kang M, Garland S, Kaldor J, McGeechan K, Skinner SR, for the HPV.edu Study Group. Effect of a school-based educational intervention about the human papillomavirus vaccine on psychosocial outcomes among adolescents: analysis of a cluster randomized trial. JAMA Network Open. 2021;4:e2129057. doi:10.1001/jamanetworkopen.2021.29057.
- Davies C, Skinner SR, Stoney T, Marshall HS, Collins J, Jones J, Hutton H, Parrella A, Cooper S, McGeechan K, for the HPV.edu Study Group. ‘Is it like one of those infectious kind of things?’ The importance of educating young people about HPV and HPV vaccination at school. Sex Educ. 2017;17(3):256–75. doi:10.1080/14681811.2017.1300770.
Appendices Appendix A
Observation Checklist
*Essential criteria in bold, desirable criteria in regular formatting
1. Immunizer checks HD-MAP applicator undamaged and foil seal is intact
2. Immunizer checks amination site for suitability
3. Immunizer removes foil cover
4. Immunizer places applicator on administration site without delay
5. Immunizer correctly presses the device onto the correct immunization site
6. Immunizer correctly triggers the HD-MAP applicator (click heard)
7. Immunizer acknowledges the click indicating that the device has deployed
8. Immunizer holds HD-MAP applicator in place for the required 10 seconds
Actual HD-MAP wear time:
9. Immunizer removes HD-MAP applicator from skin without difficulty
10. Immunizer removes HD-MAP applicator from skin without making contact with the HD-MAP area (i.e. safely avoids potential self-inoculation)
11. Immunizer places HD-MAP applicator in disposal container
Appendix B
Professional Immunizer survey
1. “I do not mind having an injection with a needle and syringe.”
2. “I found the HD-MAP applicator simple to use.”
Confidence with HD-MAP administration/ preference of vaccine delivery type and location
3. “In school clinics, I would favour vaccinating students by HD-MAP over injection.”
4. “In school clinics, I would favour vaccinating students by injection over HD-MAP.”
5. “In school clinics, I would vaccinate students by injection or HD-MAP without preference.”
6. “In school clinics, I would ask the student whether they would prefer injection or HD-MAP.”
7. “In a clinical practice setting, I would favour vaccinating adults by HD-MAP over injection.”
8. “In a clinical practice setting, I would favour vaccinating adults by injection over HD-MAP.”
9. “In a clinical practice setting, I would vaccinate adults by injection or HD-MAP, without preference.”
10. “In a clinical practice setting, I would ask adult patients whether they would prefer vaccination by injection or HD-MAP.”
11. “In a clinical practice setting, I would favour vaccination of children by injection over HD-MAP.”
12. “In a clinical practice setting, I would favour vaccination of children by HD-MAP over injection.”
13. “In a clinical practice setting, I would ask parents whether they would prefer injection or HD-MAP for vaccination of their children.”
14. “In the healthcare workplace, I would show a health care worker how to administer the HD-MAP to another worker.”
15. “In the healthcare workplace, I would show a health care worker how to self-administer the HD-MAP.”
16. “In a clinical practice setting, I would show an adult patient how to self administer the HD-MAP.”
17. “If the vaccine MAP was available for self-administration in the home, I would show an adult how to self administer the HD-MAP.”
Acceptability of Micro-Array Patch (HD-MAP) vaccines
18. “I think that HD-MAP vaccines are an improvement over injections.”
19. “For myself, I would prefer a HD-MAP vaccine over an injection.”
20. “In my practice, I would prefer a HD-MAP vaccine over an injection.”
21. “Compared to injections, HD-MAP vaccines have benefits that are important to me.”
22. “For myself, I would like vaccine HD-MAPs more than injections.”
23. “In my practice, I would like vaccine HD-MAPs more than injections.”
24. “Putting on a HD-MAP vaccine is likely be less painful than getting an injection.”
25. “Compared to HD-MAP vaccines, injections have drawbacks that matter to me.”
26. “The HD-MAP applicator is likely to administer a vaccine reliably.”
27. “I agree that a HD-MAP vaccine could offer as much protection against disease as an injected vaccine.”
Usability of Micro-Array Patch (HD-MAP) vaccines
28. “Self administration of a HD-MAP vaccine is safe, when compared to an injection from a nurse or doctor.”
29. “Self-administration of a HD-MAP vaccine will be more convenient for patients than an injection.”
30. “When administering vaccines, HD-MAP vaccines will help me save time compared to injections.”
31. “I think HD-MAP vaccines are easy to use.”
32. “I could easily tell if I administer a HD-MAP vaccine the right way.”
Perceived Safety of Micro-Array Patch (HD-MAP) vaccines
33. “Self administration of a HD-MAP vaccine on the arm at home is safe, when compared to an injection from a nurse or doctor.”
34. “I believe a HD-MAP vaccine will have the same side effects as an injection.”
35. “I believe a vaccine HD-MAP will cause more skin redness and swelling compared to an injection.”
36. “I would worry more about side effects with a vaccine HD-MAP compared to an injection.”
Healthcare Worker Survey
1. “I do not mind having an injection with a needle and syringe.”
Preference of vaccine delivery type and location
2. “At my GP’s practice, I would prefer to have the doctor or nurse give me a vaccine via HD-MAP rather than injection.”
3. “At my GP’s practice, I would prefer to have the doctor or nurse give me a vaccine by injection rather the HD-MAP.”
4. “At my GP’s practice, I would feel comfortable giving myself the HD-MAP if a nurse or doctor was nearby.”
5. “At my workplace, I would prefer to have the nurse give me the HD-MAP vaccine rather than injection.”
6. “At my workplace, I would prefer to have the nurse give me a vaccine by injection rather than the HD-MAP.”
7. “At my workplace, I would feel comfortable giving myself the HD-MAP vaccine, if a nurse was nearby.”
8. “At my workplace, I would feel comfortable giving myself the HD-MAP vaccine.”
9. “At home, I would feel comfortable to give myself the HD-MAP vaccine.”
Acceptability of Micro-Array Patch (HD-MAP) vaccines
10. “I think that HD-MAP vaccines are an improvement over injections.”
11. “I prefer a HD-MAP vaccine over an injection.”
12. “Compared to injections, HD-MAP vaccines have benefits that are important to me.”
13. “I like HD-MAP vaccines more than injections.”
14. “I think that a HD-MAP vaccine would be less painful than getting an injection.”
15. “Compared to HD-MAP vaccines, injections have drawbacks that matter to me.”
16. “The HD-MAP applicator can administer a vaccine reliably.”
17. “I think a HD-MAP vaccine could offer as much protection against disease as an injected vaccine.”
Usability of Micro-Array Patch (HD-MAP) vaccines
18. “A HD-MAP vaccine will be more convenient for me than an injection.”
19. “Overall, HD-MAP vaccines will help me save time compared to injections.”
20. “HD-MAP vaccines are easy to use.”
21. “I can easily tell if I put a vaccine patch on my arm the right way.”
22. “I think I would easily tell if the HD-MAP applicator has given me the vaccine.”
23. “Putting a vaccine HD-MAP on my arm at home is as safe as an injection from a nurse or doctor.”
Perceived Safety of Micro-Array Patch (HD-MAP) vaccines
24. “I believe that a HD-MAP vaccine will have the same side effects as an injection.”
25. “I believe that a HD-MAP vaccine will cause more skin redness and swelling compared to an injection.”
26. “I would worry more about side effects with a HD-MAP vaccine compared to an injection.”
27. “Putting a HD-MAP vaccine on my arm at home is safe, when compared to an injection from a nurse or doctor.”
Social norms regarding Micro-Array Patch (HD-MAP) vaccines
28. “I would choose a vaccine HD-MAP instead of an injection if my family thought it a good idea.”
29. “I would choose a vaccine HD-MAP instead of an injection if my friends thought it a good idea.”
30. “I would choose a vaccine HD-MAP patch instead of an injection if my doctor suggested it.”
31. “I would choose a vaccine HD-MAP patch instead of an injection if my doctor recommended it.”
Appendix C
Focus group/Interview Guideline for Professional Immunizers
Exit Interview (interviews to take place after the HD-MAP applicator has been used as part of this study).
Introductory questions
What is your training experience?
How many years immunization experience do you have, in what types of settings? [Cross-reference this response with demographics in online survey].
How many HD-MAP administrations did you gain experience with?
How many HD-MAPs did you administer to yourself??
About You
Date: ____________________
Q1: What is your name/ID number:_____________________________________________
Mobile telephone _______________________________________
Q2: Please write the Australian postcode of the town/city in which you live?__________
Q3: Do you identify as: □Female □Male□Something else
Q4: Please select your age range
Q5: In which country were you born? [Insert drop-down list]_________________________
Q6: Is English the main language spoken at home? □Yes □No
Q7: Are you of Aboriginal or Torres Strait Islander descent? □Yes □No
Q8: What is your relationship status? □Single □ Married □De-facto□Other
Q9: What is your highest level of educational qualification?
□High School Year 10
□High School Year 12
□ TAFE/apprenticeship
□Undergraduate degree
□Postgraduate degree
Q10: What are you mainly doing at the moment?
□ I work in hospital administration/management
□ I am a doctor working in a hospital
□ I am a Registered Nurse working in a hospital
□ I am an allied health professional working in a hospital
□ I am an orderly/janitor/cleaner working in a hospital
□ I am a volunteer working in a hospital
□Other, please specify:__________________________________________________________________
Q11. Which best describes your current annual household income in Australian dollars?
□ $1 to $9 999
□ $10 000 to $24 999
□ $25 000 to 49 999
□ $50 000 to 74 999
□ $75 000 to 99 999
□ $100 000 to 149 999
□ $150 000 and greater
Q12: Do you practice a religion/faith? □Yes □No
Q12a. If yes, what religion/faith?[Insert drop down list]___________________________________________________________
Q13. What is the most recent vaccine you recall having?
□Flu vaccine
□Travel vaccine
□Don’t recall
□Other, please specify?
Q14. When was the last time you had an injection (of any type)?
□Within the last 6 months
□6 months to a year ago
□Between 1 and 5 years ago
□Over 5 years ago
□ Don’t recall
Q15. Please rate your agreement with the following statement (on a scale of 0% agreement to 100% agreement)
HD-MAP Vaccine usage acceptability
What are your expectations regarding the HD-MAP device’s safety and effectiveness? [Prompt: Can you please comment on this for infants, children and adults, and safety for immunizer including risk of re-use, and risk of cross infectio.]n
If vaccination with a HD-MAP (in terms of safety, immunogenicity and cost) is the same as vaccination with a needle and syringe, would you find it acceptable to use a MAP to administer vaccines on a) adults, b) infants and children, c) pregnant patients, d) yourself, if these patients were willing to be immunized using a HD-MAP? If yes, why? If no, why?
What factors would influence your choice of whether or not to use a HD-MAP for vaccination? Can you please tell me more about that? [Prompts. Factors may include patients’ wishes, setting, age of patient, perceived pain/discomfort of HD-MAP vs injection, time taken to vaccinate, type of vaccine offered, health of patient etc., location (urban, regional, remote)]?
What would you say about the benefits and/or challenges of vaccination with a HD-MAP to a patient making a decision about their choice of vaccine delivery system for themselves or their child?
What would you tell your boss/hospital administrator about using HD-MAP-delivered vaccines? Can you please comment on HD-MAP usability, time to administer, storage/cold chain, disposal/waste volume in comparison to needle/syringe delivered vaccines.
What do you think of the esthetics (look, feel and design) of the HD-MAP when compared to needle & syringe? Can you please share your thoughts on how the look, feel and design of the MAP compared to a needle and syringe is related to your perception of the pain/discomfort you may feel when vaccinated with a HD-MAP compared to a needle and syringe? Can you please tell me more about that?
Have you previously experienced the introduction of new drug delivery devices that you can tell us about, and which would be useful for the introduction of the HD-MAP device? Why/Why not?
Have you used or are you familiar with any of these immunization products?
Intanza intradermal influenza vaccine:
□ Unknown to me
□ I have heard of it
□ I have used it
Flumist nasal influenza vaccine:
□ Unknown to me
□ I have heard of it
□ I have used it
Pharmajet needle-free injection technology:
□ Unknown to me
□ I have heard of it
□ I have used it
[Prompts: Can you please tell me more about your experience of using these products, or what benefits and challenges you have heard about them]
HD-MAP Vaccine usability
Can you please tell me about your experience of using the HD-MAP applicator on a patient? [For substudy 1, ‘patient’ refers to the colleague to whom you’ve administered a prototype vaccine HD-MAP]. How easy or difficult was the HD-MAP applicator to use on a patient compared to vaccination with a needle and syringe? Can you please tell me more about that?[Prompt: Would any changes make the HD-MAP applicator easier to use?]
Do you think preparation for HD-MAP administration should include swabbing the administration site with alcohol? Can you please tell me more about that?
Did you peel the “vaccine details” label off the HD-MAP applicator? Please tell me about your experience with doing that. Is having this peel-off label of use to you? Are there any improvements you believe are useful for the peel-off label? Please comment on the shape and size of the label, and the information included.
The MAP is held in place for 10 seconds after triggering. How does this compare to using a needle and syringe on a patient with regard to time taken to vaccinate?
How long do you think the HD-MAP could be acceptably held in place?
Did the HD-MAP applicator make a noise when you triggered it? Can you tell me about this noise, and a) your response to it? b) the patient’s response to it? What age was the patient? (Prompt: Age categories for substudy 3: Infant (<12 m imms), toddler (12/18 m imms), pre-school (4y imms), school-age, adult.
How long did you hold the HD-MAP applicator on the patient? How did you measure the 10 seconds? Was it easy to remove the applicator from the patient? How do you think the HD-MAP applicator should be held in place? Can you please tell me more about that?
Could you please comment on how competently you think lay adults could self-administer the HD-MAP applicator without supervision? Can you please tell me more about that?
Could you please comment on how competently you think lay adults could administer the HD-MAP applicator to infants and children without supervision? Can you please tell me more about that?
Could you please comment on how competently you think lay adult volunteers could administer the HD-MAP to adults, infants and children without supervision in the following locations
a) outreach settings?
b) a house-to-house campaign?
c)Aboriginal and Torres Strait Islander rural and remote communities?
What should be the criteria for selecting HD-MAP vaccinators and determining the training required? Who should be their supervisors? Can you please tell me more about that?
HD-MAP Vaccine set up
Can you please tell me about your experience of setting up the HD-MAP applicator before use after having read the instructions/seen the training video? Approximately how long did it take you to set up the HD-MAP applicator before use? Was it easy/difficult to remove the foil cover from the HD-MAP applicator?
How did your experience of setting up the HD-MAP applicator compare to setting up a needle and syringe for use on a patient? Approximately how long does it take you to set up a needle and syringe vaccine before use?
How confident are you that you could tell that the HD-MAP applicator was not/was damaged or previously discharged before use? Can you please tell me more about that?
How confident did you feel setting up the HD-MAP applicator for use on patients? What factors contributed to your confidence in setting up the HD-MAP applicator?
HD-MAP Vaccine storage
Can you please comment on how vaccines used with a needle and syringe are currently stored in your healthcare setting? The HD-MAP may not require refrigeration at 2–8°C prior to use – how much impact (positive or negative) would this have on the vaccination process?
HD-MAP Vaccine disposal
How did you dispose of the HD-MAP applicator? In the future, would you be prepared to separate the applicator and used HD-MAP and dispose of these in different waste bins if it was easy to do so? This would mean putting the used HD-MAP into a sharps waste stream, and applicator into plastic recycling stream? Can you please tell me more about that?
How does the HD-MAP compare to needle and syringe with regards to waste management? How do you currently dispose of used needle and syringes post vaccination?
Is there anything else you’d like to add about the HD-MAP that we haven’t discussed today?
Thank you for your time and for sharing your expertise
Questions for research participants (interviews to take place after HD-MAP administration as part of this study).
Healthcare Worker subjects
Audio record the interview
For the recording
(5)Please state your name and surname
(6)What is your current job?
(7)How many HD-MAPs did the immunizer administer to you?
(8)How many HD-MAPs did you administer yourself?
About You
Date: ____________________
Q1: What is your name/ID number:_____________________________________________
Mobile telephone _______________________________________
Q2: Please write the Australian postcode of the town/city in which you live?__________
Q3: Do you identify as: □Female □Male□Something else
Q4: Please select your age range:
Q5: In which country were you born? [Insert drop-down list]_________________________
Q6: Is English the main language spoken at home? □Yes □No
Q7: Are you of Aboriginal or Torres Strait Islander descent? □Yes □No
Q8: What is your relationship status? □Single □ Married □De-facto □Other
Q9: What is your highest level of educational qualification?
□High School Year 10
□High School Year 12
□ TAFE/apprenticeship
□Undergraduate degree
□Postgraduate degree
Q10: What are you mainly doing at the moment?
□ I work in hospital administration/management
□ I am a doctor working in a hospital
□ I am a Registered Nurse working in a hospital
□ I am an allied health professional working in a hospital
□ I am an orderly/janitor/cleaner working in a hospital
□ I am a volunteer working in a hospital
□Other, please specify:__________________________________________________________________
Q11. Which best describes your current annual household income in Australian dollars?
□ $1 to $9 999
□ $10 000 to $24 999
□ $25 000 to 49 999
□ $50 000 to 74 999
□ $75 000 to 99 999
□ $100 000 to 149 999
□ $150 000 and greater
Q12: Do you practice a religion/faith? □Yes □No
Q12a. If yes, what religion/faith?[Insert drop down list]___________________________________________________________
Q13. What is the most recent vaccine you recall having?
□Flu vaccine
□Travel vaccine
□Don’t recall
□Other, please specify?
Q14. When was the last time you had an injection (of any type)?
□Within the last 6 months
□6 months to a year ago
□Between 1 and 5 years ago
□Over 5 years ago
□ Don’t recall
Q15. Please rate your agreement with the following statement (on a scale of 0% agreement to 100% agreement)
All adult subjects
HD-MAP vaccine acceptability
What are your expectations regarding the HD-MAP device’s safety and effectiveness? (Prompt: Can you please comment on the safety and of the device for adults, infants, and children).
If vaccination with a HD-MAP works as well (in terms of safety and immune response) as vaccination with a needle and syringe, would you find it acceptable to receive a vaccination via HD-MAP a) from a trained health worker (immunization nurse, doctor)?; b) to vaccinate yourself?; c) to vaccinate other adults yourself?; d) to vaccinate infants and children yourself? If yes, why? If no, why?
What factors would influence your choice of whether or not to receive a HD-MAP vaccination administered by a healthcare worker (immunization nurse, doctor)? Can you please tell me more about that? (Prompts. Factors may include: equivalent efficacy in terms of safety and immune response, perceived pain/discomfort of HD-MAP vs injection, time taken to vaccinate etc.).
Is the training (trained nurse, doctor etc.) of the person applying the HD-MAP vaccine important to you? Can you please tell me more about that? Would you be comfortable receiving an HD-MAP immunization from a layperson?
How did your experience of mock vaccination with the HD-MAP differ from a vaccination with an injection? How do you think the HD-MAP would change your experience of vaccinations? (Prompts: Can you please consider the following factors in your response: pain/discomfort, convenience, ease of use?)
Can you please comment on whether you’d be more or less likely to receive immunizations using HD-MAP than needle and syringe? Why, why not? Can you also please consider your response in relation to the seasonal flu vaccine.
With what you experienced today, what would you tell about the benefits and/or challenges of vaccination with a HD-MAP to a friend or relative making a decision about their choice of vaccine delivery system for themselves?
With what you experienced today, what would you tell about the benefits and/or challenges of vaccination with a HD-MAP to a friend or relative making a decision about their choice of vaccine delivery system for a child?
What do you think of the esthetics (look, feel and design) of the HD-MAP when compared to needle & syringe? Can you please share your thoughts on how the look, feel and design of the HD-MAP compared to a needle and syringe is related to your perception of the pain/discomfort you may feel when vaccinated with a HD-MAP compared to a needle and syringe? Can you please tell me more about that?
HD-MAP Vaccine set up
Can you please tell me about your experience of setting up the HD-MAP applicator before use after having read the instructions? Approximately how long did it take you to set up the HD-MAP applicator before use? Was it easy/difficult to remove the foil cover from the HD-MAP applicator?
How confident are you that you could tell that the HD-MAP applicator was not/was damaged or previously discharged before use? Can you please tell me more about that?
HD-MAP vaccine usability
How could you tell that the HD-MAP applicator device had been used appropriately? Can you please tell me more about that? (Prompts: Can you please comment on this in relation to a) self-administration? b) Healthcare Worker administration?)
Did the HD-MAP applicator make a noise when you and the professional immunizer triggered it? Can you tell me about this noise, and your response to it?
How long did you and the professional immunizer leave the HD-MAP applicator on your a) upper arm; b) forearm? Was it easy to keep in place? Was it easy to remove? Why/Why not?
Option A
For HCW who experienced HD-MAP self-administration before experiencing PI administration of HD-MAP (followed by vaccination with N&S)
Can you please tell me about your experience of using the HD-MAP applicator on yourself? How easy or difficult was the HD-MAP applicator to administer to your a) upper arm; b) forearm? Would any changes make the HD-MAP applicator easier to use for self-administration?
Were the HD-MAP instructions easy or difficult to understand? Are there any additional instructions that may help you to effectively use the HD-MAP applicator on yourself? Do you think the Instructions For Use could be improved?
Did you need to ask the healthcare worker or research assistant any additional questions about administering the HD-MAP to yourself? Can you please tell me more about that?
Can you please tell me about your experience of having the HD-MAP applicator given by a HCW? How easy or difficult was the HD-MAP applicator to have on your a) upper arm; b) forearm? Can you please tell me more about that? Would any changes make the HD-MAP applicator easier to use for HCW-administration?
Do you have any other comments about self-administration of vaccines using the HD-MAP?
Option B
For HCW who experienced PI administration of HD-MAP before experiencing HD-MAP self-administration (followed by vaccination with N&S)
Can you please tell me about your experience of having the HD-MAP applicator given by a HCW? How easy or difficult was the HD-MAP applicator to have on your a) upper arm; b) forearm? Can you please tell me more about that? Would any changes make the HD-MAP applicator easier to use for HCW-administration?
Can you please tell me about your experience of using the HD-MAP applicator on yourself? How easy or difficult was the HD-MAP applicator to administer to you’re a) upper arm; b) forearm? Can you please tell me more about that? Would any changes make the HD-MAP applicator easier to use for self-administration?
Were the HD-MAP instructions easy or difficult to understand? Are there any additional instructions that may help you to effectively use the HD-MAP applicator on yourself? Do you think the Instructions For Use could be improved?
Do you think having the HCW administer the HD-MAP to your arm made your task to self-administer the HD-MAP easier/more difficult? Can you please tell me more about that?
Did you manage to administer the HD-MAP to yourself? At which site(s)? Did you find it easier/more difficult to administer the HD-MAP to a) your upper arm; b) your forearm? Can you please tell me more about that?
Did you need to ask the healthcare worker or research assistant any additional questions about administering the HD-MAP to yourself? Can you please tell me more about that?
Do you have any other comments about self-administration of vaccines using the HD-MAP?
All Healthcare Worker subjects
HD-MAP Vaccine disposal
How did you dispose of the HD-MAP applicator?
In the future, would you be able to separate the applicator and used patch and dispose of these in different waste bins? This would include putting the HD-MAP patch into a sharps waste stream if it was provided, and the applicator into plastic recycling stream? Can you please tell me more about that?
Benefits and challenges of the HD-MAP device
In order to find out if there is something we have missed in this discussion I will ask you to tell me about additional thought you have about HD-MAPs
Is there anything you would like to add about
The possible benefits of the HD-MAP device?
The possible challenges of the HD-MAP device?
Any potential improvements to the HD-MAP device?
Any potential improvements to the Instructions For Use?
How could the HD-MAP administration experience be improved?
Is there anything else you’d like to add about the HD-MAP that we haven’t discussed today?
Thank you for your time and for sharing your expertise.