ABSTRACT
In recent years, the Global Polio Eradication Initiative has gradually implemented a global shift in polio immunization programs. Few studies cover polio immunization program impacts on the efficacy of other vaccines. This study investigated whether polio immunization programs affected hepatitis A (HepA) and hepatitis B (HepB) vaccination efficacy. Serum samples were collected from 968 infants before the first dose of polio vaccine, 28 days after completing primary polio immunization, and at 24 months old. Infants were classified into six polio immunization program groups: 1sIPV+2bOPV, 2sIPV+1bOPV, 2sIPV+1tOPV, 1cIPV+2bOPV, 2cIPV+1bOPV, and 2cIPV+1tOPV (sIPV: Sabin inactivated poliovirus vaccine; cIPV: Salk inactivated poliovirus vaccine; b, bivalent; t, trivalent; OPV, oral polio vaccine). No significant differences existed in antibody titers against HepA virus (anti-HAV) among the polio immunization program groups at any of the three time points (pre-first dose [p = 0.412], 28 days after primary immunization [p = 0.676], 24 months old [p = 0.556]). Before the first dose (p = 0.178) and at age 24 months (p = 0.987), no significant differences existed in HepB surface antibody (HBsAb) titers between the six polio immunization program groups). Twenty-eight days after primary immunization, no significant difference existed in HBsAb titers between groups after Bonferroni correction. Following HepA and HepB immunization, anti-HAV and HBsAb positivity reached > 98% in all groups, reflecting effective immunization. Our data suggest that different polio immunization programs did not affect HepA and HepB vaccine efficacy; HepA and HepB vaccines maintained high effectiveness irrespective of polio immunization program. This trial was registered on Clinical Trials.gov: NCT03614702.
1. Introduction
The Global Polio Eradication Initiative (GPEI) was officially launched in 1988 with the goal of eradicating poliomyelitis worldwide. Since the launch of this initiative, the number of poliomyelitis cases caused by wild poliovirus (WPV) infection has decreased by more than 99%. The last case of WPV type 2 (WPV2) infection was reported in India in 1999; no further cases of WPV2 infection had been reported since that time, and the eradication of WPV2 was announced by the GPEI in September 2015.Citation1 Wild poliovirus type 3 (WPV3) has not been circulating globally since 2012, and after several years of additional follow-up, was officially declared eradicated by the World Health Organization (WHO) on World Polio Day 2019.Citation2
Oral poliovirus vaccines (OPVs) and inactivated poliovirus vaccines (IPVs) are used worldwide to prevent the transmission of polio and have high effectiveness. In comparison with IPVs, OPVs are more convenient to use, have lower production costs, elicit better oral and intestinal mucosal immunity, and are more efficacious in preventing transmission of WPVs.Citation3 In comparison with OPVs, IPVs induce stronger humoral immunity and prevent invasion of the central nervous system by polioviruses. However, intestinal mucosal immunity induced by IPVs is weaker because of the inability of these vaccines to replicate in the intestine.Citation4 In addition, OPVs incur potential risks of vaccine-associated paralytic poliomyelitis (VAPP) in rare cases. Moreover, the live attenuated polioviruses in OPVs may re-acquire transmissibility and neurovirulence through prolonged replication, leading to outbreaks of circulating vaccine-derived poliovirus (cVDPV). Following the eradication of WPV2 and to ultimately eradicate poliovirus, the GPEI converted the trivalent OPV (tOPV) to a bivalent type 1 + 2 OPV (bOPV) globally in April 2016.Citation5 The IPV was introduced into routine immunization programs to diminish the risk of reduced resistance to type 2 polioviruses because of the withdrawal of OPV2 from immunization programs.Citation6 There are two main types of IPVs in use: cIPV prepared from wild polio strains and sIPV prepared from attenuated polio strains. The Salk strain of poliovirus used to prepare cIPV is more virulent; the viral components are obtained from African green monkey kidney (Vero) cells or human diploid cells, which are inactivated with formaldehyde so that the viruses are not biologically active but maintain antigenicity. The production of cIPV is complicated by biosafety issues associated with mass production. The attenuated Sabin strain has better safety, immunogenicity, and induces stronger cross-neutralization than the Salk strain while avoiding potential hazards during preparation. Thus, the WHO recommends use of the attenuated Sabin strain for IPV production.Citation7
Prior to 2014, China used a full tOPV immunization program for polio; subsequently, IPV was gradually introduced into the national immunization program. With the successful marketing and launch of a sIPV developed by the Institute of Medical Biology, Chinese Academy of Medical Sciences in 2015, China’s domestic IPV production and supply capacity has been increased; sIPV will gradually be incorporated into the national immunization program instead of cIPV. On May 1, 2016, China and 155 other countries simultaneously switched their polio immunization programs to implement sequential immunization with one dose of IPV followed by three doses of bOPV. On January 1, 2020, the previous polio immunization program in China was officially replaced by the 2IPV+2bOPV program. In China, primary polio immunization is conducted at 2, 3, and 4 months of age, with a booster immunization at 4 years of age.Citation8 During this period, the hepatitis B (HepB) and hepatitis A (HepA) vaccines are also administered. China has included HepB in the national immunization program since 1992. Infants must be vaccinated against HepB within 24 hours of birth, at 1 month of age, and at 6 months of age.Citation8 Vaccination against HepB elicits protective antibodies against hepatitis B surface antibody (HBsAb), and quantitation of HBsAb levels can be used to analyze the impact of HepB immunization. Currently, the HepA vaccines used worldwide include inactivated vaccines (HepA-I) and live attenuated vaccines (HepA-L).Citation9 Both HepA-I and HepA-L were introduced into the Expanded Program of Immunization (EPI) of China starting in 2008.Citation10,Citation11 Two doses of HepA-I are administered at 18 and 24 months of age, while only a single dose of HepA-L is administered at 18 months of age.Citation8
In this study, we investigated whether different sequential polio immunization programs affected the effectiveness of HepA and HepB vaccines. We tested serum antibodies against HepA virus (anti-HAV) and HBsAb in a total of 968 infants categorized into six groups of polio immunization program: 1sIPV+2bOPV, 2sIPV+1bOPV, 2sIPV+1tOPV, 1cIPV+2bOPV, 2cIPV+1bOPV, and 2cIPV+1tOPV (sIPV: Sabin inactivated poliovirus vaccine; cIPV: Salk inactivated poliovirus vaccine), and performed statistical analysis on the test results.
2. Materials and methods
2.1. Study design and participants
This was a randomized, double-blind, single-center, parallel arm trial conducted in Guangxi Province, China, in 2015. A total of 1200 healthy infants aged 2 months were enrolled in the study. The inclusion criteria for the study were as follows: written informed consent from guardians; full-term birth (37 to 42 weeks gestation); birth weight more than 2500 g; normal axillary temperature; no history of immunization (except HepB vaccine) after birth; no history of other live vaccines 28 days before the start of the trial; and no history of inactivated vaccines 14 days before the start of the trial. Infants with any factors that could potentially interfere with assessment of the postvaccination immune response or with a history of allergies to vaccine components were excluded. Other eligibility criteria were consistent with those typically used in clinical studies of vaccines. The 1200 infants were randomly divided into six groups (N = 200 per group; grouping information is shown in ). Blood samples were collected before the first dose of polio vaccine, 28 days after primary polio immunization, and at 24 months of age. Because of the long study duration, some participants were lost to follow up, and the actual number of participants included in the final analysis was 968. The efficacy of HepA immunization was assessed via levels of anti-HAV antibodies. According to previous studies,Citation12–14 the threshold value of anti-HAV antibody associated with protection is 20 international units (IU/L). The efficacy of HepB immunization was assessed via levels of HBsAb. Levels of more than 10 IU/L of HBsAb are effective in preventing HepB virus (HBV) infection.
Table 1. Group information of each immunization program
2.2. Randomization and blinding
The study had a randomized, double-blind, parallel design. The original label of each vaccine was covered with a study-specific sequential number. Participants were randomized by drawing scratch cards containing individual study numbers; each study number reflected three vaccine numbers from each group to be administered to each participant. The investigator was responsible for recording the study number and vaccine number on the Original Notebook and Vaccination Card; vaccination group staff were responsible for administering vaccines according to these numbers. A new Vaccination Card was used for each vaccination. Used Vaccination Cards were kept by vaccination group staff so that they were unavailable to other investigators.
2.3. Vaccination
Subjects received the first, second, and third polio vaccines at 2, 3, and 4 months of age according to the predefined polio immunization programs (sIPV-bOPV-bOPV, sIPV-sIPV-bOPV, sIPV-sIPV-tOPV, cIPV-bOPV-bOPV, cIPV-cIPV-bOPV, or cIPV-cIPV-tOPV; polio vaccines information can be found in Table S1). HepB vaccines were administered at 0, 1, and 6 months of age and HepA-L vaccine was administered at 18 months of age according to the Chinese EPI. The vaccination programs used in this study are illustrated in .
Figure 1. Vaccination procedures used in this study.
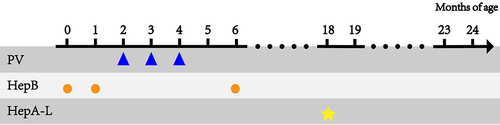
The vaccines used in this study were approved for routine use by Chinese national regulatory authorities and were administered via injection the deltoid muscle by a qualified nurse according to the package insert of each individual vaccine.
2.4. Detection methods
Both HBsAb and anti-HAV were quantitated using electrochemiluminescence kits from Roche Diagnostics GmbH (Mannheim, Germany). The lot number for Anti-HBSH assay kit was 47336501 and the lot numbers for the Anti-HAV assay kits were 47932801 and 46607001. Antibody levels were determined using a Roche Diagnostics Cobas e411 automatic electrochemiluminescence analyzer and expressed in IU/L.
2.5. Statistical analysis
Following study completion, the database was locked and data analysis was performed. Based on prior studies, anti-HAV antibody levels ≥ 20 IU/L and HBsAb levels ≥10IU/L are regarded as positive results of HAV and HBV vaccination, respectively. The Kruskal-Wallis H test was used to compare height, weight, and antibody levels between groups. P values were corrected using the Bonferroni method. Pearson’s chi-square test was used to assess differences between groups in sex, race, and antibody positivity rates. Two-sided values of p < 0.05 were considered statistically significant. All data were analyzed using IBM SPSS Statistics 21 (IBM, Armonk, NY, USA). All figures were prepared using GraphPad Prism 8.0 (GraphPad, San Diego, CA, USA).
3. Results
3.1. Baseline participant characteristics
In 2016, 1200 healthy infants were enrolled in the study at 2 months of age in Liuzhou city, Guangxi Province, China after assessing eligibility. Participants were immunized with polio, HepA, and HepB vaccines according to the study protocol. A total of 232 participants were lost to follow up by the time the third blood sample was obtained at 24 months of age. Thus, 968 serially obtained sets of blood samples were analyzed. There were no significant differences among groups in terms of sex, race, height, and weight ().
Table 2. Demographic characteristics of participants
3.2. Impact of polio immunization programs on HepA vaccine efficacy
Based on the Chinese EPI, a single dose of HepA-L is administered at 18 months of age and two dose of HepA-I are administered at 18 and 24 months of age. Participants in this study were inoculated with HepA-L at 18 months of age. Anti-HAV antibody titers in infants are shown in and Figure S1. The mean anti-HAV antibody level before polio vaccination (2 months of age) was 1863.01 IU/L, with a mean positivity rate of 76.47%. At 2 months of age, there were no significant differences in antibody positivity rates (p = 0.834) and antibody levels(p = 0.412) among the six groups. Twenty-eight days after completing primary polio immunization (5 months of age), anti-HAV antibody levels had decreased to a mean of 450.97 IU/L with a positivity rate of 74.63%. At 5 months of age, there were no significant differences in antibody positivity rates (p = 0.612) and antibody levels among the six groups (p = 0.676). After completion of HepA vaccination at 24 months of age, the mean anti-HAV antibody level was 591.65 IU/L, with a positivity rate of 99.47%. At 24 months of age, there were no significant differences in antibody positivity rates (p = 0.134) and antibody levels among the six groups (p = 0.556).
Table 3. Immunization effect of hepatitis A vaccine
3.3. Impact of polio immunization programs on HepB vaccine efficacy
The HepB immunization program in China consists of three doses at 0, 1, and 6 months of age. HBsAb levels in participants are presented in and Figure S2. Prior to polio vaccine administration (2 months of age), the mean HBsAb levels was 904.38 IU/L, with a positivity rate of 96.36% (note that two doses of HepB vaccine had been administered by this time). At 2 months of age, there were no significant differences in antibody positivity rates (p = 0.484) and antibody levels (p = 0.178) among the six groups. Twenty-eight days after completing primary polio immunization at age 5 months, the mean level of HBsAb was 1428.42 IU/L, with a positivity rate of 99.58%. At 5 months of age, there were no significant differences in antibody positivity rates (p = 0.378) among the six groups. A borderline significant difference in antibody levels among the six groups was detected using the Kruskal–Wallis H-test (p = 0.048); however, following a post hoc two-by-two paired comparison using the Bonferroni method to correct the significance level, this difference fell below the threshold for statistical significance (Table S2). The third dose of HepB vaccine is completed at 6 months of age; at 24 months of age, the mean level of HBsAb was 707.84 IU/L, with a positivity rate of 86.47%. At 24 months of age, there were no significant differences in antibody positivity rates (p = 0.938) and antibody levels (p = 0.987) among the six groups.
Table 4. Immunization effect of hepatitis B vaccine
4. Discussion
In 2016, China switched polio immunization strategies by discontinuing the use of tOPV and introducing bOPV as an alternative, with a sequential immunization program of 1 dose of IPV + 3 doses of bOPV. The IPV-bOPV sequential immunization program and the full tOPV program induced similar antibody positivity rates, while the IPV-bOPV sequential immunization program induced higher antibody levels than full tOPV immunization.Citation15 Compared with the full OPV immunization program, sequential IPV-OPV program may reduce risks of VAPPs without affecting vaccination coverage, safety, or humoral responses.Citation16 Levels of the protective antibodies against type 1 and type 3 polioviruses produced by sequential immunization programs are not lower than those induced by the all-IPV program.Citation17,Citation18 Furthermore, administration of one or two doses of bOPV after IPV can enhance intestinal immunity against type 2 poliovirus, with a cross-protective effect.Citation19
Currently, both HepA-I and HepA-L are used in China. The high efficacy and long-term duration of immunity of both vaccines have been demonstrated in previous studies.Citation9 The participants in our study were vaccinated against HepA with HepA-L. High levels of maternal antibodies against HepA in infants may result in lower immunogenicity of the vaccine and reduce vaccine-associated protection; thus, HepA vaccines must be administered after maternal antibody levels have decreased.Citation20 Anti-HAV antibody levels and positivity rates are high in newborn infants because of the existence of maternal antibodies,Citation21 remain high at 6 months of age, and then decline by 12 months of age,Citation22,Citation23 consistent with the results of our study. In our study, anti-HAV antibody levels were maintained at a high level at 2 months of age because of the presence of maternal antibodies. The levels of anti-HAV antibodies decreased as maternal antibody levels waned, while the antibody positivity rate remained stable around 75%. After completing the full course of HepA vaccination at 24 months of age, levels of anti-HAV antibodies in participants had increased and the positivity rate increased to approximately 99%. A study by Liu et al. showed that antibody positivity rates could reach more than 96.8% 6 months after HepA-L vaccination and 98% 6 months after two doses of HepA-I.Citation24 A study by J Luo et al. showed that antibody positivity could reach more than 98% 28 days after HepA-L or HepA-I vaccination.Citation9 Our results suggested that HepA vaccines may achieve better results after administration of polio vaccines following different sequential immunization programs. Different IPV-OPV sequential programs do not appear to impact the efficacy of HepA vaccines.
In our study, we found that infants had the highest HBsAb antibody levels and antibody positivity rates 28 days after the completion of primary polio immunization, when two doses of HepB vaccine had already been administered. This result demonstrates the efficacy HepB vaccination. At 24 months of age, one and a half years following completion of whole-course HepB vaccination, the HBsAb positivity rate and HBsAb levels dropped to 86.47% and 707.84 IU /L, respectively, consistent with the findings of Wang et al.Citation25 This study also showed that high levels of maternal HBsAb in infants did not suppress the long-term immunogenicity of HepB vaccine. Studies have also shown that even though HBsAb antibodies decrease over time after infants have been vaccinated with the HepB vaccine, protection can last until adolescence.Citation26,Citation27 According to recently published data, China still has the highest numbers of HBV infections in the world.Citation28 To effectively prevent HepB, adolescents and adults need to be immunized with a booster vaccine to ensure that there are sufficient levels of circulating protective antibodies against HepB. No significant differences in HBsAb levels were observed among the six groups 28 days after primary polio immunization in this study. The lower mean HBsAb levels in the 2cIPV+1bOPV group may have been caused by low levels of antibodies produced by a few participants. Thus, we conclude that different IPV-OPV sequential immunization programs did not affect the efficacy of HepB vaccines; irrespective of the polio immunization program, HepB vaccination induced a strong and protective immune response, with antibody positivity rates of about 99%.
Because of the higher antibody protective rates and lower likelihood of VAPP associated with two doses of IPV compared with a single dose of IPV, China officially replaced its polio immunization strategy with a 2IPV+2bOPV program on January 1, 2020. In our study, we assessed whether 2IPV+1bOPV impacted HepA and HepB vaccine efficacy compared with other polio immunization programs. We found no significant differences in HepA and HepB vaccine efficacy between participants receiving 2IPV+1bOPV and other polio immunization programs, suggesting that the switch to 2IPV+ 1bOPV in the Chinese polio immunization strategy is unlikely to affect HepA and HepB vaccine efficacy.
Our study had several limitations. First, all participants included in the trial were selected from Liuzhou city, China, and thus the results may not be representative of the country as a whole. Second, we lacked a tOPV full immunization group as a control to assess whether the IPV-OPV sequential immunization program would have impacted the efficacy of HepA and HepB vaccines compared with full tOPV vaccination. Third, HepA and HepB vaccines administered to all participants were provided by the national immunization program; however, vaccines produced by different manufacturers may show different results. Because the participants we enrolled were concentrated in Liuzhou city, Guangxi Province, HepA and HepB vaccines came from one or two manufacturers. In addition, serum collection at 2, 5, and 24 months were designed to monitor polio immunization efficacy according to previous studies; our study was a follow-up to the work of Ting Zhao et al.Citation29 These serum collection points were not the optimal time points (1 month after immunization) to monitor HepB and HepA vaccination efficacy. In subsequent studies, the sample size should be expanded, and populations from different regions should be selected for comparative analyses to assess whether regional differences would produce different results. In addition, studies of the impact of different polio vaccination programs on the efficacy of vaccines administered within a short period of polio vaccines, such as the measles-rubella vaccine, and Japanese B encephalitis vaccine, should be conducted.
In conclusion, our study showed that sequential IPV-OPV immunization (1IPV+2bOPV, 2IPV+1bOPV, and 2IPV+1tOPV) and sIPV/cIPV immunization did not impact the efficacy of HepA and HepB vaccine. Our results may be helpful for the preparation of combined polio and HepA or HepB vaccination strategies. Considering the urgent need to complete poliovirus eradication and the biosafety issues involved in the manufacturing of cIPV, switching of polio vaccination programs should be implemented gradually according to WHO recommendations.
Author contributions
Shiyi Chen, Yuping Zhao, Hongyuan Shi, Ting Zhao and Jing Li designed the study and extracted the data. Shiyi Chen analyzed the data and drafted the manuscript. Zhiyao Yang and Ying Li revised the manuscript. Xiaolei Yang and Guoliang Li are responsible for clinical sample collection on site. Zhifang Ying and Jianfeng Wang are responsible for the pre-processing of clinical samples. All authors read and approved the final version of the manuscript.
Supplemental Material
Download MS Word (554.3 KB)Acknowledgments
We thank the investigators at the Guangxi Provincial Center for Disease Control and Prevention, the Liujiang Center for Disease Control and Prevention, and the clinical investigators who contributed to the clinical trials and sample collection.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2021.2024063
Additional information
Funding
References
- Bandyopadhyay AS, Garon J, Seib K, Orenstein WA. Polio vaccination: past, present and future. Future Microbiol. 2015;10:791–6. PMID: 25824845. doi:10.2217/fmb.15.19.
- Global Polio Eradication Initiative. Two out of three wild poliovirus strains eradicated. Global Polio Eradication Initiative, World Health Organization, Geneva, Switzerland. 2019
- Ghendon Y, Robertson SE. Interrupting the transmission of wild polioviruses with vaccines: immunological considerations. Bull World Health Organ. 1994;72:973–83. PMID: 7867144.
- Patel M, Zipursky S, Orenstein W, Garon J, Zaffran M. Polio endgame: the global introduction of inactivated polio vaccine. Expert Rev Vaccines. 2015;14:749–62. PMID: 25597843. doi:10.1586/14760584.2015.1001750.
- Hampton LM, Farrell M, Ramirez-Gonzalez A, Menning L, Shendale S, Lewis I, Rubin J, Garon J, Harris J, Hyde T, et al. Cessation of trivalent oral poliovirus vaccine and introduction of inactivated poliovirus vaccine - worldwide, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:934–38. PMID: 27606675. doi:10.15585/mmwr.mm6535a3.
- World Health Organization. Meeting of the strategic advisory group of experts on immunization, November 2012 – conclusions and recommendations. Wkly Epidemiol Rec. 2013; 88:1–16. PMID: 23311010.
- World Health Organization. Guidelines for the safe production and quality control of inactivated poliomyelitis vaccine manufactured from wild polioviruses. Addendum to the Recommendations for the production and quality control of poliomyelitis vaccine (inactivated) WHO Technical Report Series, 2004, Annex. 2003.
- China; NHaFPCotPsRo. Immunization schedules and instructions for vaccines of the national immunization program(2016 Version). Chin J Viral Dis. 2017;7(2):81–86. PMID: 27279955. doi:10.11604/pamj.2016.23.128.8756.
- Luo J, Wang X, Ma F, Kang G, Ding Z, Ye C, Pan Y, Zhao Y, Hong S, Chen J, et al. Long-term immunogenicity and immune persistence of live attenuated and inactivated hepatitis a vaccines: a report on additional observations from a phase IV study. Clin Microbiol Infect. 2019;25:1422–27. PMID: 30496870. doi:10.1016/j.cmi.2018.11.005.
- Fangcheng Z, Xuanyi W, Mingding C, Liming J, Jie W, Qi J, Yuanping G, Wen Q, Yajuan X, Jiangsen M, et al. Era of vaccination heralds a decline in incidence of hepatitis A in high-risk groups in China. Hepat Mon. 2012;12:100–05. PMID: 22509186. doi:10.5812/hepatmon.838.
- Zhang X, An J, Tu A, Liang X, Cui F, Zheng H, Tang Y, Liu J, Wang X, Zhang N, et al. Comparison of immune persistence among inactivated and live attenuated hepatitis a vaccines 2 years after a single dose. Hum Vaccin Immunother. 2016;12:2322–26. PMID: 27494260. doi:10.1080/21645515.2015.1134069.
- Bhave S, Sapru A, Bavdekar A, Kapatkar V, Mane A. Long-term immunogenicity of single dose of live attenuated hepatitis A vaccine in Indian children. Indian Pediatr. 2015;52:687–90. PMID: 26388627. doi:10.1007/s13312-015-0697-8.
- Lemon SM. Immunologic approaches to assessing the response to inactivated hepatitis A vaccine. J Hepatol. 1993;18(Suppl 2):S15–S19. PMID: 8182266. doi:10.1016/s0168-8278(05)80372-1.
- Song YJ, Lim J, Park WS, Sohn H, Lee MS, Shin DH, Kim CB, Kim H, Oh GJ, Ki M. Seropositivity among Korean Young Adults Approximately 2 years after a single-dose vaccination against Hepatitis A virus. PLoS One. 2015;10:e0142297. PMID: 26540392. doi:10.1371/journal.pone.0142297.
- Li J, Zhang ZJZ, Pan JB, Zhang HR, Li RQ, Li MZ, Lu L, Huang F, Wu J. Surveillance of immunization effectiveness and titer of type I and type III polio vaccine in Beijing before and after the adjustment of immunization strategy in 2012-2018. Zhonghua Yu Fang Yi Xue Za Zhi. 2020;54:779–83. doi:10.3760/cma.j.cn112150-20190621-00499.
- Ciapponi A, Bardach A, Rey Ares L, Glujovsky D, Cafferata ML, Cesaroni S, Bhatti A. Sequential inactivated (IPV) and live oral (OPV) poliovirus vaccines for preventing poliomyelitis. Cochrane Database Syst Rev. 2019;12:Cd011260. PMID: 31801180. doi:10.1002/14651858.CD011260.pub2.
- O’Ryan M, Bandyopadhyay AS, Villena R, Espinoza M, Novoa J, Weldon WC, Oberste MS, Self S, Borate BR, Asturias EJ, et al. Inactivated poliovirus vaccine given alone or in a sequential schedule with bivalent oral poliovirus vaccine in Chilean infants: a randomised, controlled, open-label, phase 4, non-inferiority study. Lancet Infect Dis. 2015;15:1273–82. PMID: 26318714. doi:10.1016/S1473-3099(15)00219-4.
- Asturias EJ, Bandyopadhyay AS, Self S, Rivera L, Saez-Llorens X, Lopez E, Melar M, Gaensbauer JT, Weldon WC, Oberste MS, et al. Humoral and intestinal immunity induced by new schedules of bivalent oral poliovirus vaccine and one or two doses of inactivated poliovirus vaccine in Latin American infants: an open-label randomised controlled trial. Lancet. 2016;388:158–69. PMID: 27212429. doi:10.1016/S0140-6736(16)00703-0.
- Tang G, Yin W, Cao Y, Tan L, Wu S, Cao Y, Fu X, Yan J, Jiang X. Immunogenicity of sequential inactivated and oral poliovirus vaccines (OPV) versus inactivated poliovirus vaccine (IPV) alone in healthy infants: a systematic review and meta-analysis. Hum Vaccin Immunother. 2018;14:2636–43. PMID: 29985751. doi:10.1080/21645515.2018.1489188.
- Kanra G, Yalçin SS, Ceyhan M, Yurdakök K. Clinical trial to evaluate immunogenicity and safety of inactivated hepatitis A vaccination starting at 2-month-old children. Turk J Pediatr. 2000;42:105–08. PMID: 10936974.
- Linder N, Karetnyi Y, Gidony Y, Ohel G, Levin E, Kuint J, Davidovich N, Gidony I, Mendelson E, Barzilai A. Placental transfer of hepatitis A antibodies in full term and preterm infants. Pediatr Infect Dis J. 1997;16:245–47. PMID: 9041609. doi:10.1097/00006454-199702000-00015.
- Linder N, Karetnyi Y, Gidony Y, Dagan R, Ohel G, Levin E, Mendelson E, Brazilai A. Decline of hepatitis A antibodies during the first 7 months of life in full-term and preterm infants. Infection. 1999;27:128–31. PMID: 10219645. doi:10.1007/BF02560513.
- López EL, Contrini MM, Xifró MC, Cattaneo MA, Zambrano B, Dumas R, Rouyrre N, Weber F. Hepatitis A vaccination of Argentinean infants: comparison of two vaccination schedules. Vaccine. 2007;25:102–08. PMID: 16914234. doi:10.1016/j.vaccine.2006.07.014.
- Liu XE, Wushouer F, Gou A, Kuerban M, Li X, Sun Y, Zhang J, Liu Y, J LI, Zhuang H. Comparison of immunogenicity between inactivated and live attenuated hepatitis A vaccines: a single-blind, randomized, parallel-group clinical trial among children in Xinjiang Uighur Autonomous Region, China. Hum Vaccin Immunother. 2013;9:1460–65. PMID: 23571173. doi:10.4161/hv.24366.
- Wang Z, Zhang S, Luo C, Wu Q, Liu Q, Zhou YH, Hu Y. Transplacentally acquired maternal antibody against hepatitis B surface antigen in infants and its influence on the response to hepatitis B vaccine. PLoS One. 2011;6:e25130. PMID: 21966434. doi:10.1371/journal.pone.0025130.
- Roznovsky L, Orsagova I, Kloudova A, Tvrdik J, Kabieszova L, Lochman I, Mrazek J, Hozakova L, Zjevikova A, Pliskova L. Long-term protection against hepatitis B after newborn vaccination: 20-year follow-up. Infection. 2010;38:395–400. PMID: 20589522. doi:10.1007/s15010-010-0039-7.
- Chan PK, Ngai KL, Lao TT, Wong MC, Cheung T, Yeung AC, Chan MC, Luk SW. Response to booster doses of hepatitis B vaccine among young adults who had received neonatal vaccination. PLoS One. 2014;9:e107163. PMID: 25198289. doi:10.1371/journal.pone.0107163.
- Jing W, Liu J, Liu M. Eliminating mother-to-child transmission of HBV: progress and challenges in China. Front Med. 2020;14:21–29. PMID:31974872. doi:10.1007/s11684-020-0744-2.
- Zhao T, Mo Z, Ying Z, Huang T, Che Y, Li G, Yang X, Sun M, Jiang L, Shi L, et al. Post hoc analysis of two clinical trials to compare the immunogenicity and safety of different polio immunization schedules in Chinese infants. Ann Transl Med. 2021;9:253. PMID: 33708880. doi10.21037/atm-20-2537.
